Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malignant lymphoma, which comprises both Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), is the third most common malignancy in childhood.
Among children younger than age 15 years, there is a slight predominance of NHL; HL is more frequent among children up to age 18 years.
There are approximately 500 to 750 newly diagnosed cases of pediatric NHL in the United States each year.
NHL is more common in boys than girls and in white children than black.
There are geographic differences with respect to the frequency of histologic subtypes of NHL. Burkitt lymphoma is the predominant subtype in equatorial Africa and northeast Brazil, where it is associated with Epstein-Barr virus (EBV) in the majority of cases, in contrast to the infrequent association observed in the United States and Western Europe.
Children with inherited immunodeficiency conditions are at increased risk of developing NHL. These include those with ataxia-telangiectasia, Wiskott-Aldrich syndrome, and X-linked lymphoproliferative syndrome. Constitutional mismatch repair deficiency syndrome is also associated with an increased risk of NHL. Children with acquired immunodeficiency disorders, including the acquired immunodeficiency syndrome, and those receiving immunosuppressive therapy after bone marrow or organ transplantation are also at increased risk.
The most common subtypes of NHL in children are Burkitt lymphoma, lymphoblastic lymphoma, anaplastic large-cell lymphoma, and diffuse large B-cell lymphoma (including the mediastinal subtype).
Burkitt lymphoma is a mature B-cell lymphoma of germinal center origin, characterized by a very high proliferation rate, resulting from the activation of the MYC oncogene as a result of juxtaposition to one of the immunoglobulin genes, through one of three characteristic balanced chromosomal translocations, that is, t(8;14), t(2;8), and t(8;22).
Lymphoblastic lymphoma is typically of precursor T-cell immunophenotype and may be associated with reciprocal translocations involving a gene. Rare cases may be of precursor B-cell lineage.
Anaplastic large-cell lymphoma is a peripheral (postthymic) T-cell lymphoma characterized by large anaplastic (“hallmark”) cells expressing CD30 and, in the vast majority of pediatric cases, anaplastic lymphoma kinase (ALK), as a result of a balanced translocation involving the ALK gene, for example, t(2;5).
Diffuse large B-cell lymphomas are a biologically heterogeneous category of mature B-cell lymphomas of germinal center or postgerminal center origin, composed predominantly of large cells, with a diffuse growth pattern.
The clinical features at diagnosis are determined by primary sites of disease, which vary according to histologic subtype.
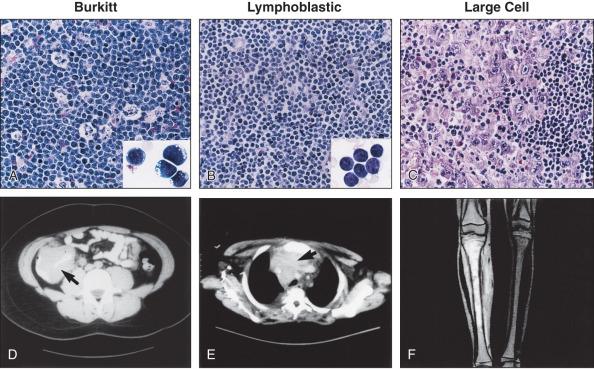
Children with Burkitt lymphoma usually present with an abdominal mass and associated gastrointestinal symptoms, but those with advanced-stage lymphoblastic lymphoma typically present with a mediastinal mass associated with a spectrum of respiratory symptoms.
Children with large-cell lymphoma or HL may present with disease in either the abdomen or mediastinum.
Infectious processes, such as bacterial adenitis, histoplasmosis, tuberculosis, and EBV infection, may simulate lymphoma.
A comprehensive characterization of the biological features of tissue will help distinguish NHL from other small round blue cell tumors, including Ewing sarcoma, neuroblastoma, and rhabdomyosarcoma.
The workup should include a history and physical examination, complete blood count, chemistry panel (including electrolytes, blood urea nitrogen, creatinine, uric acid, phosphorus, calcium, and lactate dehydrogenase [LDH]), diagnostic imaging studies (computed tomographic scan of chest, abdomen and pelvis, nuclear imaging with a positron emission tomography scan), and HIV screen.
For children with NHL, stage is usually assigned according to the St. Jude system; children with HL are staged using the Ann Arbor system.
