Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This text is primarily concerned with extrinsic deformations produced by unusual or excessive constraint of an otherwise normal fetus.
Individual deformations in this book are presented in an atlas format along with deformation sequences.
Deformations are structural defects that represent a normal response of a tissue to an external mechanical force.
Deformations are usually attributed to intrauterine molding and termed positional defects .
The mechanical pressure causing the molding can either be intrinsic (e.g., neuromuscular imbalance) or extrinsic (e.g., fetal crowding).
A deformation sequence refers to the manifold molding effects of a given deforming situation, such as the oligohydramnios sequence.
A clear distinction between deformation and malformation is critical for prognosis, management, and recurrence risk counseling.
Mechanical forces play an important role in both normal and abnormal morphogenesis. Anomalies that represent the normal response of a tissue to unusual mechanical forces are termed deformations , in contrast to malformations , which denote a primary problem in the morphogenesis of a tissue, and disruptions , which represent the breakdown of previously normal tissues. A fourth category of problems, termed dysplasias , results in abnormal structure because the tissues from which individual structures are formed are abnormal ( Fig. 1.1 ). An isolated structural defect can arise through any of these four basic categories of morphogenesis, and clarifying the nature of the underlying defect is necessary to provide proper counseling. An isolated malformation, such as an idiopathic talipes equinovarus (rigid clubfoot), can arise as a localized error in morphogenesis with a multifactorial recurrence risk and differences in birth prevalence among various racial groups and between genders. Prevalence ranges from a high of 6.8 per 1000 live births in Hawaiian and Maori people, to 1.2 per l000 in White people, to 0.74 per 1000 in US Black and Hispanic people, to 0.39 per 1000 in Chinese populations. Bilateral cases occur slightly more frequently than unilateral cases, and males are affected twice as frequently as females. Such variations in race and sex predilection occur for many different multifactorial defects, emphasizing the importance of differences in genetic background. If a clubfoot arises as part of a broader pattern of defects, it can do so through any one of these underlying categories of abnormal morphogenesis, and the recurrence risk is based on the cause of the overall pattern of abnormal morphogenesis.
A multiple malformation syndrome results in a primary developmental anomaly of two or more systems in which all of the structural defects are caused by a single underlying etiology, which can be genetic, chromosomal, or environmental (teratogenic). In some cases the cause may be unknown, but as mechanisms of abnormal morphogenesis become better understood, the number of syndromes in this category has been diminishing. Multiple structural defects can also result from a sequence in which a single primary defect in morphogenesis produces multiple abnormalities through a cascading process of secondary and tertiary problems in morphogenesis. During the evaluation of a child with multiple anomalies, it is extremely important from the standpoint of recurrence risk counseling to distinguish a malformation syndrome from multiple defects that arose from a single localized error in morphogenesis, such as spina bifida malformation sequence (when a failure in neural tube closure results in neurogenic clubfeet and bladder/bowel problems). Examples of different types of structural defects are discussed in the next paragraph.
In Fig. 1.1A , an infant with campomelic syndrome has a cleft palate, hypospadias, abnormal skeletal development, and talipes equinovarus because of a mutation in SOX9 , which resulted in a malformation syndrome. In Fig. 1.1B , an infant was born at 36 weeks after amniotic membranes ruptured prematurely at 26 weeks, resulting in oligohydramnios deformation sequence. This may have occurred spontaneously or it could have been caused by an underlying genetic connective tissue disorder such as Ehlers-Danlos syndrome, which predisposes the fetus toward early amnion rupture, ligamentous laxity, and hernias. The fetus in Fig. 1.1C has been affected by the disruption of previously normal structures because of early amnion rupture during the first trimester, resulting in strands of amnion damaging facial structures. The infant in Fig. 1.1D has an autosomal recessive skeletal dysplasia (diastrophic dysplasia) that resulted in cleft palate, malformed pinna with calcification of ear cartilage, hitchhiker thumbs, symphalangism, and talipes equinovarus with progressive scoliosis, which is particularly refractory to treatment. This condition results from a mutant sulfate transporter protein gene, which is inherited from each parent, with no signs evident in the carrier parents. To offer appropriate genetic counseling, it is important to distinguish such underlying malformation syndromes, skeletal dysplasias, and genetic connective tissue disorders from isolated defects that resulted solely from fetal disruption or late gestational constraint. Syndromes and dysplasias often have a genetic basis.
Deformations often involve either the craniofacial region or the limbs, and they are usually attributed to intrauterine molding and termed positional defects . The mechanical pressure causing the molding can either be intrinsic (e.g., neuromuscular imbalance) or extrinsic (e.g., fetal crowding). When a prolonged decrease in fetal movement occurs resulting from either type of problem, joints may develop contractures because joints require movement to form in a normal way. Thus there are predominantly two types of deformational problems: those in which the causative forces are caused by an intrinsic problem within the developing fetus (e.g., a neuromuscular insufficiency or a central nervous system developmental defect) and those in which the deformation was produced by mechanical forces that were extrinsic to an otherwise normal fetus.
