Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chronic heart failure has long been recognized as a cause of considerable mortality and morbidity in adults. The early recognition of heart failure in the 17th and 18th centuries was that of edema, anasarca, and dyspnea, which was appropriately attributed to blood “backing up” behind an impaired pump, the heart. Early descriptions of heart failure in children were usually in the setting of rheumatic fever. It was not until 1936 that Abbott mentioned “isolated” cardiac insufficiency as a cause of death in children, although we now recognize that chronic heart failure and cardiomyopathy are indeed important causes of morbidity and mortality in children. Among children with cardiomyopathy who entered a national population-based registry in Australia between 1987 and 1996, freedom from either transplant or death at 5 years after diagnosis was only 83% for those with the hypertrophic and 63% for those with the dilated form of the disease.
The concepts that underlie our understanding of chronic heart failure in both children and adults have changed considerably in recent years. This chapter summarizes some current concepts related to key pathophysiologic processes in chronic heart failure and examines the outcomes of its treatment.
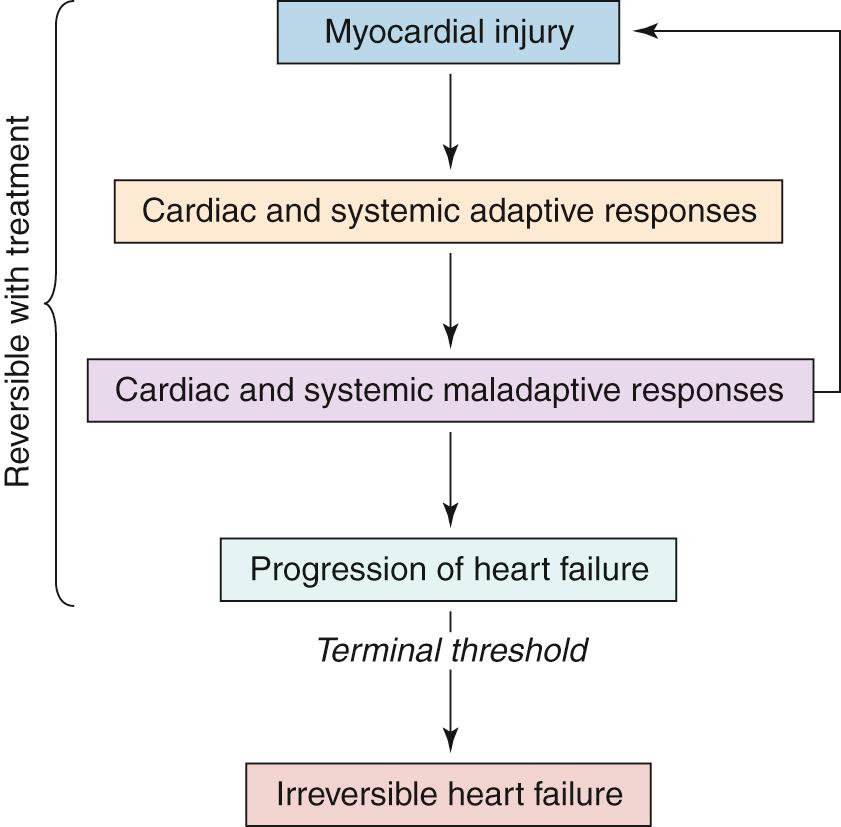
Although, for centuries, heart failure was considered to be the result of a severe and irreversible injury to the heart leading to an irremediable abnormality of the ventricle's systolic function, it is now recognized that the syndrome of heart failure reflects a more complex, dynamic, and progressive process that can no longer be defined in simple hemodynamic terms and that affects not only on the heart itself but also a myriad of extracardiac physiologic processes.
The current framework considers heart failure―whether due to ischemia, infection, altered cardiac load, or tachycardia―to be a condition in which a primary insult to the heart results in a cascade of secondary responses affecting the heart as well as related organs. It appears that irrespective of the precise nature of the primary insult, for the most part the secondary responses and clinical evolution share common features, so that the progression of heart failure represents an ordered, predictable, coordinated cascade of events. Although this may initially be reversible, it can, in the absence of treatment, result in terminal heart failure and ultimately death.