The treatment plan is determined on the basis of histology, stage, immunophenotype, and in some cases, clinical symptoms such as fever, weight loss, and night sweats.
Children with advanced-stage Burkitt lymphoma are generally treated with intensive cyclophosphamide-based regimens given over a relatively short period of time, but children with lymphoblastic lymphoma are generally treated with regimens derived from strategies for children with acute lymphoblastic leukemia.
Among children with large-cell lymphoma, the treatment plan varies with respect to tumor cell immunophenotype.
Involved-field radiation therapy has a role in certain cases of HL but is rarely indicated for children with NHL.
Children with refractory or recurrent disease are generally considered to have a poor prognosis and are therefore candidates for intensive or novel salvage treatment regimens.
Those with chemosensitive disease are potential candidates for an intensification phase with hematopoietic stem cell rescue.
The most concerning late effects of therapy include cardiac toxicity, infertility, and the development of a second malignancy.
The risks for these complications are determined in part by the components of initial therapy.
The most important predictors of treatment outcome for children with NHL are treatment protocol and tumor burden, as reflected by stage and serum LDH.
Substantial clinical and laboratory advances have improved our understanding of both the pathogenesis and treatment of the malignant lymphomas of childhood and adolescence, which comprise Hodgkin lymphoma (HL) and the non-Hodgkin lymphomas (NHLs). These are the third most common type of cancer in children in the United States, comprising approximately 15% of newly diagnosed cases in this age group each year. Among children younger than 15 years of age, the NHLs account for approximately 60% of cases. However, when children up to age 18 years are included, there is a slight predominance of HL.
The NHLs of childhood are markedly different from those of adulthood. Whereas diffuse high-grade extranodal subtypes account for the majority of pediatric cases, low- and intermediate-grade lymphomas are predominant in adults. These differences are probably due in part to age-related maturational changes in the immune system and consequently in the types of cells susceptible to malignant transformation. The differences between adults and children with respect to histologic subtype underlie the differing clinical features, staging, and treatment strategies in these age groups. The age-related differences in biology across the pediatric, adolescent, and young adult age groups and their respective impacts on treatment have been reviewed.
The clinical presentation, staging, histologic subtypes, and treatment strategies in children and adults with HL are less dissimilar; however, some differences are worth noting. Epidemiologic studies have suggested three distinct forms that depend on age: the childhood form in patients 14 years of age or younger, the young adult form (ages 15 to 34 years), and an older adult form in individuals 55 to 74 years of age. Among the four histologic subtypes of HL described in the Rye classification system, the nodular sclerosing subtype is most common in children, occurring in 40% of younger children and 70% of adolescents. Mixed-cellularity HL occurs in approximately 30% of cases and is more common in children with HIV infection and in those less than 10 years of age; it is frequently associated with an advanced stage (with extranodal extension) at presentation. Lymphocyte-predominant HL accounts for approximately 10% to 15% of pediatric cases, is usually associated with localized disease at presentation, and occurs more commonly in younger patients and in boys. The fourth subtype of HL, lymphocyte depletion, is very rare in children.
Despite excellent event-free survival (EFS) rates in HL, there are well-recognized challenges related to the sequelae of therapy, which include endocrine dysfunction, chemotherapy-induced sterility, radiation-induced abnormalities in bone growth, chemotherapy- and radiation-related second cancers, and late cardiac deaths. Most current studies are exploring strategies that maintain an excellent treatment result while reducing late effects. For example, a combined-modality approach with low-dose radiation and combination chemotherapy may reduce the bone growth abnormalities associated with high-dose extended-field radiation therapy. However, because therapy is modified in an attempt to reduce the risk of late effects, the rates of EFS may be compromised. For example, attempts at reducing alkylating agent–related late effects in patients with advanced-stage disease by substituting other chemotherapeutic agents have lowered EFS rates. There have been some recent clinical trials that have also attempted to reduce toxicity without compromising outcome. In this regard, among children with favorable-risk HL and a complete early response to vincristine, doxorubicin, methylprednisolone (VAMP) chemotherapy, a high rate of 2-year EFS could be achieved with limited use of radiotherapy. The results of the Children's Cancer Group (CCG) 5942 study, which examined the benefit of adding involved field radiation therapy (IFRT) for patients who achieved a complete response to chemotherapy, were analyzed and revealed a statistically significant improvement in EFS for those who received IFRT; however, there was no associated improvement in overall survival. The refinement of a risk-adapted treatment approach to the management of pediatric HL remains a challenge. Current studies are examining the benefits of incorporating noel immunotherapeutic agents into frontline therapy in an effort to both improve outcome and limit unwanted side effects of therapy. These and other issues regarding the management of children with HL are discussed in Chapter 102 . The remainder of this chapter focuses on the NHLs of childhood.
