Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
S. Schu is a consultant for St. Jude Medical Inc. for training and education. T. Vancamp reports no conflict of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Neuromodulation has gained significant acceptance as a standard treatment modality for intractable chronic neurological disorders. More specifically, spinal cord stimulation (SCS) has a history dating back to its first application in 1967 ( ). Since then it has been successfully used for the treatment of chronic neuropathic pain syndromes like chronic low-back and leg pain, failed back surgery syndrome (FBSS), complex regional pain syndromes (CRPS), and cardiovascular pathologies like peripheral vascular disease and refractory angina pectoris ( ). Metaanalyses and systematic reviews support the use of SCS, and long-term results have shown to be favorable in randomized controlled trials (RCTs) when compared to reoperation and conventional medical management (CMM) ( ).
Traditional neurostimulation of implantable pulse generators (IPGs) from most manufacturers use the same waveform, herein called “tonic stimulation.” Tonic waveforms are characterized by a simple, rhythmic, charge-balanced design, and the only modifications that can be altered, within a limited output, are the frequency, pulse width, electrode configuration (neutral, anode, or cathode), and amplitude. These traditional neurostimulation devices, initially adapted from cardiac pacemaker designs, have not changed much over the years. In most devices the output in all channels is the same, with the exception of one provider (Boston Scientific Neuromodulation Inc., Valencia, CA., USA) that allows individual channel output control. IPGs traditionally used constant voltage output (e.g., Medtronic Inc., Minneapolis, MN, USA) and were later followed by constant current regulated devices (e.g., Boston Scientific and St. Jude Medical Neuromodulation Inc., Plano, TX, USA). Although there have been attempts in the relevant literature to prove the superiority of one form of neurostimulation output over the other (constant voltage vs. constant current), the real question of whether one system is consistently more likely to have a better clinical outcome over the other has never been robustly investigated ( ).
Tonic stimulation has some challenges, even if proper patient selection has been performed and candidates seem clearly eligible for SCS. There is a substantial heterogeneity regarding pain relief with tonic stimulation, with up to 20%–30% of patients being classified as poor responders or nonresponders ( ). This failure of the therapy or lack of analgesic benefit is compounded by the inability to produce adequate paresthesia coverage in hard-to-reach areas (e.g., lower back), changes in intensity of paresthesia produced by changing body positions and movements, and intolerance to paresthesia sensations in some patients.
The concept of burst stimulation arose from treating patients with tinnitus via auditory cortex stimulation. Early on it was noted that pure-tone tinnitus, but not noise-like tinnitus, was suppressed by tonic stimulation ( ). White-noise tinnitus may be caused by an augmented neuronal burst firing in the extralemniscal (nontonotopic) system, whereas pure-tone tinnitus may result from increased neuronal tonic firing in the lemniscal (tonotopic) system ( ). Electrical cortical burst stimulation also exerts an effect on the medial geniculate system, which predominantly fires in burst ( ). Based on anatomic pathways and pathophysiologic characteristics, the clinical similarities between tinnitus and pain, and the “know-how,” the experience with burst stimulation for tinnitus was translated to burst stimulation of the spinal cord, herein called burst SCS, in an attempt to modulate the medial pain pathways ( ).
Serendipitously, it was noticed that burst SCS needed to be applied at a subthreshold, paresthesia level to be comfortably tolerated by patients. Even though the applied energy levels of burst SCS were below the threshold for paresthesia sensation, its use resulted in pain control. This early use of burst SCS led to a series of preliminary studies to research its potential further ( ). Because burst SCS leads to paresthesia-free pain control, it has naturally led to placebo controlled studies ( ).