These secondary responses to cardiac injury may, at least in the initial phase, be adaptive and designed to preserve the flow of blood to the vital organs. Thus, in response to a regional injury of the myocardium, global function is maintained by invoking a number of compensatory mechanisms. The regional function of the uninjured myocardium increases and the ventricle hypertrophies as growth factors within the myocyte accelerate the synthesis of protein and growth of the myocyte. As described later, a reduction in perfusion pressure within the kidneys is detected by receptors in the renal arterioles, activating the complex renin-angiotensin-aldosterone system to cause constriction of the efferent arterioles so that the glomerular filtration pressure is maintained along with a balance of salt and water. Increased activation of the neuroendocrine system, manifest by the systemic release of neurohormones such as noradrenaline and adrenaline, maintains cardiac output through chronotropic and inotropic activity.
With time, these mechanisms become maladaptive as the patient progresses into a phase of decompensated heart failure. The increase in left ventricular mass together with dilation of the ventricle augments mural stress within the myocardium and its consumption of oxygen, potentially worsening the myocardial injury. Chronic activation of the renin-angiotensin system results in edema, the elevation of pulmonary arterial pressure, and increased afterload. Sympathetic activation increases the risk of arrhythmia and sudden death. Although these changes may be reversed by successful treatment, it has been suggested that such treatment must be initiated before the patient reaches the so-called terminal threshold, after which recovery of left ventricular function is not possible ( Fig. 65.1 ).
The ability to accurately describe the function of the heart, its metabolic demands and its interactions with the vasculature, is of paramount importance in analyzing the mechanisms of circulatory failure and the effects of interventions in patients with myocardial disease. In clinical practice our assessment of cardiac function is usually limited to the indirect estimation of ventricular systolic and end-diastolic pressure, ejection fraction, and, in some echocardiography laboratories, the assessment of mural stress. However, a complete evaluation of cardiac function would extend further, ideally to include an indicator of ventricular systolic and diastolic performance that is relatively independent of load, an assessment of the myocardial consumption of oxygen, and an examination of the relationship between ventricular performance and cardiac load.
Since Suga presented his analysis of the instantaneous pressure-volume relationship and subsequently developed the concept of time-varying elastance, there has been heightened interest in the use of the pressure-volume relationship in assessing ventricular performance. This has been especially true in recent years with the introduction of the conductance catheter technique, which allows high-fidelity online measurements of ventricular pressure and volume at fast acquisition speeds.
