Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A choledochal cyst is a congenital dilatation of the biliary tract. The dilatation can be found along any portion of the biliary tract. However, the most common site is the choledochus. The diameter of the bile duct varies according to the child’s age. The normal diameter of the common bile duct (CBD) is seen in Table 44.1 . Any diameter of the bile duct greater than the upper limit for age should be considered abnormal.
| Age (Years) | Range (mm) | Mean (mm) |
|---|---|---|
| ≤4 | 2–4 | 2.6 |
| 4–6 | 2–4 | 3.2 |
| 6–8 | 2–6 | 3.8 |
| 8–10 | 2–6 | 3.9 |
| 10–12 | 3–6 | 4.0 |
| 12–14 | 3–7 | 4.9 |
Different classifications have been proposed for choledochal cyst (CC). However, the Todani classification has been the most widely accepted ( Fig. 44.1 ). According to this classification, CCs are classified into five types:
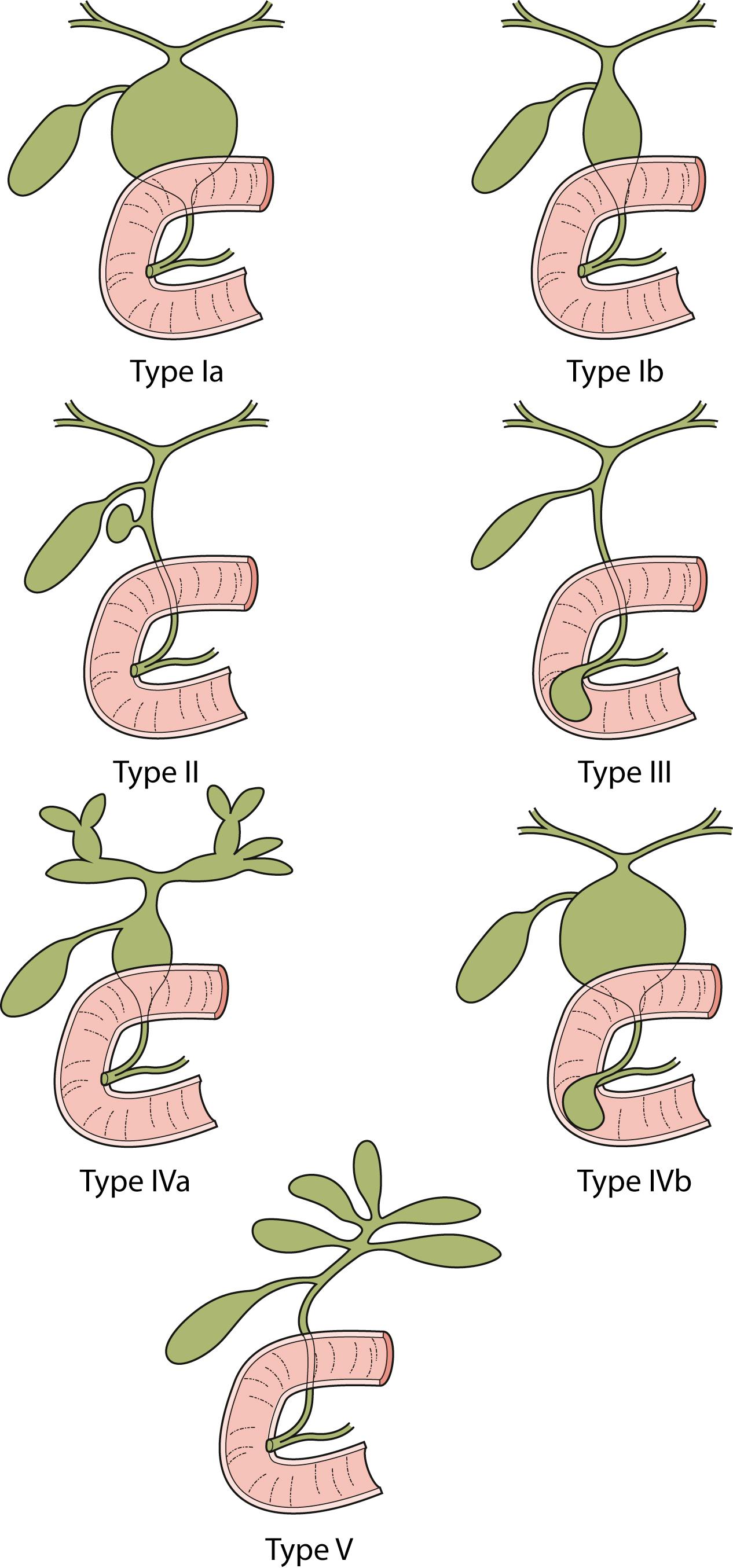
Type I
Ia: Cystic dilatation of the CBD
Ib: Fusiform dilatation of the CBD
Type II: Diverticulum of the CBD
Type III: Choledochocele (dilatation of the terminal CBD within the duodenal wall)
Type IV
IVa: Multiple cysts of the extrahepatic and intrahepatic ducts
IVb: Multiple extrahepatic duct cysts
Type V: Intrahepatic duct cyst (single or multiple, as in Caroli disease).