The two predominant types of deformations are depicted in Fig. 1.2 , and the distinctions between these two types are based on historical and physical findings. In Fig. 1.3 , the overall pattern of intrinsic deformation due to neuromuscular insufficiency is quite similar for both congenital myotonic dystrophy ( Fig. 1.3A ), an autosomal dominant disorder with a 50% recurrence risk, and Pena-Shokeir syndrome ( Fig. 1.3B ), an autosomal recessive disorder with a 25% recurrence risk. Myotonic dystrophy results from amplification of a trinucleotide repeat in the 3′ untranslated region of a protein kinase gene on chromosome 19. Normally, there are 5–30 copies of this repeat. When there are 50–80 repeats, the result is mild disease; when there are more than 2000 repeats, infants are severely affected with congenital myotonic dystrophy, as depicted in Fig. 1.3A . This severe pattern is seen in the offspring of affected women only and becomes more likely as the mutant allele is transmitted through successive generations, a phenomenon termed anticipation .
The pattern of intrinsic neuromuscular deformation is recognizably different from the pattern seen with extrinsic deformation resulting from oligohydramnios deformation sequence, as demonstrated in Fig. 1.1B . In Fig. 1.3C , the facial effects of intrinsic deformation caused by Möbius sequence (deficient cranial nerve VI and VII function) also differ from the extrinsic facial deformations seen in Fig. 1.3D , in which prolonged oligohydramnios and a persistent transverse lie resulted in marked facial compression. Disruptive defects occur when a previously normally formed structure is partly or completely destroyed. This can result from either amniotic bands (see Fig. 1.1C ) or an interruption of blood supply ( Fig. 1.4A–B ); in some cases of early amnion rupture, both mechanisms may occur ( Fig. 1.4C ). When one monozygotic, monochorionic twin dies in utero, interruption of blood supply to fetal structures can lead to infarction, necrosis, hypoplasia, and/or resorption of tissues distal to a point at which thromboembolic debris lodge in the circulation of the surviving twin (see Fig. 1.4A ).
Extrinsic forces may result in a single localized deformation, such as a positional foot deformation, or they may cause a deformation sequence. A deformation sequence refers to the manifold molding effects of a given deforming situation. A good example of one such deformation sequence is the oligohydramnios sequence, in which oligohydramnios is responsible for a number of associated deformations (see Figs. 1.1B, 1.2B, and 1.3D ). The major cause of such deformations is uterine constraint of the rapidly growing, malleable fetus in late gestation. Most of these molded deformations have an excellent prognosis once the fetus is released from the constraining environment. However, they usually merit prompt treatment at the appropriate time to achieve the best result (usually the earlier, the better). Such treatment often involves the use of gentle mechanical forces to attempt to reshape the deformed structure into a more normal form.
A clear distinction between deformation and malformation is critical to providing parents with a clear understanding of their infant's problem and its prognosis, management, and recurrence risk. Such a clear distinction may not always be possible, and the study of structural defects in twins demonstrates the complexity of making such distinctions. Twin studies represent a unique approach to understanding both genetic and environmental etiologic contributions to the development of congenital anomalies. Twins share identical intrauterine environments, but their genetic constitutions are either homogeneous, as in monozygotic twins, or heterogeneous, as in dizygotic twins. Monozygotic twins often share the same placenta and placental vasculature, which makes them more vulnerable to vascular disruption. Late fetal crowding makes deformations much more common in twins, and deformations are equally likely in both types of twinning. Because the fetal head accounts for much of the volume in late gestation, it is particularly susceptible to extrinsic, constraint-related deformations. Among 801 infants with deformational plagiocephaly who were treated with an external orthotic device, 69 (8.6%) were of multiple-birth origin. Because there is less space within the lower portion of the uterus, the bottom twin in vertex presentation is most likely to develop torticollis-plagiocephaly deformation sequence ( Fig. 1.5 ).
Monozygotic twinning results when a fertilized zygote splits into two embryos during the first 14 days after fertilization; this splitting process is associated with an increased likelihood of early malformations, many of which lead to the threefold higher mortality rate seen in monozygotic twins compared with dizygotic twins or singletons. Among craniofacial anomalies, structural defects of the malformative or disruptive type occur more frequently in monozygotic twins compared with dizygotic twins. Malformations are often concordant, whereas disruptions and deformations are not. Among 35 twin pairs in which one or both twins exhibited craniofacial anomalies, 21 pairs were monozygotic and 14 pairs were dizygotic. Malformations were more common among monozygotic twins compared with dizygotic twins, and concordance occurred predominantly in the monozygotic group, with seven of eight pairs being concordant. Among 20 twin pairs with discordant anomalies for whom there was information regarding fetal position, the affected infant was usually vertex and inferior in the pelvis, and deformational craniofacial anomalies were often associated with early fetal head descent during the third trimester.
