Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Paediatric liver biopsies present a unique set of diagnostic problems for the pathologist, many of which become clinically apparent in the first few months of life as neonatal cholestasis . Among the important disorders one must consider in evaluating neonatal liver biopsies are extrahepatic biliary atresia , paucity of intrahepatic bile ducts (syndromatic and non-syndromatic types), metabolic diseases , viral hepatitis and the hepatic effects of parenteral nutrition ( Table 13.1 ). Common to many of these conditions are the histological features of cholestasis and giant-cell hepatitis (formation of multinucleated hepatocytes). Because these features are not specifically diagnostic of any one neonatal liver disease, the pathologist must be acquainted with other biopsy changes by which to establish or suggest the diagnosis. In many instances, assays of metabolic enzymes and products in serum and liver tissue take diagnostic precedence over routine histopathological interpretation. Electron microscopy may be required to assess the structure of organelles or storage material in hepatocytes or Kupffer cells, particularly when lysosomal storage disorders are being considered. Consultation with investigators dealing with mitochondriopathies, mutations of bile-salt transport proteins and expression of proteins involved in blood vessel and bile-duct morphogenesis (e.g. Jagged proteins and Notch receptors) should be considered if special studies are needed to determine the cause of neonatal cholestasis. Childhood liver tumours are discussed in Chapter 11 .
| Aetiology | Histological features |
|---|---|
| Extrahepatic biliary atresia | Ductular reaction; ductular cholestasis; portal and periportal fibrosis |
| Paucity of intrahepatic bile ducts | Loss of interlobular bile ducts (bile duct-to-hepatic artery ratio < 1) |
| Neonatal hepatitis | Portal and lobular mononuclear cell inflammation; apoptotic bodies |
| Metabolic disorders | Steatosis; fibrosis or cirrhosis; storage product in liver cells or Kupffer cells (see specific disorder) |
| Parenteral nutrition | Ductular reaction; portal fibrosis or cirrhosis |
∗ Many of the conditions shown in Table 13.1 are associated with histological cholestasis and formation of giant multinucleated hepatocytes, in addition to the diagnostic features listed.
Histopathological examination of neonatal liver biopsies may benefit from a systematic checklist of questions by which the major diagnostic concerns in neonatal liver disease can be evaluated. A simplified, stepwise set of seven questions can be asked:
Is the acinar structure normal for age? As described in Chapter 3 , the hepatic plates are two cells thick until 5 or 6 years of age and should not be misconstrued as a pathological change. As with adult biopsies, the presence of fibrosis, nodularity or cirrhosis should be noted early in the biopsy evaluation and correlated with other histological features which may define the aetiology.
Are cholestasis and giant cells present? As indicated earlier, neither of these is diagnostically specific. If present, the next interpretive steps should be examination of portal tracts for evidence of biliary tract obstruction (e.g. atresia) and of portal tracts and parenchyma for evidence of hepatitis.
Are histological changes of hepatitis present? Mononuclear cell infiltrates within acini and portal tracts associated with liver-cell degeneration should be sought when considering cytomegalovirus, Epstein–Barr virus, rubella or hepatitis virus infections.
Are the interlobular bile ducts normal? This question has three major ramifications. Abundance of bile ducts usually signifies some form of biliary obstruction, such as extrahepatic atresia or choledochal cyst. Paucity of bile ducts ( ductopenia , vanishing bile-duct syndrome ) may be due to developmental, metabolic or infectious causes. Lastly, malformations of bile ducts comprise a spectrum of problems related to abnormal remodelling of the embryonic bile-duct plate (fibropolycystic diseases).
Does the biopsy specimen contain iron or copper? Although rare, neonatal haemochromatosis and Indian childhood cirrhosis (copper toxicosis in young children) are serious liver diseases with high mortality rates that must be excluded. In the older child and adolescent, Wilson’s disease ( Ch. 14 ) must not be overlooked. It should be noted, however, that fetal and neonatal liver contains much higher copper levels than that of adults, with an irregular tissue distribution. Mild siderosis is also within the spectrum of normal findings in the fetal and neonatal liver.