Forme fruste is a special variant of CC characterized by pancreaticobiliary malunion, but little or no dilatation of the extrahepatic bile duct. Children present with symptoms similar to those in patients with a CC. Excision of the extrahepatic bile duct is recommended in these children because of the likely eventual development of cancer due to chronic pancreaticobiliary reflux.
Type I CCs predominate. Together with type IVa cysts, they account for more than 90% of cases. Caroli disease is characterized by segmental saccular dilatation of the intrahepatic bile ducts. It may affect the liver diffusely or be localized to one lobe.
There are many theories to explain the development of a CC. However, none of these can explain the formation of the five different types of CC. CCs seem to be either congenital or acquired. Congenital cysts develop during fetal life. These appear to develop as a result of a prenatal structural defect in the bile duct. Shimotake et al. found that the total number of ganglion cells within the wall of these CCs is significantly lower than in control specimens. Also, smooth muscle fibers are more abundant in the cystic type than in the fusiform type.
CCs, which develop later in life, are considered “acquired.” The theory of the long common biliopancreatic channel proposed by Babbitt has been widely accepted to explain the formation of this type. Normally, the terminal CBD and pancreatic duct unite to form a short common channel, which is well surrounded by the Oddi sphincter. This normal anatomic arrangement prevents the reflux of pancreatic fluid into the bile duct. If this common channel is long and part of it is not surrounded by the normal sphincter, pancreatic secretions can reflux into the biliary tree ( Fig. 44.2 ). Proteolytic enzymes from the pancreatic fluid are activated and can cause epithelial and mural damage that leads to mural weakness and dilatation of the choledochus. This theory is supported by the fact that high concentrations of