Non-Hodgkin lymphomas may occur at any age in childhood but are unusual in children younger than age 3 years; the median age at diagnosis is approximately 10 years. There are approximately 500 to 750 new cases of pediatric NHL in the United States each year. In contrast to HL, which has a bimodal age distribution with peaks in early and late adulthood, the incidence of NHLs increases steadily with age. NHL occurs almost twice as commonly in whites as in blacks and two to three times more often in boys than in girls; the explanation for these differences has yet to be elucidated.
There are specific populations at increased risk for the development of NHL. These include individuals with primary immunodeficiency syndromes, including ataxia-telangiectasia (A-T), Wiskott-Aldrich syndrome, X-linked lymphoproliferative syndrome (XLP), common variable immunodeficiency, Nijmegen syndrome, and autoimmune lymphoproliferative syndrome (ALPS). More recently, an increased risk for the development of NHL has been reported to occur in children with constitutional mismatch repair deficiency syndrome (CMMRDS). It is important that these syndromes be recognized so that appropriate therapy can be designed. For example, in the management of children with A-T who develop a malignancy, IFRT and radiomimetics such as bleomycin should be avoided. Children with A-T are also at increased risk for the development of late-onset hemorrhagic cystitis after exposure to cyclophosphamide. Boys with XLP are at increased risk for developing fatal infectious mononucleosis, B-cell lymphomas, or both. XLP should be considered in any boy with a high-grade B-cell lymphoma whose brother has had either fatal infectious mononucleosis or B-cell lymphoma and in any boy who has had two primary B-cell lymphomas. Children who have received immunosuppressive therapy (e.g., recipients of bone marrow or organ transplants) and those with the acquired immunodeficiency syndrome (AIDS) are also at a higher risk for developing NHL. The overall prevalence of lymphomas among children with HIV infection is approximately 1.6%. Among HIV-positive children with hemophilia, NHL is 36 times more frequent than in HIV-negative children with factor 8 deficiency. The majority of the HIV-associated NHLs have a B-cell immunophenotype with either aggressive B-cell/Burkitt or large-cell morphology. Proliferative lesions of mucosa-associated lymphoid tissue (MALT), which may be either benign or malignant, have also been described in children with HIV infection. Although deficient T-cell function has been implicated in these congenital and acquired immunodeficiency states, further study is required to fully clarify the mechanisms of pathogenesis.
There are well-recognized geographic differences in both the incidence and the distribution of histologic subtypes of NHL. For example, NHLs are very rare in Japan but are very common in equatorial Africa. More specifically, Burkitt lymphoma accounts for approximately half of all childhood malignancies in equatorial Africa and is the predominant NHL subtype in northeastern Brazil and areas of the Middle East. In contrast, lymphoblastic lymphomas are the predominant histologic subtype in southern India. In some parts of the world, the distribution of histologic subtypes of childhood NHL has yet to be firmly established.