The original concept of the gate-control theory, introduced by , postulates that stimulating large myelinated Aβ fibers suppresses pain transmission through small unmyelinated C fibers and small myelinated Aδ fibers. It is believed by some that tonic stimulation produces pain control by activation of large Aβ fibers that, in turn, inhibit small fibers (C, Aδ). However, not all working mechanisms of action (MOAs) of tonic stimulation are well understood or simply ascribed to gating mechanisms. Descartes assumed that there were only ascending pathways, but we now know there are also descending pathways and a combination of segmental (antidromic) and supraspinal mechanisms (orthodromic) is the most plausible explanation for the MOA of tonic stimulation ( ). For further explanations of the MOA for tonic stimulation see Chapter 31 .
Spontaneous rhythmic burst firing can be observed in high-threshold, unmyelinated C fibers when larger myelinated fibers degenerate ( ). Like tonic stimulation, localized electroencephalography (EEG) has demonstrated that burst SCS exerts a similar effect on the lateral pain pathway that shares some of the structures that are involved in somatosensory perception, encoding discriminatory components of pain ( ). An animal model showed that burst stimulation produces a significantly greater reduction in visceral nociception when compared to tonic stimulation, even at paresthesia subthreshold levels, due to a lack of increasing spontaneous activity of neurons in the gracile nucleus ( ).
Other MOAs may be involved in burst SCS for pain control, including the selective effect of burst on Aβ fibers, similar to what has been described for sine-wave stimulation at 2 kHz and augmenting the gate-control effect ( ). Studies of frequency-dependent neuronal activation have not been performed with the square-wave stimulation used in burst SCS, but increases in burst SCS pulse frequency may selectively activate more Aβ fibers and even inhibit larger numbers of wide dynamic range (WDR) and nociceptive-specific (NS) neurons. However, higher pulse frequencies may require accompanying changes in other parameters, as frequency alone may not directly correspond to decreased neuronal firing ( ).
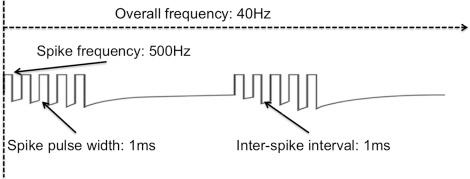
The classical description of burst stimulation involves a unique design, in that burst trains are made up of five spikes, each with a 1 ms pulse duration and an interspike interval of 1 ms, and firing at 500 Hz. These burst packages are delivered at an overall lower frequency of 40 Hz ( Fig. 52.1 ) ( ). The design of this energy or charge delivery by itself may be an important factor to consider. In an open-label double-blinded RCT study, investigators could not find a difference between burst stimulation with spike frequency at 1000 Hz and pulse width = 500 μs when compared to a 500 Hz frequency, 1000 μs pulse width stimulation design in 15 patients ( ). Pain scores on the Pain Vigilance and Awareness Questionnaire (PVAQ) and Pain Catastrophizing Scale (PCS) did not show statistically significant differences between the two groups, which suggests that charge delivery may be as important as the way stimuli are delivered (packets of burst vs. tonic) ( ). Thus 500 Hz tonic SCS should have a different clinical effect on neuropathic pain than 500 Hz burst SCS, which has been confirmed by a placebo controlled RCT ( ).
In a preclinical neuropathic pain model of cervical radiculopathy it was shown that altering the electrophysiological parameters could exert different effects on reduction in neuronal responses to noxious stimuli ( ). Changes in neuronal response following burst SCS were mediated by the stimulation parameters, thus pointing out the importance of the stimulation design as previously mentioned. The number of pulses per burst, duration of pulses, and amplitude each significantly correlated to changes in neuronal responses after burst SCS, and frequency had a significant effect on the percentage of recorded neurons that responded. Furthermore, this study showed that there is a correlation between charge per burst delivered to the spinal cord and the decrease in responses of both WDR and NS neurons after burst SCS.