Monozygotic twins with placental vascular interconnections may be predisposed toward vasculodisruptive defects in the surviving twin when one twin dies in utero (termed twin disruption sequence ). Among monochorionic twin pregnancies with one dead twin (see Fig. 1.4A–B ), only 57% of the co-twin survivors were unaffected, with 19% of the co-twins dying in utero or during the neonatal period and 24% surviving with serious sequelae (e.g., cerebellar infarcts, porencephaly, hydranencephaly, microcephaly, spinal cord infarcts, hemifacial microsomia, gastroschisis, limb-reduction defects, renal cortical necrosis, atresia of the small bowel, and cutis aplasia). Among 18 twins with twin disruption sequence, most deaths occurred in the second or third trimester, and intrauterine death in the first trimester rarely caused damage to the surviving co-twin. Survivors had a poor prognosis resulting from microcephaly, spastic tetraplegia, severe intellectual disability, and therapy-resistant epilepsy.
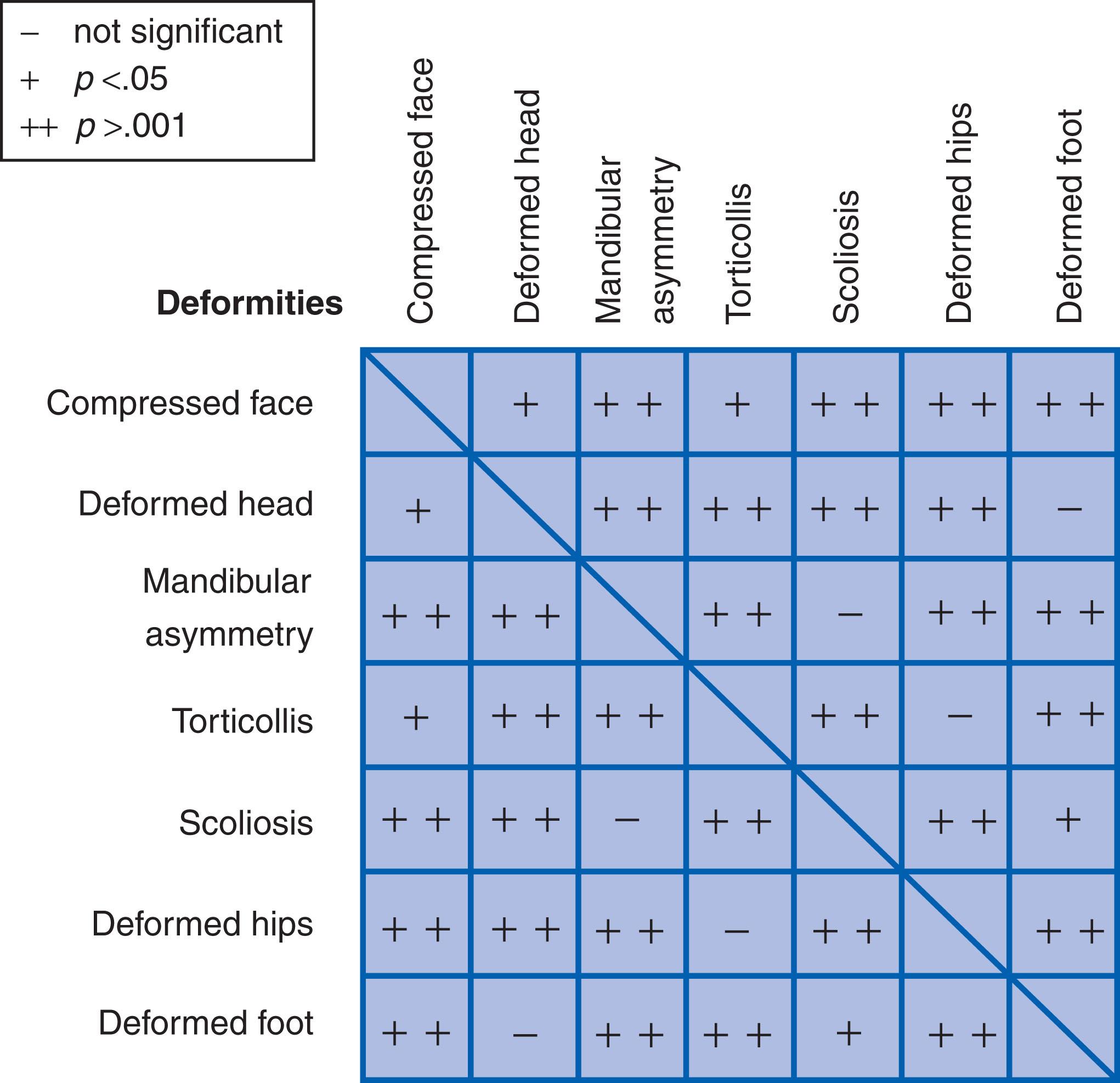
In general, deformations have a much more favorable prognosis than malformations. The collective frequency of all types of extrinsic deformations of late fetal origin is about 2% of live births. This frequency is based on the work of neonatologist Dr. Peter Dunn, who correlated clinical records of 4754 infants born in the Birmingham Maternity Hospital (United Kingdom) with maternal medical history and infant follow-up. This population study documented a 3.6% incidence of malformation, with or without deformation, and among the malformed infants in Dunn’s sample, 7.6% were also deformed. The deformation rate was 26.7% for infants with urinary tract malformations and 100% for those with bilateral renal agenesis. Of 11 infants born to mothers with premature rupture of membranes, none were malformed but 10 were deformed. Among the group of infants with postural deformations, Dunn noted that 33% had two or more deformations and that there were significant associations between facial deformity, plagiocephaly, mandibular asymmetry, muscular torticollis, scoliosis, developmental dysplasia of the hip (DDH), and talipes equinovarus. About one-third (32%) of the deformed infants had been in breech presentation. First-born infants were significantly more likely to be deformed than subsequently born infants (54% vs. 35%). Thus it is particularly important to look for deformations in the infants of primigravida mothers and in infants with malpresentation.