Has the biopsy specimen been studied by diastase–periodic acid–Schiff (PAS) or immunoperoxidase staining to exclude α 1 -antitrypsin deficiency? The expression of α 1 -antitrypsin deficiency is variable, and biopsies may not show diagnostic staining of retained enzyme within liver cells prior to 13–15 weeks of age. This condition should be histologically excluded whenever possible.
Are storage cells present? Abnormal storage products in liver cells or Kupffer cells may be seen in various metabolic diseases which cause hepatomegaly and failure to thrive. These should be sought on routine haematoxylin and eosin (H&E) as well as special stains.
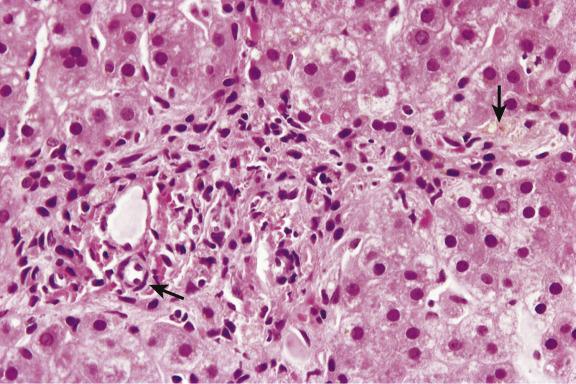
Inflammation and hepatocellular damage in the neonatal period may result from infections, from inborn errors of metabolism and from primary disorders of immune dysregulation. Infections include type B hepatitis, cytomegalovirus infection and rubella among others; inborn errors of metabolism include α 1 -antitrypsin deficiency, galactosaemia and bile-acid synthetic defects. Immune dysregulatory conditions include autoimmune hepatitis (AIH), as well as rare disorders affecting specific lymphoid cell populations. An example of the latter is the unusual case of paediatric acute liver failure (PALF), in which overrepresentation of CD103-positive Trm (resident memory T cells) and CD8 (cytotoxic T cells) can result in a severe hepatitis and acute liver failure. Disorders at the ultrastructural or molecular level may also need to be considered, such as neonatal hepatitis due to depletion of mitochondrial DNA. A diagnosis of neonatal hepatitis is therefore a signal for further investigation. The histological picture is broadly similar whatever the cause. There is a variable degree of hepatocellular swelling and multinucleation, cholestasis and portal inflammation ( Fig. 13.1 ). Lobular inflammation may be mild. Liver-cell necrosis and swelling result in collapse and distortion of the reticulin framework. Fibrosis is sometimes already well developed, as in neonatal haemochromatosis ( Ch. 14 ) or the severe perinatal liver disease which may rarely be seen in Down’s syndrome ( Chapter 15 ). Giant multinucleated hepatocytes are commonly seen, whatever the cause of the hepatitis ( Fig. 13.2 ). The outcome of neonatal giant-cell hepatitis is resolution, liver failure, cirrhosis or a chronic cholestatic course. The variety of different outcomes is well illustrated in α 1 -antitrypsin deficiency.