There may be also a more optimal endogenous opioid release in the dorsal horn, with a maximal release at 500 Hz stimulation ( ). Hypothetical selective activation of mixed electrical and chemical synapses at 500 Hz may be involved as well, permitting subthreshold oscillatory synchronization of functionally connected areas (via gap junctions) ( ). Furthermore, burst SCS may modulate the μ-opiodergic, antinociceptive, descending pain-modulating pathways that activate off cells in the rostroventral medulla, thereby preventing further pain signals from reaching the cortex. It also may be possible that low-threshold tactile C fibers are activated, which have an antinociceptive function ( ). Activation of the nucleus raphe magnus descending inhibitory pathways preferentially attenuates C fiber activity more than Aδ fiber-mediated activity ( ).
Burst stimulation, in contrast to tonic stimulation, also seems to have an effect on the attention to pain and changes in pain that are mediated via the anterior cingulate cortex (ACC), which is part of the medial pain system ( ). Thus burst SCS exerts not only a modulatory effect on the lateral discriminatory pathway, but also an effect on the medial affective–attentional pathway. Indeed, changes in PVAQ scores demonstrate that burst is significantly better than both placebo and tonic SCS in altering the patient’s attention to pain and pain changes ( ). Burst SCS also is significantly better than tonic SCS in addressing the concept of worst and least pain, further supporting its effect on the medial pathway. Source-localized EEG data obtained in patients supports these findings. Burst SCS showed a significantly larger alpha activity in the dorsal ACC and activation for both alpha and beta oscillatory activity in the dorsolateral prefrontal cortex when compared to tonic SCS ( ).
A study by suggests that both burst and tonic SCS, without a statistically significant difference, result in suppression of nociception by attenuating dorsal horn neuronal hyperexcitability and tactile allodynia. In their study they also found that a GABA B (gamma-aminobutyric acid B) antagonist blocks the effects of tonic stimulation but not the effects of burst stimulation, suggesting that the effects of burst stimulation are not GABA dependent. In contrast to tonic stimulation, which mitigates injury-induced decreases in GABA, burst has no mitigating effect on such decreases. Burst stimulation may not activate GABAergic signaling mechanisms and may elicit a unique MOA for the benefits it exerts ( ).
In a case report looking at the effect of different SCS modalities, investigators found that both paresthesia-inducing and paresthesia-free SCS affect somatosensory evoked potentials in the same manner, suggesting that the inhibitory effect during paresthesia-free modalities is independent of the generation of action potentials, with a probable MOA at the stimulation site ( ).
In a rat model of radicular pain, the investigators compared the effects of tonic and burst SCS on pain, thalamic neuronal firing, and spinal neuropeptide expression ( ). Both tonic and burst SCS attenuated sensitivity and reduced thalamic firing for all stimuli. Both spinal substance-P and calcitonin gene-related peptide were lower for tonic and burst SCS when compared to no SCS. This study suggests that there is a similar working MOA for the parameters researched at both the spinal and supraspinal levels. In yet another study using quantitative sensory testing in 18 patients, it was found that both burst and tonic SCS increase mechanical detection thresholds, confirming findings in animal studies. Furthermore, burst tended to modulate thermal perception ( ).
In a rat study comparing tonic and burst stimulation on trigeminal allodynia using occipital nerve stimulation (ONS), it was found that both stimulation forms significantly improved symptoms, but there was a latent positive response associated with burst stimulation that made it superior to tonic stimulation ( ). Also the burst group exhibited a longer-lasting therapeutic carry-over effect than tonic stimulation.
In assessing effects of peripheral nerve stimulation (PNS) on nine subjects with ONS for migraine or trigeminal neuropathic pain and one coccygeal stimulation, investigators found an increased trend in heat pain threshold for burst when compared to tonic stimulation, with significantly greater heat pain thresholds for subjects with ONS when comparing burst to no stimulation ( ). Pressure pain thresholds were significantly greater in tonic stimulation mode when compared to no stimulation, showing that PNS is different from SCS. As the authors conclude, this may be caused by prolonged effects or an alternative pathway involved in PNS. As with tonic stimulation, not all MOAs underlying burst stimulation have been elucidated, necessitating further in-depth investigations to solve this puzzle. For a further discussion of the MOA of burst SCS see Chapter 14 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here