In a study of 1,455,405 Spanish infants born alive between 1976 and 1997, 26,290 (1.8%) had major or minor anomalies detected at birth; of this group, 3.67% also had deformations. Deformations were found four times more frequently (14.62%) among stillborn infants with anomalies. These authors distinguished three distinct patterns of deformations. One pattern featured polyhydramnios, thin skin without dermal ridges, and multiple deformations resulting from fetal akinesia-hypokinesia sequence caused by various intrinsic neuromuscular defects. A second pattern resulted in oligohydramnios with redundant thick skin and multiple deformations caused by intrinsic renal or urinary tract defects, or due to extrinsic loss of amniotic fluid. A third pattern of deformation was associated with normal amounts of amniotic fluid and was caused by various types of extrinsic fetal compression. Differences between the 0.07% frequency of extrinsic deformations in this latter study and the 2% frequency noted by Dunn reflect major differences in the classification of minor postural deformations. In Dunn’s earlier studies, 90% of the postural deformations he observed resolved spontaneously, and such transient defects were probably not counted in the Spanish study. Alternatively, with more accurate gestational dating and size determination, the incidence of large-for-gestational-age and postmature infants has been decreasing, thereby lowering the risk for late gestational constraint.
This text is primarily concerned with extrinsic deformations produced by unusual or excessive constraint of an otherwise normal fetus. Individual deformations in this book are presented in an atlas format along with deformation sequences. Because it may sometimes be difficult to exclude an underlying connective tissue dysplasia, examples of such problems are also discussed as part of the differential diagnosis. Because the major emphasis of this text is extrinsic deformations caused by uterine constraint, this category will be considered first. Next, consideration will be given to the interaction between extrinsic and intrinsic factors in the genesis of some deformations. Finally, intrinsic deformations caused by a fetal neuromuscular insufficiency are discussed.
The presumption in this category is that there is no primary problem within the fetus and that the deformations are secondary to extrinsic forces that have deformed an otherwise normal fetus. The most common cause of extrinsic deformation is uterine constraint. About 2% of babies are born with an extrinsic deformation, 90% of which resolve spontaneously; hence, these are relatively common problems. Usually the amount of amniotic fluid is adequate to cushion the fetus, allowing for normal growth and mobility before 36–37 weeks of gestation. Thus one of the major functions of the amniotic fluid is to distend the uterus and enable the fetus to move freely and to grow with equal pressure in all regions with no excessive or localized constraint. As the fetus becomes more crowded within the uterus during late gestation, it will usually settle into a position in which the largest moving fetal parts, the relatively bulky legs, have more room in the upper portion of the uterus. Thus the fetus tends to assume a vertex presentation. The implication that most extrinsic deformations are produced during late fetal life is supported by the observations of Nishimura, who found that both hip dislocation and clubfoot are rare findings in aborted fetuses before 20 weeks of gestation.
After about 35–38 weeks of gestation, the human fetus tends to grow out of proportion to the size of the uterine cavity ( Fig. 1.6 ). During this period of rapid late fetal growth, the relative proportion of amniotic fluid decreases, causing the fetus to become increasingly constrained. Uterine constraint of rapidly growing, malleable fetal structures may result in mechanically induced deformations. Because the cause of one deformation may often have led to other constraint-related problems in the pliable fetus, there may be a nonrandom occurrence of more than one deformation in the same child. Dunn found that 33% of newborn infants with one deformation problem had additional deformations ( Fig. 1.7 ). In this text, the combination of multiple deformations resulting from the same extrinsic cause is termed a deformation sequence .
