Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Botulinum toxin type A injections represent the single most frequently performed minimally invasive aesthetic procedure in the United States, totaling an estimated 7.7 million procedures in 2019 alone, according to the American Society of Plastic Surgery. Neuromodulators represent a cornerstone of minimally invasive surgery with 150% more procedures performed annually than the next three leading procedures (soft tissue fillers, chemical peels, and laser hair removal) combined. Their popularity only continues to surge with an over 850% increase in volume in the past two decades. Moreover, noninvasive procedures serve as an important gateway to attract patients to more invasive options; one study estimated 15% of patients who sought noninvasive treatment underwent an invasive surgery an average of 19 months later. Given their critical role, mastery of neuromodulator technique has become an essential skill for any plastic surgeon.
Botulinum toxin derives from the bacterium Clostridium botulinum , which produces seven serotypes (A, B, C1, C2, D, E, F, and G). , Although both botulinum toxin A and B are commercially available for use, botulinum A (BTA) represents the workhorse of injectable neuromodulators and the only one currently approved for cosmetic use. Initially reported in the 1970s as a novel method to weaken extraocular muscles for treatment of strabismus, BTA gained traction throughout the 1980s for facial spasmodic disorders including blepharospasm and hemifacial spasm. In 1989 the U.S. Food and Drug Administration (FDA) officially approved the use of onabotulinum toxin A, commercially known as Botox , for the treatment of facial spasmodic disorders.
Botox’s relative obscurity as a niche ophthalmologic drug was short-lived, however. In the late 1980s, Dr. Carruthers, an ophthalmologist by training, noted the incidental improvement of dynamic glabellar rhytids in patients receiving Botox for management of blepharospasm. Recognizing a potential application in aesthetic surgery, she partnered with her husband, a dermatologist, and the pair published the first landmark study showing its use for improvement in dynamic glabellar frown lines in 1992. A decade later, the FDA officially approved cosmetic use of Botox in the treatment of glabellar rhytids; this was followed by approval for the treatment of crow’s feet in 2013 and forehead lines in 2017. ,
BTA ameliorates dynamic wrinkles by inducing flaccid paralysis. The protein disrupts the synaptosomal-associated protein 25, located on the presynaptic cell membrane. This inhibits synaptic vesicles containing acetylcholine from fusing with the presynaptic membrane, blocking release of the neurotransmitter into the synaptic cleft. , , Although motor endplate activity ceases within hours of injection, maximal clinical effects take up to 2 weeks to manifest because of spontaneous, nonvesicle associated release of acetylcholine. The toxin’s results are irreversible; attenuation of effects is attributed to sprouting of new axons and the formation of new neuromuscular junctions. ,
Four formulations of BTA are approved for aesthetic use by the FDA: abobotulinum toxin A (Dysport), incobotulinum toxin A (Xeomin), onabotulinum toxin A (Botox), and most recently prabotulinum toxin A (Jeuveau). Although each drug shares the common active ingredient responsible for muscle paralysis, the three differ in purification and processing, yielding slight variation in the products.
Each drug has its own unique dosing regimen, with no universally accepted exchange ratio. Although the conversion ratio of Xeomin-to-Botox is generally considered as 1:1, the ratio of Dysport-to-Botox remains under debate. , The reported Dysport-to-Botox ratios range from 1.3:1 to 11:1, although the majority of studies settle on a ratio somewhere between 2:1 or 3:1. , , ,
The lack of consensus for conversion standards undermines clinical efforts to scientifically compare the different products. , Some studies, including a randomized control study by Lowe et al., report Botox affords a superior result to Dysport in terms of glabellar line severity with a conversion rate of 2.5 units Dysport:1 unit Botox. However, these studies are criticized for being underpowered or inadequately randomized in terms of patient age. , Other studies by the same author contend Dysport is, in fact, better; Lowe et al. reported that Dysport is significantly more effective than Botox for upper face lines, but this time with a 4:1 ratio. A different study by Karasi et al. argues that Dysport used in a 3:1 ratio of Botox has a longer duration of action when measured by serial electromyography. This lack of conversion consensus makes it difficult to compare products directly. As a result, there is no overwhelming evidence arguing the superiority of one product to another.
Besides relative efficacy, the diffusion profiles of each drug are also contested. Diffusion differs from spread; spread refers to the more technique-dependent movement of the product from the original site of injection. Differences in spread could be attributed to the volume, speed, or angle of injection. Diffusion refers to the movement of the toxin beyond the original site as a result of kinetic dispersion, dictated by the toxin subtype’s unique properties as well as the property of the surrounding tissues. For example, diffusion can differ with the receptor density of a particular area, since areas with a high density of receptors will bind more of the toxin, limiting overall diffusion. This was demonstrated by Hexsel et al. when examining hyperhidrosis. Three women received 5-unit injections at three points on their back at three depths (2, 3, and 4 mm), and the field effect of each injection (the area where the toxin prevented sweating) was measured. Interestingly, location mattered more than depth, with injections at the midline having a smaller field effect no matter the depth. This highlights how the same dose in a different anatomic area can elicit a different response because of receptor density.
Diffusion is not a desirable quality with BTA. Relatively less diffusion allows for precise, intentional product placement and decreases the risk of the toxin spreading systemically and causing adverse side effects. Differences in diffusion are sometimes attributed to the different molecular weights of each of the toxins, which are attributed to the different protein load of each toxin. When BTA is produced naturally by bacteria, it is complexed to a series of proteins that help to protect the toxin from destruction inside the gastrointestinal tract. For equivalent clinical doses, the protein content of Dysport is 40% lower than Botox (5 ng protein per 100 units Botox vs. 4.35 ng per 200 units Dysport). Xeomin is reported to be completely free of complexing proteins with an estimated weight of 0.44 ng per 100 unit vial.