activated pancreatic amylase and/or lipase have been found in patients with CC and long pancreaticobiliary channels. In an experimental study, CC was produced by creating a choledochopancreatic end-to-side ductal anastomosis. In addition, a high incidence of a common channel has been detected in CC patients.
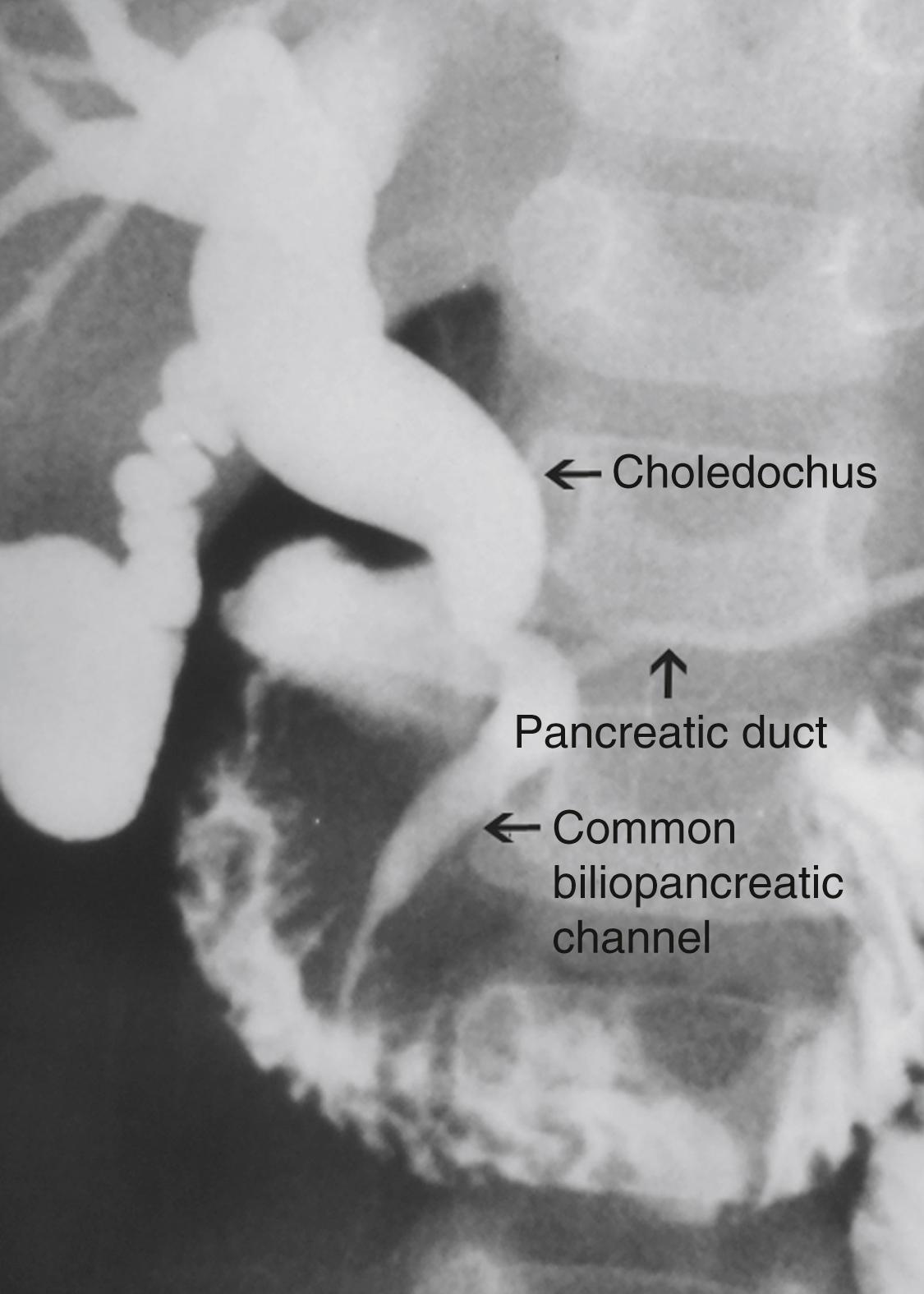
Obstruction at the level of the pancreaticobiliary junction may be an associated causal factor in choledochal dilatation. An experimental model for the study of cystic dilatation of the extrahepatic biliary system has been produced by ligation of the distal end of the CBD in the newborn lamb. High biliary pressure in patients with CC have also been recorded during operative correction.
In adults, an anomalous union of the pancreaticobiliary duct is defined when the common pancreaticobiliary channel is longer than 15 mm or when its extraduodenal portion is more than 6 mm. In a study of 264 infants and children undergoing endoscopic retrograde cholangiopancreatography (ERCP), the maximal length of the common channel was found to be 2.7 mm among children 3 years or younger, 4 mm among children 4–9 years old, and 5 mm between 10 and 15 years old.
A genetic basis for CC is suspected because its female predominance and different prevalence among countries in Asia and Europe versus United States. Using “trio-based” exome-sequencing of 31 patients with CC, Wong et al. recently reported 13 genes that were recurrently mutated at different sites.
An inflammatory reaction within the CC is noted in most cases. It is minimal in infants and gradually becomes more significant as the patient gets older. The degree of mucosal ulcerations and pericystic inflammation becomes more severe after repeated bouts of cholangitis. Stones or debris may be found in the CC, along with a dilated intrahepatic bile duct and a common biliopancreatic channel. Liver histology varies from normal to cirrhosis, depending on the patient’s age and degree of cholangitis.