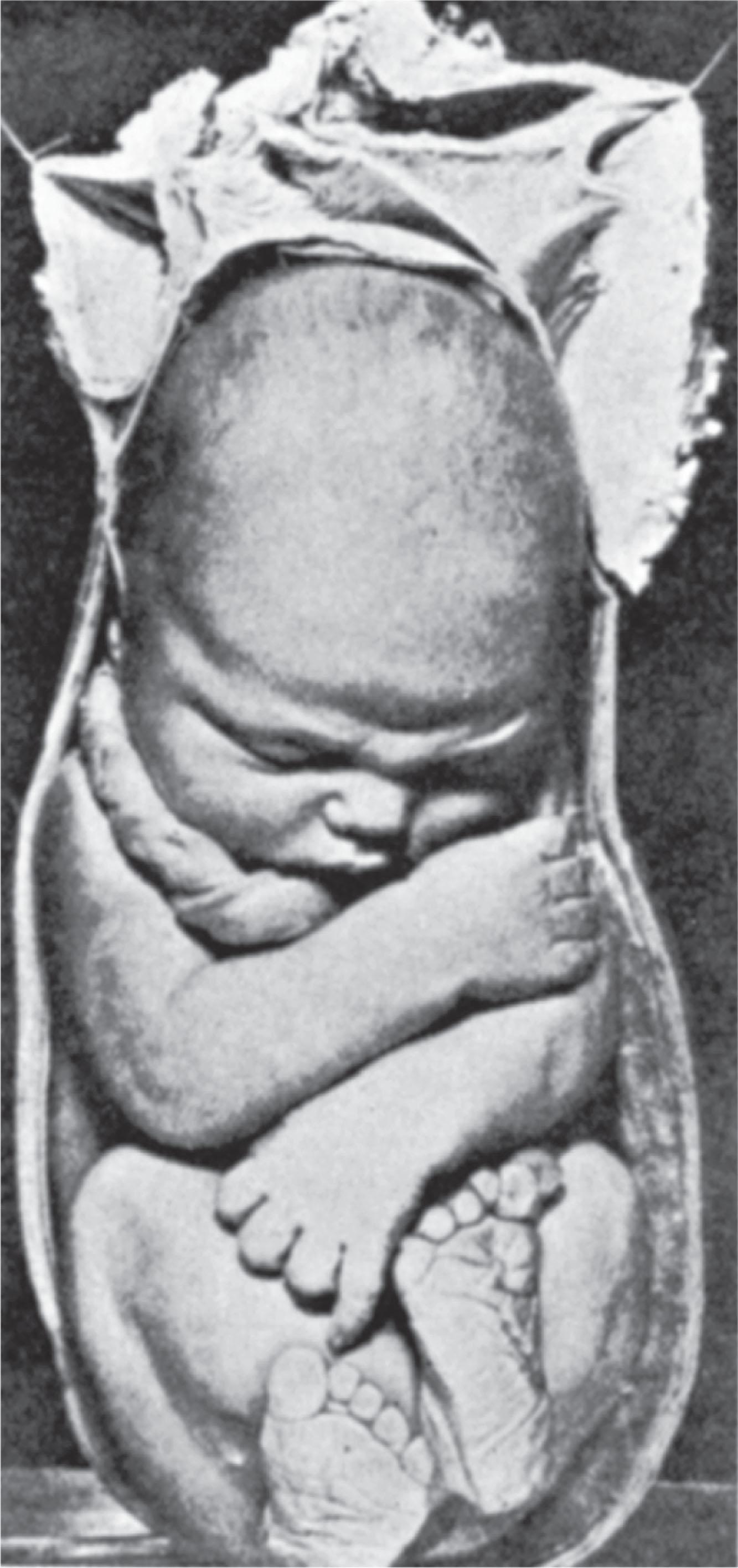
Uterine constraint tends also to reduce the rate of late fetal growth and is one cause of prenatal growth deficiency. Such newborn babies frequently show deformational evidence of uterine constraint other than growth deficiency alone. Any situation that tends to overly distend the uterus may be associated with early onset of labor and premature birth. This is a major concern for multiple-gestation infants, who simply distend the uterus prematurely, and also for the woman with uterine structural malformations or extensive uterine myomas. When fetal growth has been constrained during late gestation, catch-up growth is usually initiated promptly after delivery so that most term fetuses reach their genetically determined growth percentiles by 6–8 months of age.
If the basic type of deformation is not clearly determined at the time of birth, then the early postnatal course usually provides valuable clues as to whether the deformations noted at birth are extrinsic or intrinsic in causation. The otherwise normal infant who has been constrained in late fetal life tends to show progressive improvement in growth and form after being released from the deforming situation upon delivery. If growth has been slowed by constraint in late fetal life, the infant tends to show catch-up growth toward their genetic potential in early infancy, beginning within the first 2 months. If the growth of particular structures has been restrained, they tend to show catch-up growth toward normal form, such as with the nasal and mandibular growth restriction seen following prolonged face presentation. Joints that have been externally constrained prenatally tend to show progressive increases in their range of motion after birth, often showing more dramatic resolution with neonatal physical therapy. This is clearly evident with positional torticollis or positional foot deformations but not with structural neck malformations (e.g., Klippel-Feil malformation) or rigid clubfoot. If there is a lack of catch-up growth and/or little or no return toward normal form after birth, then further evaluation is indicated to search for a more intrinsic problem that might be responsible for the deformation(s).
As long ago as Hippocrates, it was known that uterine constraint could cause fetal deformation. Aristotle carried out experiments demonstrating that crowding of development caused limb deformations in chicks; however, little was written about constraint deformations until the Renaissance era. In 1573 Ambroise Paré of France wrote:
Narrowness of the uterus produces monsters by the same manner that the Dame of Paris carried the little dog in a small basket to the end that it didn’t grow.
In 1921, von Reuss’s text Diseases of the Newborn contained an entire chapter on postural deformations, but modern texts about the newborn have more limited coverage of this subject. Full understanding of the mechanisms and types of fetal deformations is vitally important for clinicians who deal with problems of aberrant morphogenesis. A number of investigators have made major contributions to the knowledge and understanding of extrinsic deformations during the past century. A few brief summations and quotations from representative investigators will serve to emphasize some of their important observations and interpretations.