There are also geographic differences in the clinical and biological features of some NHLs. For example, whereas Burkitt lymphoma in equatorial Africa (endemic Burkitt lymphoma) frequently involves the jaw, abdomen, orbit, paraspinal area, and central nervous system (CNS), the common sites of involvement associated with Burkitt lymphoma in the United States and Western Europe (sporadic Burkitt lymphoma) include the abdomen, bone marrow, and nasopharynx. The predominant chromosome 8 breakpoints, as well as the breakpoints within the immunoglobulin heavy-chain gene (on 14q32) differ between sporadic and endemic cases. In addition, sporadic cases have more complex karyotypic abnormalities than endemic cases in addition to the classic translocations, suggesting distinct mechanisms of malignant transformation. Whereas the endemic cases are associated with IgM expression, the sporadic cases generally are not. The sporadic and endemic cases also differ with respect to Epstein-Barr virus (EBV) association. The overlap of the lymphoma belt in equatorial Africa with the malaria belt prompted speculation that an infectious agent might be involved in lymphomagenesis. This led to the discovery of the EBV and its association with African Burkitt lymphoma. Although a direct role in pathogenesis has not been demonstrated, the circumstantial evidence for its involvement is compelling. It has been suggested that as a B-cell mitogen, EBV increases the target pool of cells potentially susceptible to malignant transformation. Supporting this hypothesis is evidence that Rag gene expression can be induced by EBV, theoretically increasing the likelihood of a translocation occurring in immature B cells that are about to rearrange their immunoglobulin genes. The potential role of Epstein-Barr nuclear antigen (EBNA)-1 in pathogenesis has been suggested by experiments demonstrating that lymphomas develop in mice transgenic for EBNA-1. Moreover, an identified EBNA-1 variant has been shown to be associated with the majority of Burkitt lymphoma cases studied, prompting investigators to speculate that this tumor-associated mutation alters EBNA-1 function in a way that directly or indirectly provides a growth advantage for the lymphoma cell. A more direct role for EBV in lymphomagenesis is suggested by studies of the EBV-positive Burkitt lymphoma cell line, Akata, which loses its malignant phenotype with spontaneous loss of EBV; however, the malignant phenotype is regained with EBV reinfection. EBV association has been reported in approximately 90% of the endemic (i.e., African) Burkitt tumors and in approximately 15% of sporadic cases (United States and Western Europe). Aberrant and disrupted expression of the EBV genome has recently been reported in cases of sporadic Burkitt lymphoma that were EBV-negative by conventional EBNA screening. This observation, coupled with the 50% rate of EBV association in Burkitt tumors in other parts of the world (including Brazil, Russia, Argentina, and Chile), suggest a widespread role for this virus in lymphomagenesis.
The classification systems applied to pediatric NHL are similar to those used for lymphomas occurring in adults. In the National Cancer Institute (NCI) Working Formulation for clinical use, published in 1982, which subclassified NHLs based on their morphologic appearance and clinical aggressiveness into three grades (low, intermediate, and high), most of the childhood NHLs would be designated as high-grade lymphomas ( Fig. 94.1 ). The Revised European-American Lymphoma (REAL) Classification introduced in the mid-1990s and the World Health Organization (WHO) Classification initially published in 2001 and updated in 2008 and 2016 define the different categories of NHL as distinct clinicopathological entities, with well-defined morphologic, immunophenotypic, and genetic features, named, when feasible, according to their postulated counterparts in the normal lymphoid system. This has led to the definition of several lymphoma subtypes within the high-grade lymphoma category previously assigned to most pediatric lymphomas. These include Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), precursor B or T lymphoblastic lymphomas, and anaplastic large-cell lymphoma (ALCL). The most recent WHO Classification has added multiple entities as biologically and clinically distinct subtypes of DLBCL, reflecting the significant progress made in better characterizing the lymphomas previously included under this general category (see further below). Some low-grade lymphoma subtypes, including follicular center cell lymphoma and marginal zone lymphoma, may also occur in children where they appear to have distinct clinicopathological features, albeit with less frequency. The WHO classification of pediatric NHLs is summarized in Table 94.1 .
| COMMON PEDIATRIC LYMPHOMAS |
| B-Cell Lymphomas |
| B lymphoblastic leukemia and lymphoma Burkitt lymphoma Burkitt-like lymphoma with 11q aberration (provisional entity) DLBCL, NOS Activated B-cell type Germinal center type T-cell/histiocyte rich-large B-cell lymphoma Primary mediastinal (thymic) large B-cell lymphoma ALK+ B-cell lymphoma High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 rearrangements High-grade B-cell lymphoma, NOS Large B-cell lymphoma with IRF4 rearrangement (provisional entity) B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical Hodgkin lymphoma |
| T-Cell Lymphomas |
| T lymphoblastic leukemia and lymphoma Anaplastic large-cell lymphoma (ALK positive or ALK negative) Peripheral T-cell lymphoma, unspecified |
| UNCOMMON PEDIATRIC LYMPHOMAS |
| Pediatric-type follicular lymphoma Hepatosplenic T-cell lymphoma Pediatric nodal marginal zone lymphoma (provisional entity) Extranodal marginal zone B-cell lymphoma of MALT lymphoma Mycosis fungoides Subcutaneous panniculitis-like T-cell lymphoma Adult T-cell leukemia and lymphoma (HTLV-1 associated leukemia/lymphoma) Primary cutaneous CD30 positive T-cell lymphoproliferative disorders Extranodal NK/T-cell lymphoma, nasal type EBV-positive T-cell lymphoproliferative disorders of childhood Systemic EBV-positive T-cell lymphoma of childhood Hydroa vacciniforme-like lymphoproliferative disorder |
Modern classification systems define Burkitt lymphoma as a mature B-cell neoplasm composed of monomorphic medium-sized cells (“small noncleaved cell”), with an extremely short doubling time, resulting from MYC gene translocations. Burkitt lymphoma and leukemia (classically known as acute lymphoblastic leukemia [ALL] L3 in the French–American–British classification) form a biological continuum. B-cell lymphomas with a similar proliferation rate and genetic features, previously termed “small noncleaved cell lymphoma, Burkitt-like,” have also been incorporated in this clinicopathological entity as one of its morphologic variants (see later).