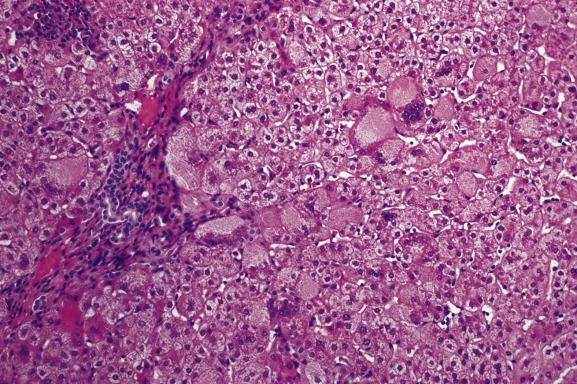
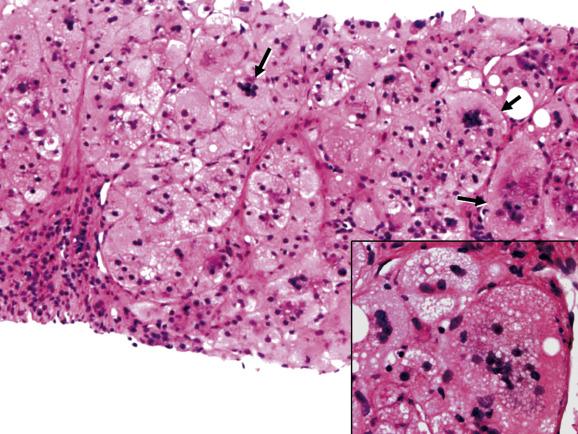
From a histological point of view, the main differential diagnosis of neonatal hepatitis is extrahepatic biliary obstruction, which may require surgical treatment. Giant multinucleated hepatocytes, an altered reticulin structure and little or no ductular reaction are more prominent in hepatitis than in biliary obstruction, while cholestasis is usually more severe in atresia and there is typically a ductular reaction.
Extracellular biliary atresia (EHBA) results from inflammation and destruction of all or part of the extrahepatic bile-duct system in utero or in the perinatal period. Pathological studies of atretic bile-duct segments show chronic inflammation and obliterative fibrosis, sometimes with a few remaining bile-duct cells seen on routine stains or cytokeratin immunostaining. Satisfactory bile drainage and an improved outcome after the Kasai portoenterostomy have sometimes been associated with identification of bile ducts with lumens of 150 μm or greater at the proximal resection margin. Optimal surgical results are obtained if the Kasai procedure (hepatic portoenterostomy) is performed within the first 8 weeks of life, with approximately 30% surviving into adulthood with their native liver. Many patients, nevertheless, later require liver transplantation.
The pathogenesis of the destructive process in extrahepatic atresia remains speculative, but considerations have included viral infections (reovirus type 3, rotavirus), exposure to toxins (e.g. outbreaks of biliary atresia in Australian livestock from ingestion of the plant isoflavonoid biliatresone ), abnormalities in regulatory T cells, abnormal remodelling of the embryonic bile-duct plate and disorders of Jagged protein/Notch receptor and Hedgehog signalling. DNA microarray studies suggest a gene profile of abnormal cell signalling and transcription regulation. There may be associated congenital abnormalities, including polysplenia, intrahepatic biliary cysts, laterality defects and cardiovascular, musculoskeletal and genitourinary defects in approximately 20% of cases with more severe and earlier disease (the ‘embryonic’ form of biliary atresia). Heterozygous missense mutations in the CFC1 gene, a determinant gene for left–right axis, have been identified in some BA polysplenia syndrome patients. By contrast, the majority of patients have the ‘perinatal’ form without such anomalies. Expression of various regulatory genes appears to differentiate the two types. The process of biliary atresia is a dynamic one which may also involve the intrahepatic bile ducts, and result in progressive fibrosis even after Kasai surgery.
Liver biopsy shows cholestasis and portal tract changes resembling those of large bile-duct obstruction in the adult ( Ch. 5 ). Portal tracts are enlarged by oedema and fibrosis (which varies depending on the age at biopsy), a striking ductular reaction and infiltrating neutrophils with fewer chronic inflammatory cells ( Fig. 13.3 ). Native interlobular bile ducts are usually intact and can be identified near hepatic arterioles. Bile-containing portal macrophages are often also present. Ductular structures may contain inspissated bile (ductular cholestasis; Fig. 13.3 ) and occasionally resemble the embryonic bile-duct plate described by Jörgensen ( Figs 13.4 and 13.5 ). A prominent ductular reaction is the major histological point of distinction from neonatal hepatitis. There is panlobular cholestasis with accentuation in zone 3. Giant cells are common, but not as numerous or as striking as in neonatal hepatitis. The lobular architecture remains intact, except in patients diagnosed late in the disease who may then show secondary biliary cirrhosis ( see Fig. 5.11 ). Many histologic parameters have been evaluated in previous studies in order to determine the key predictors of EHBA. The most recent investigation of infants with cholestasis enrolled in a study sponsored by the Childhood Liver Disease Research Network (CHiLDReN) found that bile plugs in portal bile ducts/ductules and portal stromal oedema were the strongest independent histologic predictors of obstruction. The study also found five histologic features that were independent predictors of EHBA by multivariate analysis ( Box 13.1 ). Portal fibrosis is a histological parameter that worsens with age, so that higher stages (stage 3–4) are noted in older infants. It should be kept in mind that in this large-scale study, 10% of biopsies of proven EHBA cases lacked features of biliary obstruction.