Thompson : This Scottish naturalist and mathematician held the Chair of Natural History in St. Andrews for 64 years. He wrote:
We are ruled by gravity. … There is freedom of movement in the plane perpendicular to gravitational force. … Gravity affects stature and leads to sagging of tissues and drooping of the mouth.
von Reuss : This early Viennese neonatologist made the following statement about contractural deformities:
…the deformities occur probably most frequently from pressure on the part of the uterine wall; sometimes marks of pressure may be observed on the skin.
Chapple and Davidson : These pediatricians noted that the “position of comfort” of the deformed infant soon after birth tends to be that which had existed in utero, and repositioning the baby into the “position of comfort” might allow the clinician to more readily deduce the causative compressive factors that resulted in the deformation.
Browne : Denis Browne, an orthopedist at Great Ormond Street Children’s Hospital in London, recognized that deformed infants were often the product of an “uncomfortable” pregnancy, implying uterine constraint. He emphasized the need for controlled forces, using functional growth, in the correction of such deformations.
Dunn : This English neonatologist was responsible for resurrecting and extending knowledge regarding the importance of extrinsic deformation. He stated that:
Intrauterine forces capable of moulding the fetus increase throughout pregnancy as the infant grows, the mother’s uterus and abdominal wall are stretched, and the volume of amniotic fluid diminishes. At the same time the ability of the infant to resist deformation increases as the rate of fetal growth slows, the skeleton ossifies, and leg movements become more powerful. All these factors are, of course, themselves directly or indirectly under the influence of heredity and are involved in a dynamic interplay throughout fetal life. Nature plays her hand to the limit. The price paid for a larger and more mature infant at birth, better able to withstand the stresses of extra uterine life, is a 2% incidence of deformities.
Box 1.1 summarizes some of the factors that increase the likelihood of fetal constraint in utero; these factors are presented in more detail in this section.
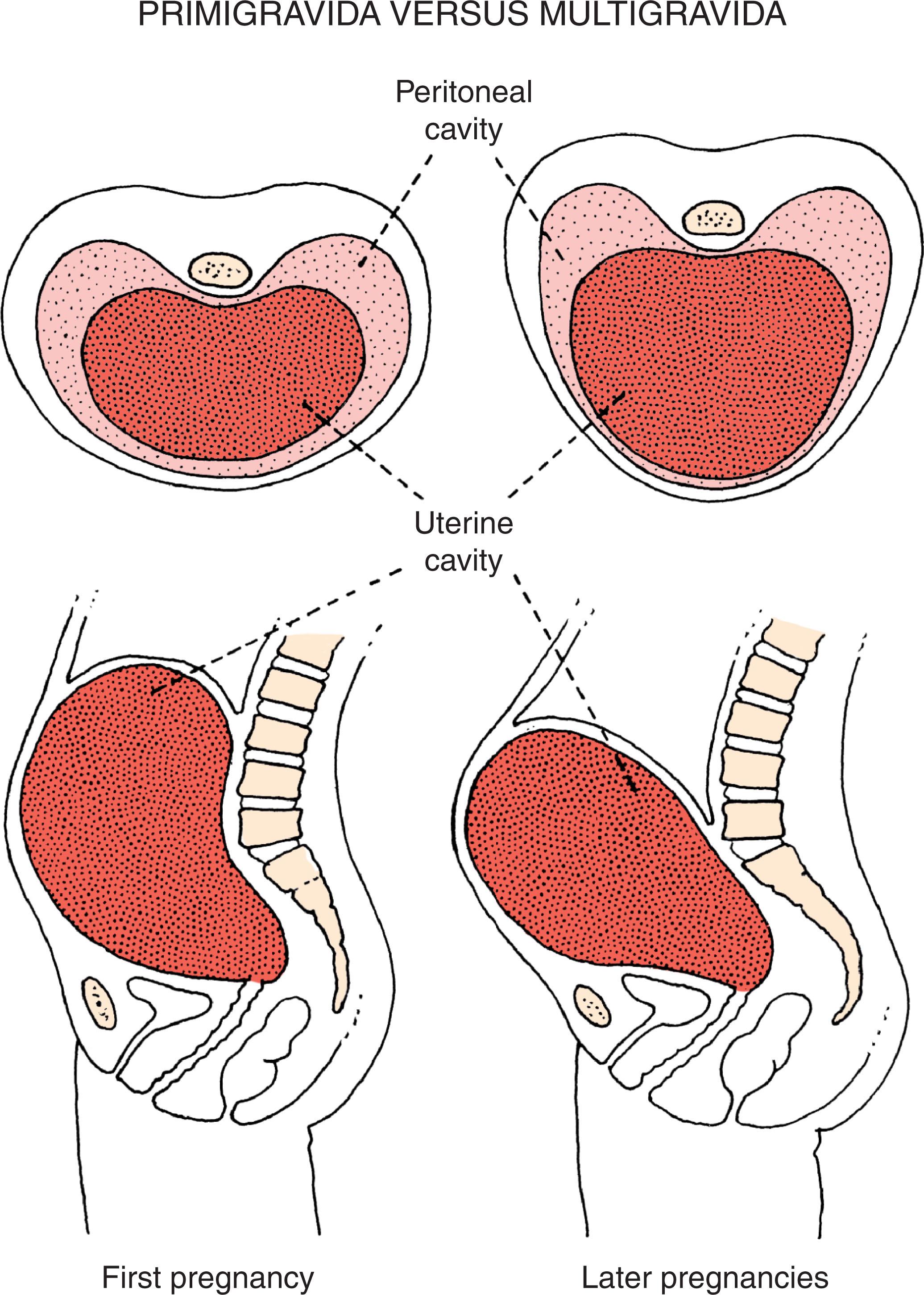
The first fetus usually experiences more constraint than later offspring because it is the first to distend the mother’s muscular uterus and abdominal wall. As a consequence, constraint-related deformations are more common in infants born to primigravida mothers than to multigravidas. Fig. 1.8 shows the form of the uterus in a primigravida compared with a multigravida, emphasizing the difference in shape and size of the structures surrounding the fetus. The greater magnitude of late fetal constraint in the primigravida is one major reason why the first-born infant is normally smaller at birth than later-born offspring. Even though the first-born infant tends to be 200–300 g smaller than later offspring at birth, they are of comparable