Histologically, Burkitt lymphoma classical variant, present in endemic cases, as well as in many of the sporadic cases, is characterized by a diffuse growth pattern, uniform medium-sized cells (typically equal in size to the nuclei of adjacent histiocytes), with coarsely clumped nuclear chromatin and one to three nucleoli. When observed in Wright-Giemsa–stained smears, these cells are large, with moderate amounts of deeply basophilic cytoplasm and prominent cytoplasmic vacuoles. These tumors typically contain numerous mitotic figures, and, matching the high cell turnover rate, they have numerous “tingible-body” macrophages with pale cytoplasm that contain ingested apoptotic cellular debris and impart a characteristic “starry-sky appearance” on low magnification. Burkitt lymphomas that are otherwise typical but show a greater degree of pleomorphism in nuclear size and shape and one to two large nucleoli in the neoplastic cells were classified as the atypical Burkitt/Burkittlike variant in the previous WHO classification (2001). These lymphomas represent a subset of the tumors previously designated as “small noncleaved cell lymphoma, Burkitt-like.” This morphologic variant can be seen in sporadic cases and in immunodeficiency-associated Burkitt lymphoma. Finally, Burkitt lymphomas with plasmacytoid differentiation (eccentric nuclei with a single central nucleolus, basophilic cytoplasm that contains abundant monotypic immunoglobulin on immunohistochemical staining) represents a third morphologic variant. This latter variant can also be seen with immunodeficiency-associated lymphoma. The most recent WHO Classifications (2008 and 2016) no longer defines morphologic variants of Burkitt lymphoma but rather recognizes the wide morphologic spectrum of a single disease.
The immunophenotype of Burkitt lymphoma, regardless of its morphologic variants, is that of a mature B cell of germinal center or postgerminal center type. Burkitt lymphoma cells strongly express CD19, CD20, CD22, and CD24, as well as CD10 and BCL6, and are negative for BCL2, CD5, CD23, CD34, and TdT. The neoplastic cells also express surface immunoglobulin (typically IgM, less commonly IgA or IgG) with light-chain (κ or λ) restriction. Staining for proliferation markers (Ki-67) highlights a growth fraction of nearly 100% in all the histologic variants. Recent studies have demonstrated that avian myelocytomasis viral oncogene homolog ( MYC ) gene expression detected by immunohistochemical staining highly correlates with MYC gene rearrangements when it has nuclear localization and is present in a high percentage (>70%) of the lymphoma cells. Demonstration of this high proliferation rate is required for the diagnosis of Burkitt lymphoma. Rare cases may exhibit a precursor B-cell immunophenotype associated with the characteristic Burkitt chromosomal translocations. These cases show morphologic and immunophenotypic features intermediate between a classical Burkitt lymphoma and a precursor B cell. The latter includes expression of CD34 and TdT and lack of surface immunoglobulin expression. Recognition of these cases as Burkitt lymphoma or leukemia typically requires integration with the cytogenetic findings.