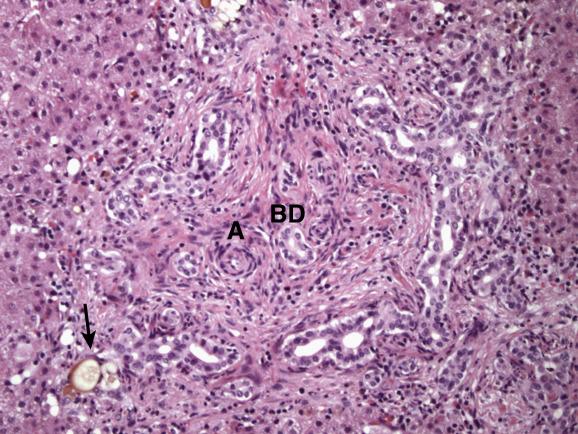
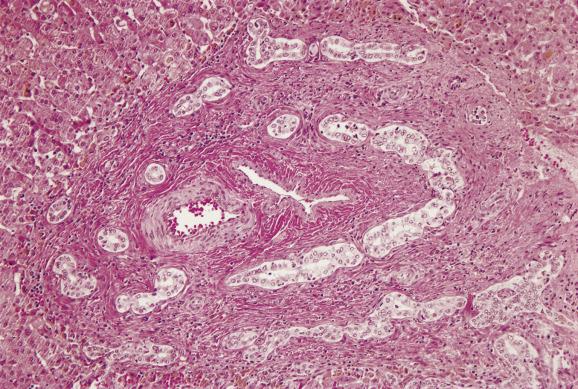
Alpha 1 -antitrypsin deficiency and total parenteral nutrition cause identical changes to EHBA and therefore require appropriate exclusion before rendering a pathological diagnosis of biliary atresia.
Although rare, these cystic lesions of the extrahepatic biliary tree should be considered in the differential diagnosis of paediatric jaundice (and rarely in adults). If liver biopsy is undertaken, histologic features of acute and chronic bile-duct obstruction are present, but these do not define the site or cause of obstruction; biliary atresia is a major differential diagnosis in this setting. Five types of choledochal cyst (CCs) are recognised radiologically. Excision is the treatment of choice. Resected cysts show lining epithelium overlying a stromal connective tissue layer rich in smooth muscle actin-positive myofibroblasts. This histology is distinct from the occasional cyst seen in biliary atresia duct remnants, which show a thick, compact collagen layer facing the cyst lumen, without overlying epithelium.
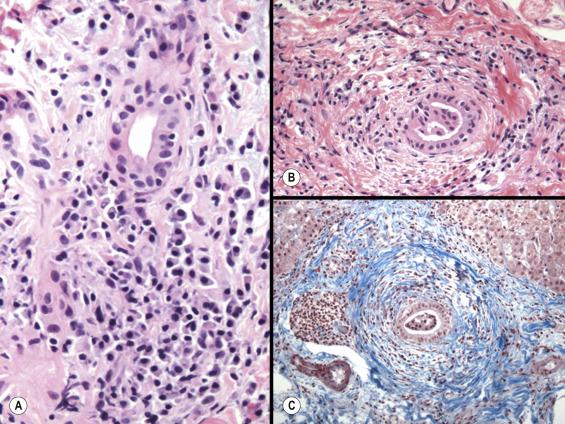
This hybrid condition affecting children and adolescents combines the cholangiographic and cholestatic abnormalities of sclerosing cholangitis with prevailing autoimmune features, including positive serum anti-nuclear and/or smooth-muscle antibodies, elevated serum gamma globulin, abnormal serum aminotransferases and the histopathology of AIH (i.e. lymphoplasmacytic portal inflammation with interface hepatitis). A prospective study from King’s College Hospital, London, UK, found inflammatory bowel disease in 44% of the autoimmune sclerosing cholangitis (ASC) patients. Biopsy features can vary over time, toggling between the hepatitic features of AIH on one occasion, biliary features and the progressive periductal fibrosis of primary sclerosing cholangitis on another, or both, regardless of the presence of biliary lesions on cholangiography ( Fig. 13.6 ). Current guidelines recommend cholangiography as part of the workup of children with autoimmune liver disease in order to exclude ASC.