size by 1 year of age. Thus the mild late fetal growth deficiency in the offspring of a primigravida is transient, and such infants tend to catch up to their genetically determined rate of growth within the first few months after birth. The first-born infant is also more likely to become constrained in an unusual position, such as a breech presentation, and to have consequent deformations relating to malpresentation.
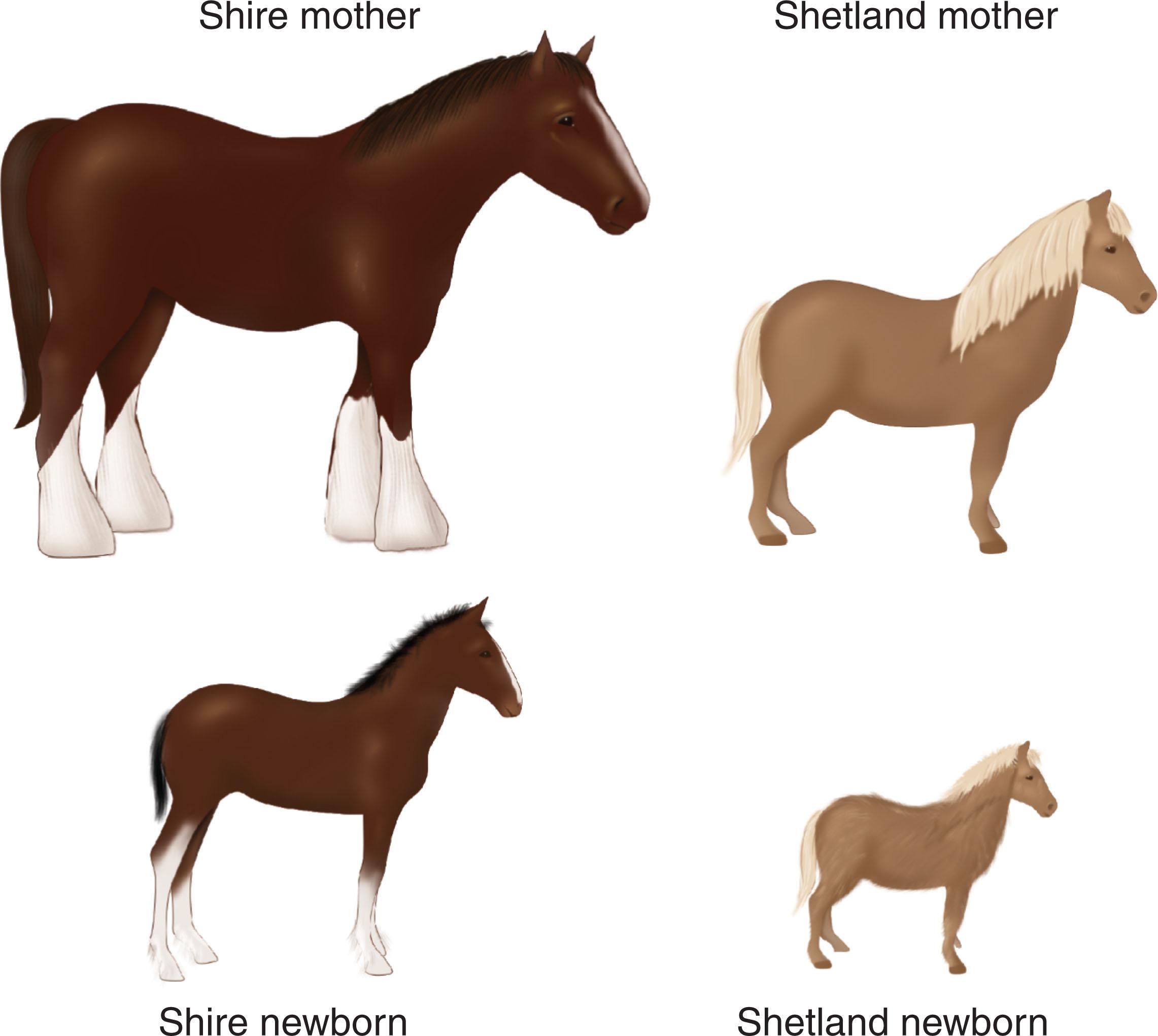
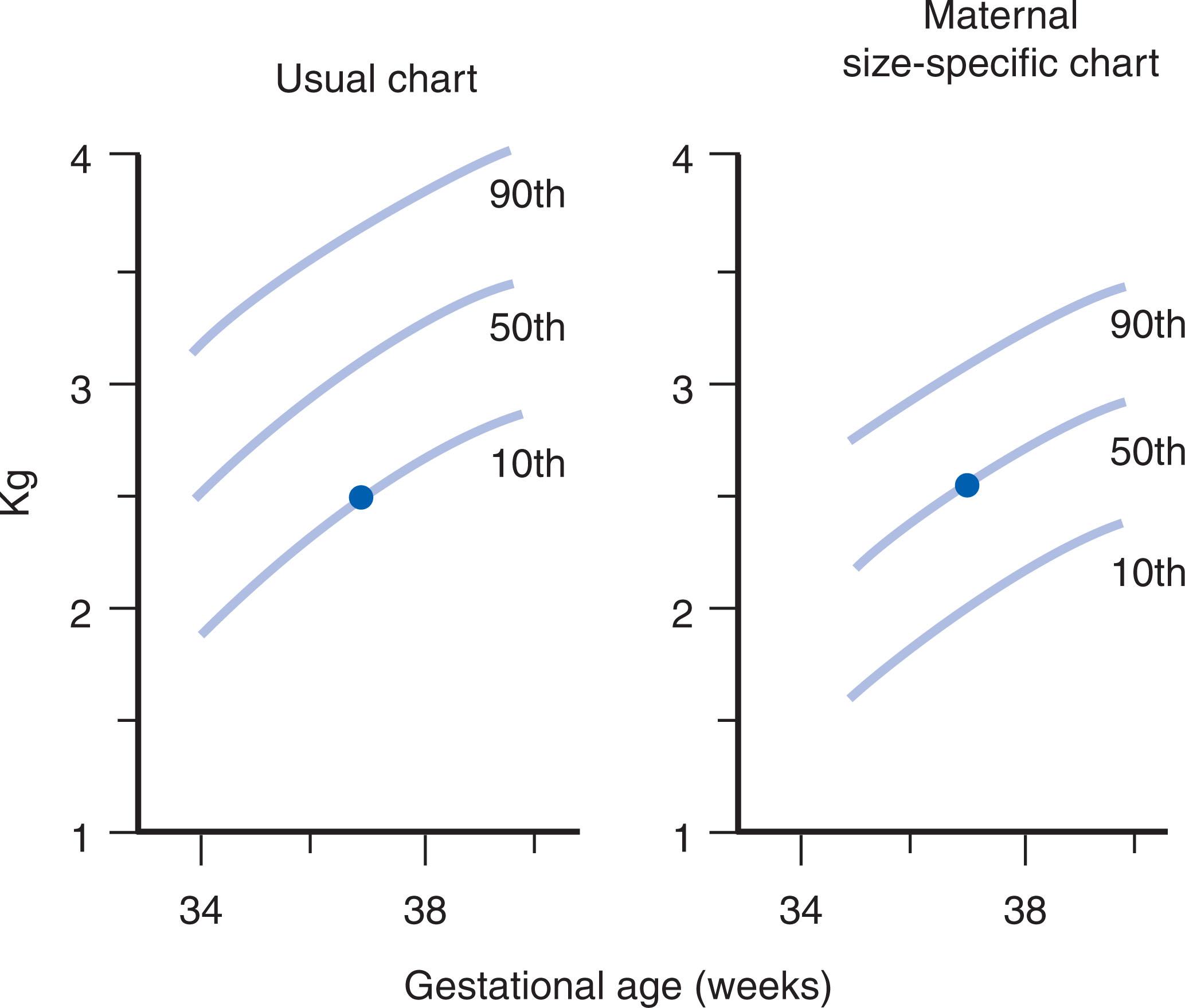
The smaller the mother in relation to fetal size, the greater the likelihood will be of deforming uterine constraint in late fetal life. Deformations are more common in offspring born to small women than in those born to larger women. This impact is readily evident in terms of birth size. Maternal size has a much greater impact on birth size than does paternal size; however, by 1 year of age, the length of the infant relates equally to maternal and paternal stature. Much of this maternal impact on birth size appears to relate to the transient effect of small maternal size in restraining late fetal growth. After birth, the infant moves into their own genetic pace of growth, which is usually evident by 6–8 months of age. The effect of maternal size on prenatal growth is illustrated in Figs. 1.9 and 1.10 . Many infants born with torticollis-plagiocephaly deformation sequence are born to parents with obvious size discrepancies (i.e., small mothers and large fathers).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here