The classic work of Wiggers, which describes changes in left ventricular pressure and volume during the cardiac cycle, has provided the foundations for our current understanding of ventricular function. In Wiggers’ schema, the cardiac cycle begins with the onset of depolarization on the electrocardiogram, which is soon followed by an increase in pressure within the ventricle. When left ventricular pressure exceeds left atrial pressure, the mitral valve closes. The aortic valve remains closed while aortic pressure still exceeds left ventricular pressure, and ventricular volume therefore remains constant; so-called isovolumic contraction exists. When left ventricular pressure exceeds aortic diastolic pressure, the aortic valve opens and the ventricle begins to eject. Consequently the volume of the left ventricle falls ( Fig. 65.2 ).
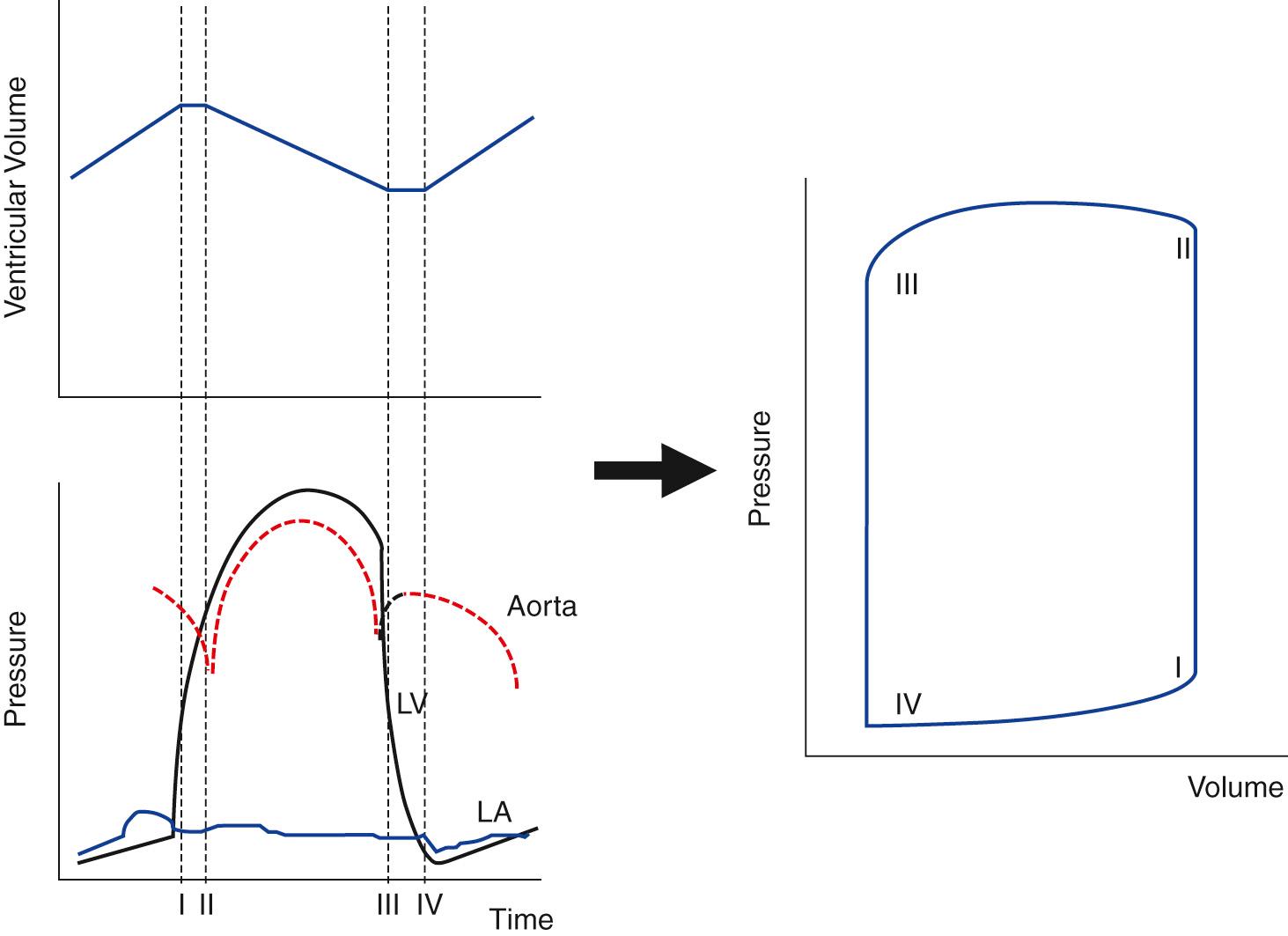
Diastole is traditionally assumed to begin with closure of the aortic valve; however, the decay in ventricular pressure (relaxation) begins before this event. After aortic valve closure, ventricular pressure continues to decay rapidly―an energy-requiring mechanism―together with the passive release of myocardial elastic forces generated during contraction. As the ventricular pressure continues to decay, the mitral valve initially remains closed. The period of relaxation during which ventricular volume remains constant is termed isovolumic relaxation.
When ventricular pressure falls below atrial pressure, the mitral valve opens and ventricular filling begins. During the early period of ventricular filling, the ventricle's pressure falls. This anomalous relationship between pressure and volume is thought to result from restorative forces, which attempt to restore the shape of the ventricle to that at end-diastole. After this time, both pressure and volume increase in the ventricle, which exhibits elastic behavior. Later in diastole the rate of ventricular filling is further augmented by atrial contraction.
Up to this point, we have considered the temporal changes in left ventricular pressure and volume; however, the essence of the pressure-volume analysis is to consider the relationship between ventricular pressure and volume, represented by the pressure-volume loop. The latter has four characteristic phases. Beginning at the bottom right hand corner (I to II in Fig. 65.2 ), an initial upstroke represents the rapid increase in ventricular pressure, with little volume change; this is isovolumic contraction. There is then a rapid fall in ventricular volume as ventricular ejection proceeds to the end-systolic point (II to III). Ventricular pressure then rapidly falls, with little volume change, as the ventricle enters the isovolumic relaxation phase (III to IV). Finally, ventricular volume increases to its end-diastolic level, reflecting ventricular filling (IV to I).
Suga noted that at a constant inotropic state, alterations in ventricular load resulted in a population of pressure-volume loops in which, at any time in the cardiac cycle, the pressure-volume points follow a straight line. It was proposed, therefore, that cardiac contraction could be modeled as a time-varying elastance, with maximal elastance occurring at end-systole (end-systolic elastance), represented by the upper left-hand corner of the pressure-volume loop ( Fig. 65.3 ).