Females are affected more often than males. In our series of 400 cases, the female-to-male ratio was 3.2:1. Clinical presentations differ according to the age of onset and the type of cyst. An abdominal mass or jaundice is a common finding in an infant with CC, whereas abdominal pain is more often seen in older children. The cystic form usually presents with an abdominal mass, whereas the fusiform type is usually found in patients presenting with abdominal pain. Choledochal cysts diagnosed antenatally are more likely cystic in nature.
Clinical manifestations among our 400 cases included abdominal pain (88%), vomiting (46%), fever (28%), icterus (25%), discolored stool (12%), abdominal tumor (7%), and the classic triad (2%).
Complications such as perforation and hemobilia are rare. However, pancreatitis is common. Malignant change is a late complication, mostly seen in adults.
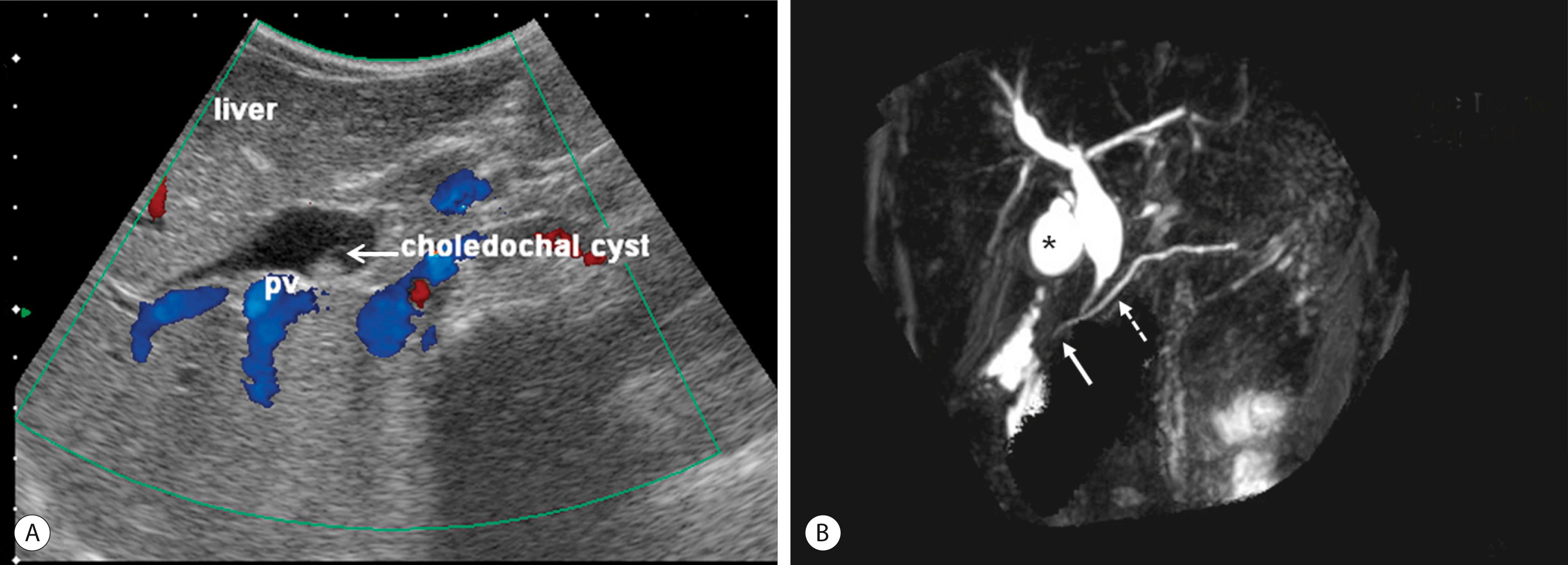
Ultrasonography (US) is the initial imaging method of choice ( Fig. 44.3A ). Contour and position of the CC, the status of the proximal ducts, vascular anatomy, and hepatic echotexture can be evaluated on US.
ERCP allows excellent definition of the cyst as well as the entire anatomy, including the pancreatobiliary junction. However, this investigation is invasive and has complications such as pancreatitis, perforation of the duodenal or biliary tracts, hemorrhage, and sepsis.
Magnetic resonance cholangiopancreatography (MRCP) is highly accurate in the detection and classification of the cyst (see Fig. 44.3B ). The overall detection rate of MRCP for a CC is very high (96–100%) and should be considered a first-choice imaging technique for evaluation.
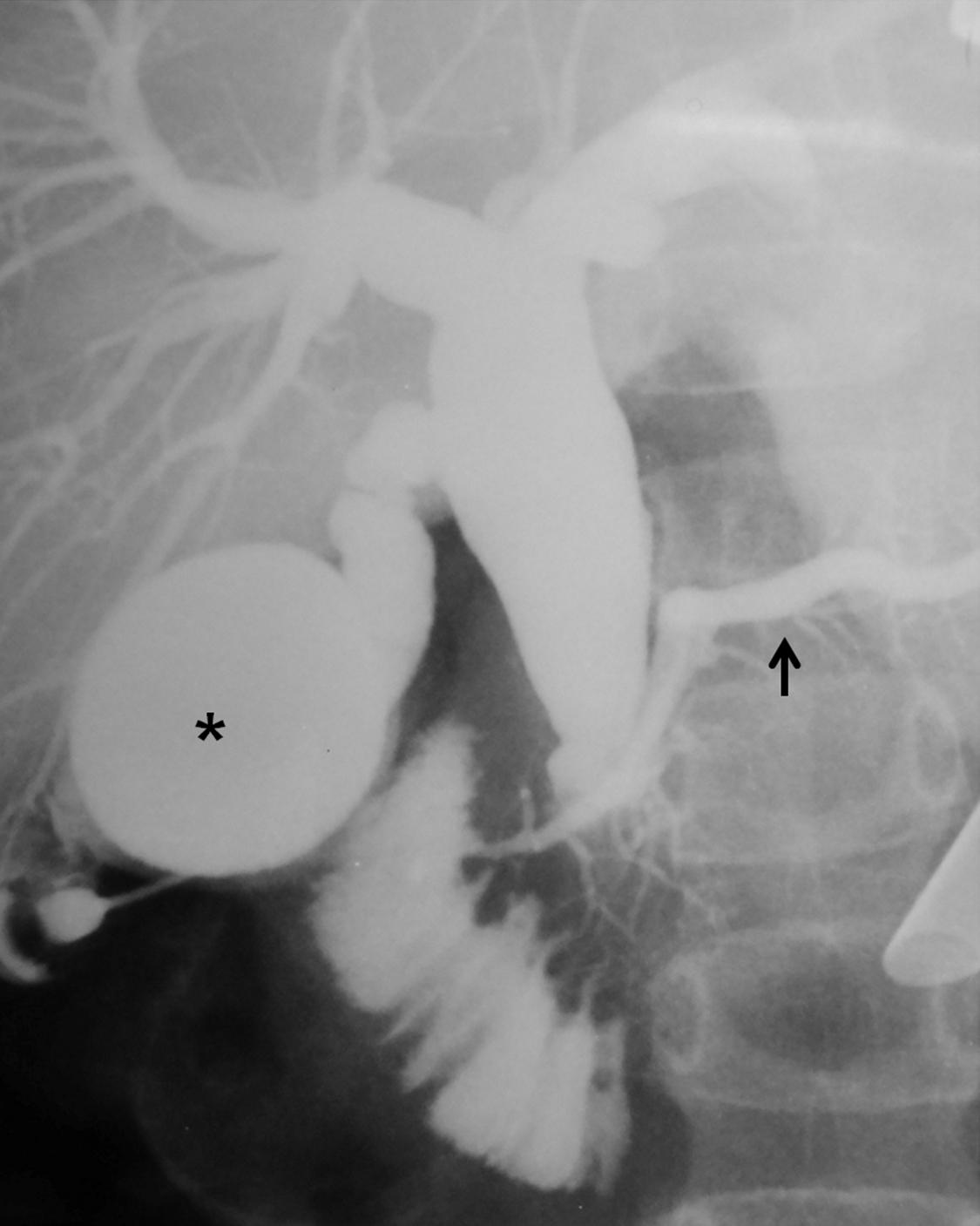
Intraoperative cholangiography is indicated when the anatomic detail of the biliary tract cannot be demonstrated by MRCP or ERCP ( Fig. 44.4 ). Contrast-enhanced computed tomography (CT) may be indicated in some patients with pancreatitis or if an associated tumor is suspected.