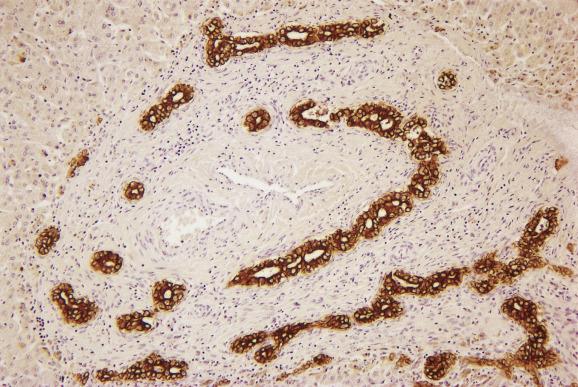
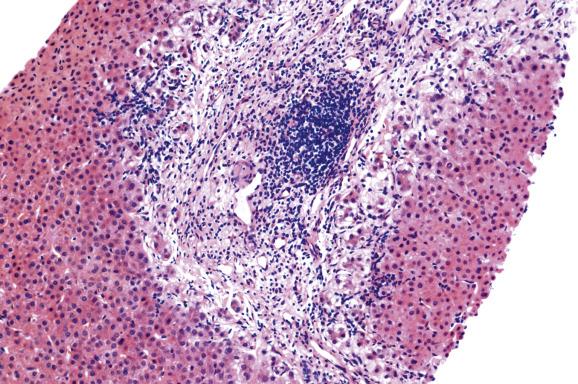
Langerhans cell histiocytosis (LCH), a clonal proliferative disorder of CD1a/CD207 dendritic cells, may present with isolated liver involvement or can be part of a multisystem disease involving several organs. The peak incidence is in children 1 to 4 years of age (the mean age of diagnosis in adults is 30 years, although the disease may be overlooked and only diagnosed years later). The main presentations of liver involvement by LCH are sclerosing cholangitis, bile duct paucity or, later, biliary cirrhosis ( Fig. 13.7 ). Infiltrating Langerhans cells with ample pink cytoplasm and kidney-bean- or boomerang-shaped vesicular nuclei with small nucleoli are seen individually or in granuloma-like clusters within hepatic sinusoids as well as in portal tracts and can be identified with Langerin, S-100 and CD1a immunostains. The bile-duct lesions show periductal fibrosis and oedema with surrounding acute and chronic inflammatory cells and variable numbers of Langerhans cells.