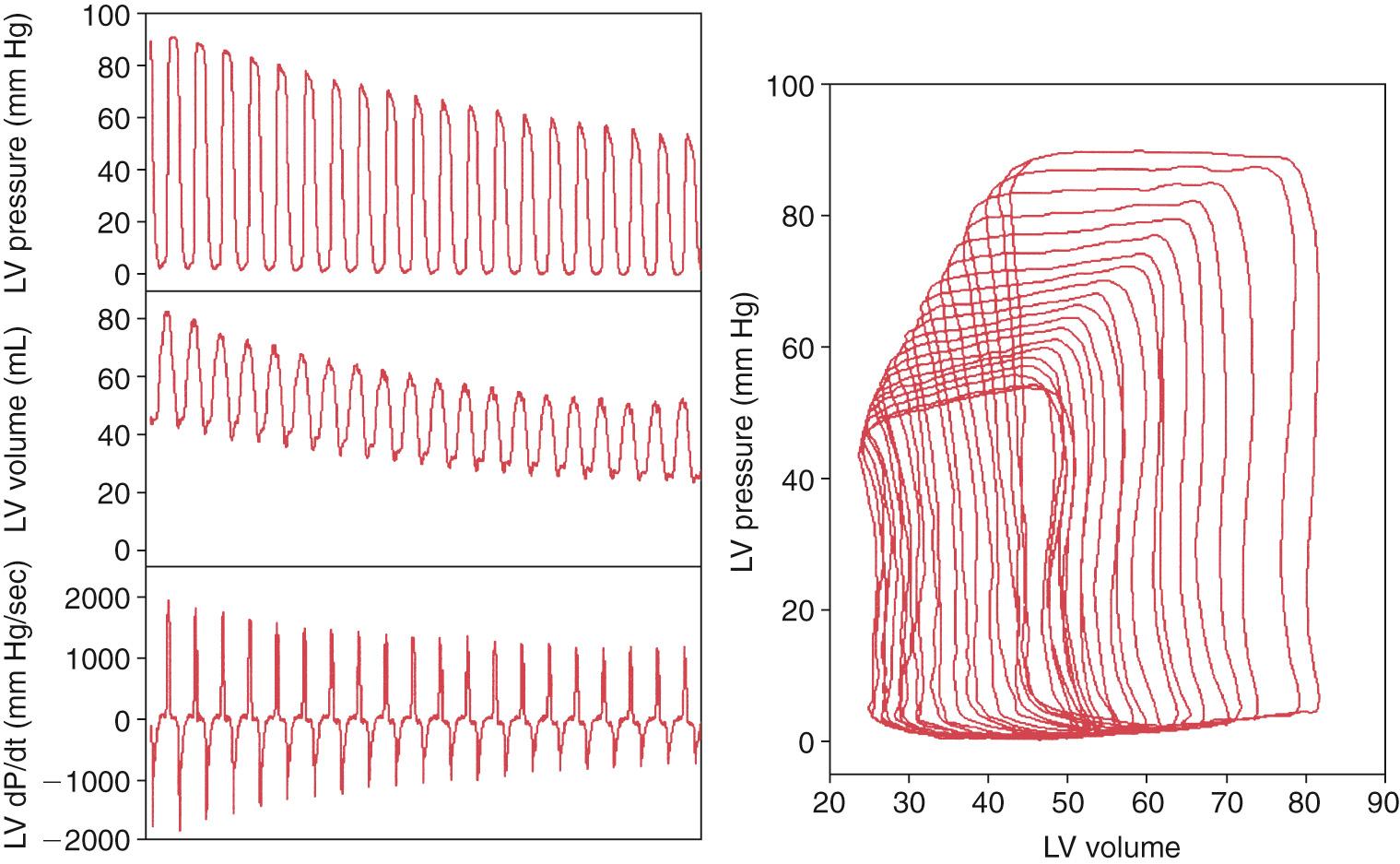
While the end-systolic pressure-volume relationship has been considered the definitive measure of ventricular contractility, the importance of other indices should not be underestimated. It is important to emphasize that there is no single gold-standard measure, which will encompass the complex physiologic processes that determine myocardial contractility, rather, there are a number of measures, each of which provides individual pieces of a complex jigsaw.
It must be appreciated that the pressure-volume relationship provides a wealth of information about cardiovascular physiology beyond end-systolic elastance. The intricate coupling between the ventricle and the vasculature is an extremely important clinical determinant of cardiovascular function. Although many treatments for heart failure are aimed at augmenting ventricular systolic performance, it is clear that without the ability of the vasculature to convert within itself the increased pressure work of the ventricle into flow work, these therapeutic strategies would be of little benefit. One measure of the efficiency of ventriculovascular coupling, based on an examination of the pressure-volume relationship, examines the coupling between end-systolic elastance and arterial elastance to illustrate how the arterial response determines the physiologic effect of an increase in contractility during inotropic stimulation.