Cystoduodenostomy and cystojejunostomy have been abandoned due to cholangitis, stone formation, and malignant degeneration. External drainage is indicated for a perforated cyst in patients whose condition is too unstable to perform cystectomy and a bilio-enteric anastomosis.
Cyst excision and a bilio-enteric anastomosis is the preferred approach for most patients. The cyst should be excised at the level of the common biliopancreatic channel orifice at its distal end and approximately 5 mm from the confluence of the right and left hepatic ducts at the proximal end. Postoperative malignancy in a residual cyst on either the hepatic duct side or from the distal part has been reported. A review from the English language and Japanese literature of 23 patients with carcinomas of the bile duct developing after CC excision found that malignancy developed in the intrapancreatic remnant of the bile duct or CC in 6 patients, in the remnant of the CC at the hepatic side in 3 patients, in the hepatic duct at or near the anastomosis in 8 patients, and in the intrahepatic duct in 6 patients. Abdominal pain and pancreatitis due to leaving a remnant of the cyst in the pancreatic head also have been described. Operative correction can be performed safely in all age groups.
Biliary infection should be treated before operation. A prolonged prothrombin time secondary to cholestasis should be corrected with intravenous vitamin K. Drugs for elimination of ascaris are given in areas where ascaris is prevalent.
Many surgeons use hepaticojejunostomy, whereas others prefer hepaticoduodenostomy. Fat malabsorption and duodenal ulcer are the main concerns with a hepaticojejunostomy. In addition, the operative time is longer in comparison with hepaticoduodenostomy. Complications after Roux-en-Y hepaticojejunostomy, such as a twist of the Roux limb, intestinal obstruction, and duodenal ulcers, have been reported. On the other hand, cholangitis and gastritis due to bilious reflux are the main concerns with hepaticoduodenostomy. However, the operative time is shorter in comparison with hepaticojejunostomy. A hepaticoduodenostomy is considered more physiologic because the bile drains directly into the duodenum. This anastomosis is performed above the transverse colon mesentery, which may help prevent intestinal obstruction from adhesions.
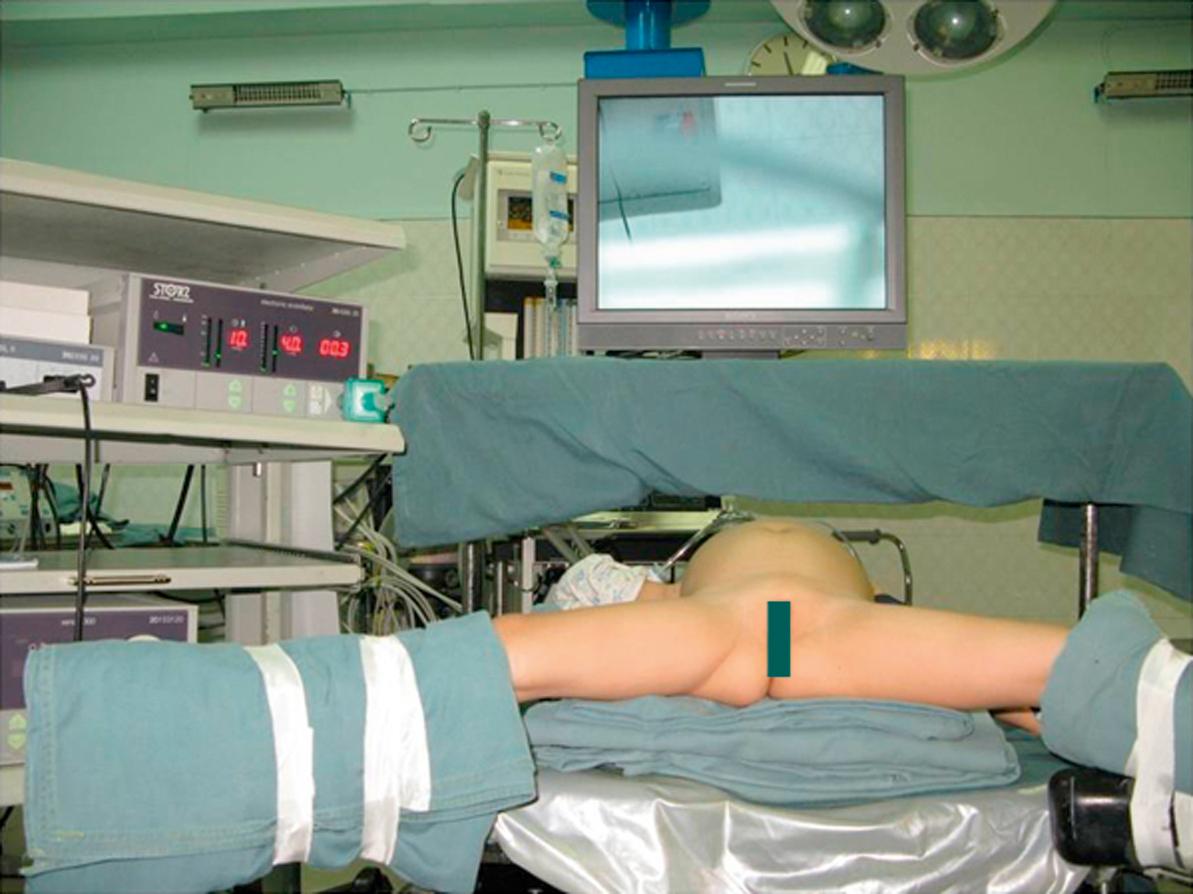
Endotracheal intubation and general anesthesia are standard. Epidural analgesia can provide excellent postoperative pain relief. Broad-spectrum intravenous antibiotics are best given at induction of anesthesia and continued for 5 days postoperatively. A nasogastric tube, rectal tube, and urinary catheter are used to decompress the stomach, colon, and bladder. The patient is placed in a 30° lithotomy position ( Fig. 44.5 ). The surgeon stands or sits at the lower end of the operating table between the patient’s legs.