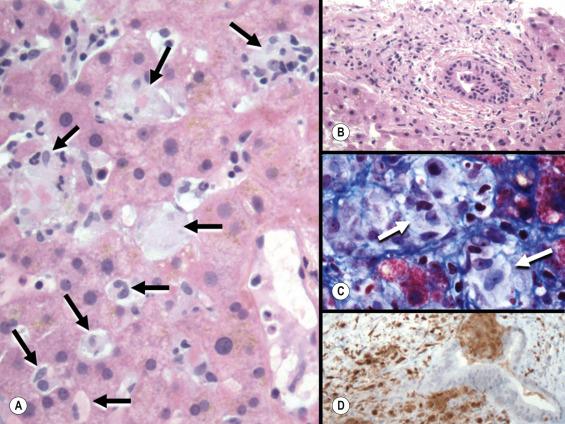
Two varieties of intrahepatic bile-duct paucity (formerly called intrahepatic biliary atresia) are recognised in childhood: syndromatic and non-syndromatic . In syndromatic paucity (Alagille’s syndrome or ALGS, arteriohepatic dysplasia), loss of small intrahepatic bile ducts is associated with abnormal facies, vertebral anomalies and various other malformations. The pathogenesis is linked to mutations in the Jagged 1 gene ( JAG1 on 20p12.2) that produce a structurally abnormal ligand for binding in the Notch signalling pathway which is involved in cell–cell interactions in differentiation and the development of intrahepatic bile ducts. Recent data have shown that 94% of cases with phenotypic features of ALGS have JAG1 mutations and a small number show mutations for its receptor Notch2. There is associated impairment of branching and elongation of hilar ducts distally into the liver periphery. Increased mortality in these patients is linked to the presence of intracardiac congenital heart disease. In non-syndromatic paucity, duct loss is not associated with facial or other anomalies. In some patients it may be related to a definable cause such as α 1 -antitrypsin deficiency or cytomegalovirus infection, while in others there is no detectable aetiological factor. The exact time of onset of bile-duct injury is difficult to establish accurately and probably varies from case to case. Some patients have active destruction of ducts in the first few weeks of life and later stabilise, potentially with few symptoms or only mild chronic cholestasis, into young adulthood. In others, cirrhosis and liver failure may develop within months or many years later. It has been speculated that there may be a small subgroup of patients with non-syndromatic paucity in which cholestatic disease first presents in adulthood ( idiopathic adulthood ductopenia ).
Histologically, in both forms of intrahepatic duct paucity there is canalicular cholestasis and chronic periportal cholestasis. Portal tracts show a variable degree of fibrosis and small bile ducts are scanty or absent ( Fig. 13.8 ). Step sections and cytokeratin 7 or 19 immunostaining may be needed for thorough assessment of duct numbers which, as in primary biliary cirrhosis, should approximately correspond to the number of arteries of similar size. A ductular reaction is usually not a prominent feature, in contrast to extrahepatic biliary atresia. Immunohistochemical staining for clusters of differentiation marker CD10 (neutral endopeptidase) is normally identified on bile canaliculi, but is absent before the age of 24 months and also in Alagille’s syndrome, which can be helpful when used in the appropriate clinical setting. Inflammation is often slight or even absent, but lymphoid aggregates may be seen in the place of bile ducts ( Fig. 13.9 ). Secondary biliary cirrhosis develops in some patients. Alpha 1 -antitrypsin deficiency should be looked for in all patients with paucity of ducts. Duct paucity has also been described in association with LCH. As primary sclerosing cholangitis can also present in childhood, it should be considered in the differential diagnosis.
The term fibropolycystic diseases covers a number of congenital abnormalities involving bile ducts, many of them related to an abnormal remodelling of the embryonic ‘bile-duct plate’. They include congenital hepatic fibrosis, Caroli’s disease (congenital dilatation of the intrahepatic bile ducts), microhamartoma (von Meyenburg complex), choledochal cyst, and both infantile and adult forms of polycystic disease. The first four of these carry an increased risk of carcinoma of the biliary tree. The bile-duct plate, first seen at approximately 8 weeks of gestation, is a layer of primitive small cells encircling the portal tract mesenchyme ( Figs 13.4 and 13.5 ). Progressive involution of most of these cells, with acquisition of strong cytokeratin 7 and 19 positivity in those remaining, is the process by which mature interlobular bile ducts of the portal tracts are formed. Persistence of portions of the ductal plate and abnormal remodelling (the ‘ductal plate malformation’ described by Jörgensen ) lead to ectatic and irregularly shaped bile ducts set in dense fibrous stroma, the basic histopathological feature common to all fibropolycystic diseases. Mutations in genes encoding proteins found on primary cilia of bile-duct epithelium and resultant ciliary defects in mechanical, chemical and osmotic sensing underlie the characterisation of many of these diseases as ‘ciliopathies’ .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here