The pressure-volume relationship can also provide important information regarding the energetic state of the ventricle. In many critically ill patients with myocardial disease, the relationship between myocardial oxygen demand and supply is already precarious; it is therefore imperative that any potentially desirable augmentation of ventricular performance should not be offset by adverse effects on myocardial metabolism and energetics. Suga demonstrated that the total energy consumption of the ventricle can be quantified by the specific area in the pressure-volume diagram that is bounded by the end-systolic and end-diastolic pressure-volume relations and the systolic pressure-volume trajectory. The scope of the pressure-volume diagram therefore extends beyond cardiac mechanics to include cardiac energetics and mechanoenergetic coupling under varying contractile conditions.
The use of the pressure-volume relationship to assess the diastolic properties of the ventricle is based on the assumption that throughout the period during diastole when both volume and pressure are increasing, the ventricle exhibits elastic behavior. As a result, at any point during this time, the slope of the relationship between pressure and volume represents ventricular compliance. As the normal pressure-volume relation at this time is curvilinear, chamber compliance becomes lower as filling proceeds, indicating that the cavity has become stiffer. The pressure-volume curve during this part of diastole is usually assumed to be exponential and to show behavior characteristic of Lagrangian stress, so that, if pressure is plotted logarithmically and volume linearly, then a linear relationship will be obtained; it is then possible to calculate its slope and intercept.
There are few data addressing the changes that occur in the ventricular pressure-volume relationship in children with myocardial failure. However, studies in adults have shown that assessment of the pressure-volume relationship can be used to determine the effects of progressive myocardial failure on integrated cardiovascular performance.
Studies investigating the matching of ventricular properties to arterial load are particularly important in this respect. In normal subjects with an ejection fraction of 60% or more, ventricular elastance is nearly double arterial elastance. This condition affords an optimal coupling between ventricular work and oxygen consumption. In patients with moderate heart failure and ejection fractions of 40% to 59%, ventricular elastance is almost equal to arterial elastance, a condition affording maximal stroke work from a given end-diastolic volume. However, in patients with severe heart failure, with ejection fraction of less than 40%, ventricular elastance is less than half of arterial elastance, which provides a suboptimal relationship between ventricular work and either oxygen consumption or stroke volume. These studies suggest that ventriculoarterial coupling is normally set toward maximizing work efficiency in terms of the relationship between left ventricular work and oxygen consumption. As heart function becomes impaired in patients with moderate cardiac dysfunction, ventricular and arterial properties are initially matched in order to maximize stroke work at the expense of work efficiency. However, as cardiac dysfunction becomes severe, the ventricle and vasculature become uncoupled, so that neither the stroke work nor work efficiency are near maximum for patients with severe cardiac dysfunction.
Whereas up to this point we have considered the function of the left ventricle as a whole, regional dyssynchrony of left ventricular function is frequently observed in patients with heart failure, in whom it results in inefficiencies in the contraction of left ventricle, a decreased cardiac output, and increased risk of sudden cardiac death ( Fig. 65.4 ). Recently, new therapies aimed at restoring mechanical synchrony in such patients have been shown to result in improvements in symptoms and outcomes.
Before discussing the cellular mechanisms associated with the development of myocardial failure, it is necessary to examine the structure and function of the normal cardiomyocyte. This section examines some of the principles related to this topic, although it will not provide a comprehensive review of the multitude of intracellular and intercellular messengers; these have been considered in some excellent specialist reviews. Cardiac myofibers are composed of groups of muscle cells (cardiac myocytes) connected in series and surrounded by connective tissue. Each cardiomyocyte is bounded by a thin bilayer of lipid (the sarcolemma) and contains bundles of myofibrils arranged along its long axis. These myofibrils, in turn, are formed of repeating sarcomeres, the basic contractile units of the cell, composed of thick and thin filaments, which provide the myocyte with its characteristic striated pattern. The thick filaments consist of interdigitating molecules of myosin and the myosin-binding proteins, while the thin filaments consist of monomers of α-actin as well as the regulatory proteins α-tropomyosin and troponins T, I, and C. A third filament within the myofibril is the giant protein titin.