The first laparoscopic operation for CC was reported in 1995. This approach quickly became popular and has become the routine approach in many centers. The laparoscopic approach is preferred for most types of CC: I, II, and IV. Relative contraindications are in patients with perforation, previous biliohepatic surgery, or especially newborns with damaged hepatic functions.
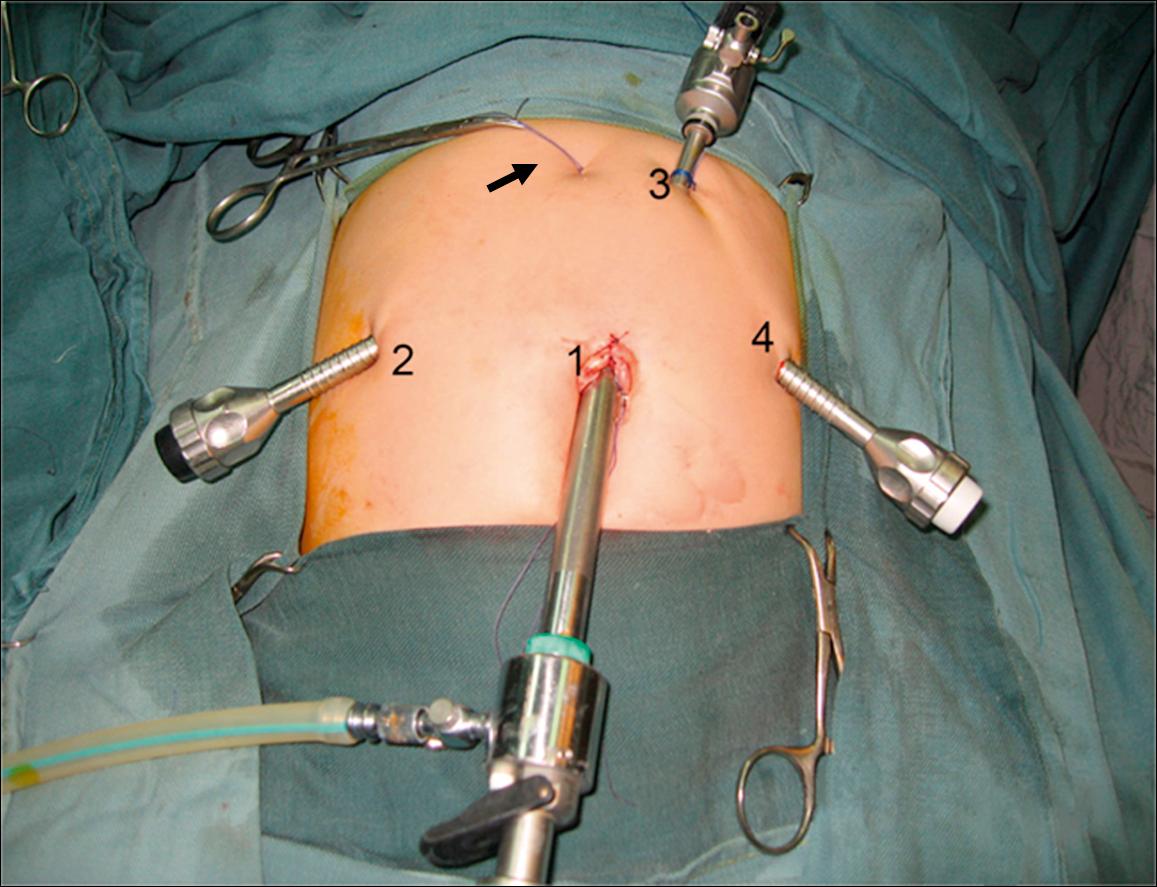
A 10-mm cannula is inserted through the umbilicus for the telescope. Three additional 5- or 3-mm ports are introduced for the working instruments: one in the right flank, another in the left flank, and one in the left hypochondrium ( Fig. 44.6 ). Carbon dioxide pneumoperitoneum is maintained at a pressure of 8–12 mmHg. The liver is secured to the abdominal wall with a suture placed around the round ligament ( Fig. 44.7A ). The cystic artery and the cystic duct are identified, clipped, and divided. A second traction suture is placed at the junction of distal cystic duct and gallbladder fundus to elevate the liver and expose the hepatic hilum (see Fig. 44.7B ).
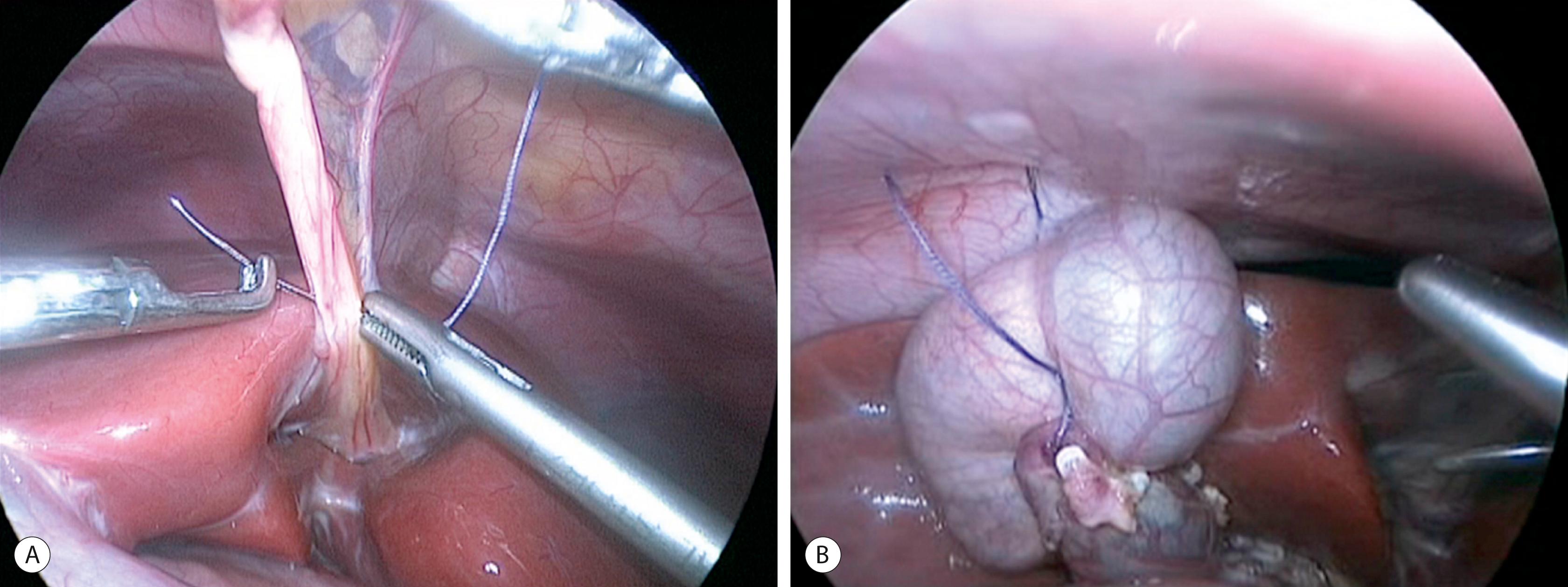
The appearance of the cyst, liver, and spleen is noted. Intraoperative cholangiography via the gallbladder can be performed if the anatomy has not been clearly defined preoperatively. With a large cyst, bile can be aspirated through a catheter, which reduces the cyst size to facilitate the pericystic dissection.