Genetically, Burkitt lymphoma is characterized by several translocations that rearrange the MYC gene on chromosome 8q24, bringing it under the controlling sequences (enhancers) for immunoglobulin heavy- or light-chain genes and leading to overexpression of this gene in the neoplastic B cells. These translocations include the t(8;14)(q24;q32) seen in 85% to 90% of the cases that involves the MYC and IgH gene loci and the less common variants, t(2;8)(p11;q24) and t(8;22)(q24;q11.2), that juxtapose MYC to the immunoglobulin light-chain genes, Ig kappa (on 2p11) and Ig lambda (on 22q11), respectively. The breakpoints involving MYC and IgH are highly variable and cluster in regions different between endemic and sporadic Burkitt lymphoma. The IgH breakpoints most often involve the VDJ region in endemic Burkitt lymphoma and the switch region Sµ in the sporadic cases, suggesting that the neoplastic transformation occurs at different stages of B-cell development in these two disease subtypes. Similarly, the MYC breakpoints appear to cluster in two different regions that correlate with the location of the IgH breakpoints. This high variability in breakpoints leads to difficulty in generating polymerase chain reaction (PCR) assays that can detect this translocation. Long-range PCR combined with nested PCR strategies have allowed detection of the t(8;14) and of the variant translocations. Approximately 70% to 80% of the sporadic cases have additional chromosomal abnormalities, the most common of which involve chromosomes 1 (1q), 6 (6q), 13 (13q), 17, and 22. Some of these alterations (e.g., those of chromosome 13q) appear to have a negative impact on prognosis in pediatric Burkitt lymphoma.
The biology of Burkitt lymphoma has been extensively characterized. MYC , a widely studied oncogene, initially discovered because of its involvement in the t(8;14), appears to play a central role in malignant transformation and the biological behavior of Burkitt lymphoma. MYC overexpression has been described in up to 50% of all human cancers, occurring through both epigenetic and genetic mechanisms (that include chromosomal translocations and genomic amplifications). In Burkitt lymphoma, MYC is deregulated primarily by mutation/translocation and by overexpression. Its oncogenic properties have been demonstrated both in vitro and in vivo by using a variety of animal models.
MYC is a nuclear transcription factor. It is the founding member of a family of transcription factors of the basic helix-loop-helix-leucine zipper (BHLH-LZ) classes. MYC operates as a heterodimeric complex with a cofactor named MAX to bind specific DNA sequences, thereby transcriptionally regulating hundreds to thousands of target genes. These genes are involved in diverse programs, including cell cycle, cell growth, apoptosis, protein translation, cell adhesion, various metabolic pathways, angiogenesis, and DNA repair. MYC can promote cell proliferation through cyclins D, B1 and A, and CDK4 that lead to G1 to S progression. It also inhibits differentiation, increases protein synthesis, and suppresses genes that encode cytoskeletal and cell adhesion molecules, thus contributing to neoplastic transformation. An interesting aspect of MYC biology, with major implications in neoplasia, is its role in apoptosis. MYC sensitizes cells to apoptosis; therefore it appears that mechanisms that would block this downstream effect are necessary in the process of malignant transformation, allowing for cell proliferation to take precedence over cell death. Apoptosis can be inhibited by a variety of proteins, such as BCL2, BCL-X L , cFLIP, mTOR, Mdm2/Hdm2, Twist, Bmi1, and Cul7. Apoptosis promoters include a variety of proteins typically regarded as tumor suppressors, such as P53, ARF, PUMA, ATM, BAX, BID, BIM, Fas/CD95, and 4EBP1. Extensive studies of tumor cell lines and tumors arising in animal models, as well as of primary human tumor samples, have shown that in most cases, MYC overexpression is accompanied by either the overexpression of one of the apoptosis suppressors (e.g., BCL2) or by inactivation of one of the proapoptotic factors (e.g., P53 or ARF). In animal models, alterations in P53-related pathways seem to play a major role in the biology of Burkitt lymphoma. The relevance of these findings to human Burkitt lymphoma is currently under study. Limited studies performed in pediatric sporadic Burkitt lymphoma suggest that these findings may be applicable to primary tumor samples as well.
A subset of MYC-negative BL with abnormalities of 11q has been described more recently and has been included as a provisional entity in the most recent WHO classification. These lymphomas represent about 3% of BLs occurring in immunocompetent patients but are much more frequent in the setting of immunosuppression post solid organ transplantation, in which they represent 43% of all BLs. They have morphologic and clinical features and a gene expression signature similar to the MYC+ BL, and they harbor a recurrent pattern of 11q abnormalities, including interstitial gains at 11q23.2-q23.3 and telomeric losses at 11q24.1-qter.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here