Cardiac myocytes are joined at each end to adjacent myocytes at the intercalated disc. This disc contains gap junctions (containing connexins) that mediate electrical conduction between cells and mechanical junctions, composed of adherens junctions and desmosomes. The myocyte also contains an extensive and complex network of proteins linking the sarcomere with the sarcolemma and, in turn, with the extracellular matrix. This highly organized cytoskeleton provides support for subcellular structures and transmits mechanical and chemical signals within and between cells by activating phosphorylation cascades.
Myocardial activation is dependent on excitation-contraction coupling. This is mediated through the release of calcium into the myocyte from the extracellular space but more importantly from intracellular stores, particularly from an intracellular network of membranes, the sarcoplasmic reticulum. It appears that the generation of an action potential facilitates the influx of calcium from the extracellular space through the so-called L-type calcium channels, which are particularly concentrated in specialized areas of the sarcolemma (transverse tubules) and invaginate into the cell to reach its interior, close to receptors on the surface of the sarcoplasmic reticulum (the so-called ryanodine receptors). The increase in the intracellular concentration of calcium, which results from its influx from the extracellular space, triggers further release of calcium from the sarcoplasmic reticulum. The calcium activates myocardial contraction through its interaction with regulatory proteins on the myofibrils. Conversely, diastole is heralded by the reuptake of calcium into the sarcoplasmic reticulum through the activation of an energy-dependent mechanism residing with the so-called sarcoplasmic reticulum calcium ATPase, which itself is regulated by a number of stimulatory and inhibitory proteins, in particular the inhibitory protein phospholamban.
The ambient level of myocardial activation is modulated by the actions of catecholamines through their interaction with specific receptors on the surface on the myocyte. Stimulation of these receptors invokes a series of complex intracellular phosphorylation cascades that modulate not only the rate of influx of calcium from the extracellular space but also the release and reuptake of calcium from the sarcoplasmic reticulum and the affinity of the myofibrillar proteins for calcium.
Central to the function and homeostasis of the cardiomyocyte is the mitochondrion. As the heart is the organ in the body with the highest rate of oxygen uptake and an enormous demand for the continuous synthesis of adenosine triphosphate by oxidative phosphorylation, cardiac myocytes have a very high density of mitochondria. This central role for the mitochondrion as the power source for the cell and its position as the major site for the transformation of energy within the myocyte has been well established. Energy is stored in the form of high-energy phosphate bonds in adenosine triphosphate. The free energy necessary for the formation of the adenosine triphosphate by the phosphorylation of adenosine diphosphate is derived from the oxidation of nicotinamide adenine dinucleotide by the electron transport chain.
In addition to playing a central role in the metabolism of oxygen, it is now recognized that the mitochondrion plays a crucial role in both apoptosis and necrosis through its so-called permeability transition pores. The mitochondrion contains all the necessary machinery for apoptosis and is now acknowledged to be a key determinant of whether a myocyte will live or die after a pathologic insult. Central to this determination is the role of reactive oxygen species, generated by the diversion of electrons from the electron transport chain. Although it has long been established that excessive levels of superoxide may result in damage to biologic molecules―for example the sarcolemma and intracellular proteins―it is also established that reactive oxygen species play a central signaling role within the cell, which may not only regulate the key metabolic pathways within the cell but also prevent apoptosis and cellular necrosis. It is clear, therefore, that the mitochondrion and reactive oxygen species play a central role as executioner or savior in determining the viability of the cardiomyocyte.
Having considered the basic physiology of the cardiac myocyte, it is of interest to consider that any of these elements―the myofibrils, the sarcolemma, the gap junctions, the cytoskeleton, the mechanism of excitation-contraction coupling, the adrenergic receptors, or the mitochondria―may contribute to the pathogenesis of heart failure. This role may be a primary one; for example, the abnormality of the mitochondrion seen in a patient with a so-called mitochondrial cardiomyopathy ; the abnormality of the key component of the cytoskeleton, dystrophin, in the patient with muscular dystrophy ; or the mutation in a sarcomeric protein in a patient with hypertrophic cardiomyopathy. Abnormalities of these elements may also occur secondary to a primary insult originating outside the myocyte. They may thus represent the final common pathway in the development of heart failure and the maladaptive myocardial response to a host of primary external insults. Thus the development of heart failure secondary to ischemia-reperfusion injury may be associated with abnormalities of the myocardial mitochondrion in association with alterations in the expression and function of the adrenergic receptors. In patients with viral myocarditis, changes in the function of the mitochondrion may herald the onset of apoptosis, whereas enteroviral proteases may cleave dystrophin, leading to a secondary impairment of the mitochondrion's function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here