The duodenum is retracted downward using a dissector inserted through the left lower port. The mid-portion of the cyst is dissected circumferentially. Separation of the cyst from the hepatic artery and portal vein is meticulously performed until a dissector can be passed through the space between the posterior cyst wall and the portal vein going from left to right. The cyst is then divided at this site.
The inferior part of the cyst is separated from the pancreatic tissue down to the common biliopancreatic duct using a 3-mm dissector for cautery and dissection. Protein plugs or calculi within the cyst and common channel are washed out and removed. The inferior part of the cyst is opened longitudinally and inspected to identify the orifice of the common biliopancreatic channel. A small catheter is then inserted into the common channel. Irrigation with normal saline via this catheter is performed to eliminate protein plugs until clear fluid returns and the catheter can be passed down into the duodenum ( Fig. 44.8A ). A cystoscope can be used to measure the length of the common channel and remove protein plugs in it if its diameter permits. The distal CC is then clipped and divided at the level of the orifice of the common channel (see Fig. 44.8B ).
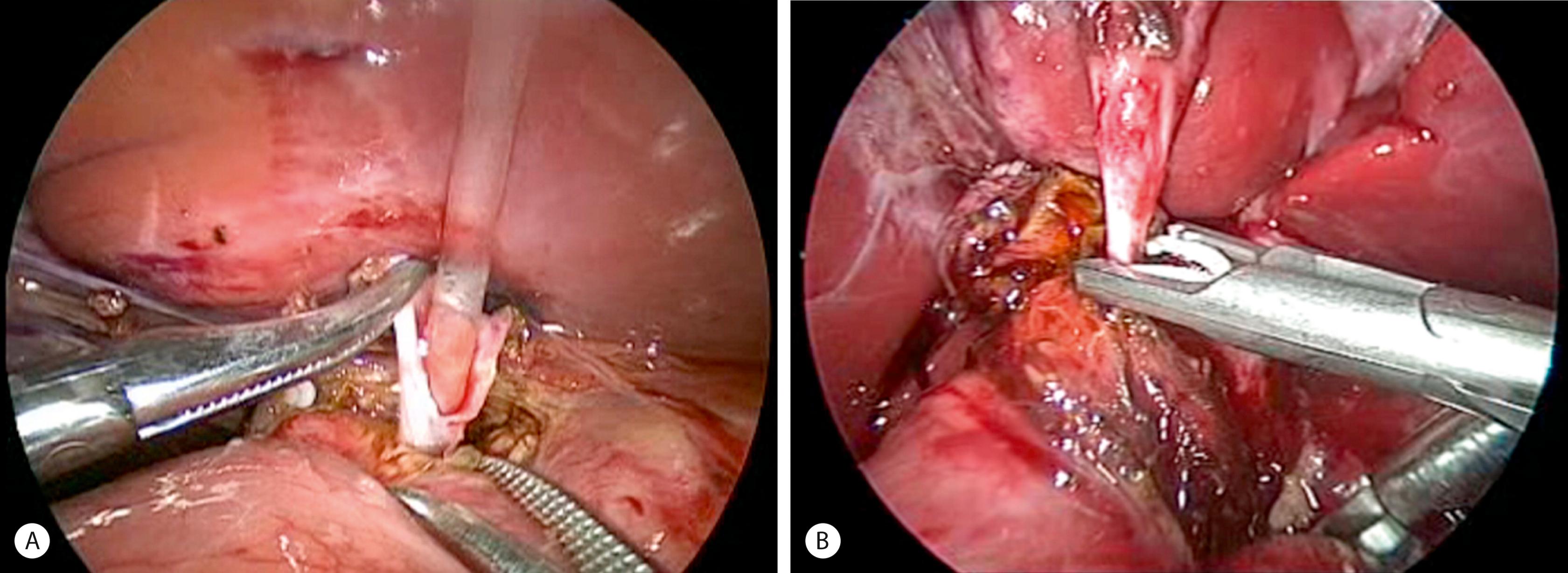
The cephalad portion of the cyst is further dissected to the common hepatic duct. The cyst is initially divided at the level of the cystic duct. After identifying the orifice of the right and left hepatic ducts, the rest of the cyst is removed, leaving a stump approximately 5 mm from the bifurcation of the hepatic ducts. Irrigation with normal saline through a small catheter inserted into the right and then into the left hepatic duct is performed to wash out protein plugs or calculi until the effluent is clear.
If the cyst is intensely inflamed with extensive pericystic adhesions, the cyst is opened by a transverse incision on its anterior wall. The dissection of the cyst wall from the portal vein is then carefully performed while viewing the cyst from inside and outside. After dividing the mid-portion of the cyst, the upper and lower parts of the cyst are removed as previously described.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here