Early studies initially postulated that larger protein complexes could limit the diffusion of BTA. One such study was a randomized control study of 12 patients by Cliff et al. where 4 units Botox were injected into the one side of a forehead, 12 units of Dysport on the contralateral side, and saline in the center. The resulting anhidrotic halo was measured to yield the field effect (and thus the extent of diffusion). Dysport was found to have a larger field effect by a mean of 77%. Another, similar study by Trindade et al. used this same anhidrosis model to measure the extent of diffusion. Twenty patients received one medial and one lateral injection of Botox to one side of face and Dysport to the other, with laterality randomized. Patients were also randomized in respect to conversion ratios, receiving either 2.5:1, 3:1, or 4:1. Anhidrosis was subsequently measured over time for up to 6 months, indicating that the anhidrosis halo was larger for Dysport in 93% of comparisons. As expected, the larger conversion ratio (4:1) had a larger field effect than 2.5:1, inferring the positive association between increasing dosage and increasing rate of diffusion.
The evidence for these assertions is far from definitive, however. Previously mentioned studies are relatively small, and their results are not contingent upon diffusion alone; the differential field effects may also be a result of injection technique and dosing used.
In vivo studies suggest that differences in molecular weights are not responsible for differences in diffusion. In one such study, the dissociation of Botox was analyzed using ion-exchange chromatography under various physiologic pHs. At more acidic pHs (such as those that would be encountered in the gut), Botox remained bound to its complexing proteins. However, at higher pHs, such as those encountered when injected to muscle, it dissociated from complexing proteins in less than a minute. This may suggest that complexing proteins have little impact when using the toxin for aesthetic, injectable purposes.
There are multiple clinical studies, which also assert there is no difference in diffusion. Carli et al. compared the diffusion rates of Botox, Dysport, and Xeomin by measuring neural cell adhesion molecule (N-CAM) in a mouse model. N-CAM is nearly absent from adult muscle in the mice model but reappears when muscle is denervated; subsequently, they used N-CAM expression to determine the extent of diffusion. When they injected 0.25 units of Botox, 1 unit Dysport, or 0.25 units of Xeomin into the tibialis anterior of mice, they found no significant difference between the products in respect to diffusion. In humans, similar results could be seen. In a clinical study of 59 female patients, a forehead anhidrosis model was used to determine extent of diffusion, similarly to previous studies. Patients were randomized as to which side of the forehead received which toxin as well as the conversion ratio either 2:1 or 2.5:1 Dysport to Botox. Similar effects were seen at the lower dose equivalence (2:1) with a larger area seen at the higher dosage (2.5:1).
This underscores how crucial the chosen dosing equivalents are when designing these studies. Given the small samples, variation with technique, and lack of consensus dosage equivalents, there is not conclusive evidence to state that there is a difference in diffusion rates of the different toxins. All these studies support a few key facts: diffusion varies by anatomic location and diffusion is dose dependent.
Although the relative protein load may not necessarily impact the rate of diffusion, it does have potential implications for antigenicity. If the immune system develops neutralizing antibodies (NAbs) against the toxin, then the toxin can be neutralized, which ultimately results in treatment failure. This was especially a problem with the original formulation of Botox used before 1998, which contained five times the amount of associated complexing proteins (25 ng per 100 units, vs. 5 ng). In a study by Jankovic et al. analyzing Botox use for cervical dystonia, the old formulation was six times more likely to elicit antibody formation.
Risk factors for development of NAbs include large doses per injection, large cumulative doses, shorter intervals between injections, and use of “booster” injections 1 to 2 weeks after treatment. , In particular, it appears more common with use of botulinum toxin B (commercially available as Myobloc), which is used for cervical dystonia but not FDA approved for aesthetic purposes. Up to 44% of patients receiving Myobloc for cervical dystonia develop antibodies.
Thankfully, the development of NAbs with BTA appears far rarer and infrequently leads to a loss of treatment efficacy. Naumann et al. analyzed the relative incidence of NAbs formation in a meta-analysis of 2240 patients in 16 clinical studies receiving Botox for five different indications. Subjects received an average of 3.8 treatments (range 1–15) with total doses varying from 10–20 units in glabellar lines to 20–500 in cervical dystonia. Development of NAbs were 1.28% in cervical dystonia (4/312), 0.46% in primary axillary hyperhidrosis (4/871), 0.32% in poststroke spasticity (1/317), 0.28% in glabellar lines (2/718), and 0% in overactive bladder (0/22). Of the 11 patients that developed NAbs, only three became clinically unresponsive to BTA.
Reported rates of NAb formation with Dysport and Xeomin also appear to be low. The rates with Dysport range from 0% in glabellar lines to 1.2% in cervical dystonia. Reported rates of Xeomin are less clear. In theory, Xeomin should be less antigenic since it has been formulated to exclude complexing proteins. Several studies support this premise. In a comparison of New Zealand white rabbits with Xeomin, Dysport, and Botox, Xeomin was the only toxin, which did not induce NAb formation. In humans, similar results were seen. Patients with upper limb spasticity were injected with Xeomin followed by either five repeat Xeomin injections or five placebo injections. At 89 weeks after Xeomin injections, no patient developed NAbs. However, during the clinical development program of Xeomin in the United States, 12 out of 1080 patients developed NAbs (1.1%). This rate may not reflect the true antigenicity, though. The 12 patients who developed NAbs were previously treated with a different BTA formulation and subsequently, their immune systems may have already been primed against the toxin. Although rigorous clinical studies comparing NAb formation rates between the formulations are lacking, existing studies suggest that NAb formation is generally a rare event, especially for patients receiving aesthetic dosing of BTA.
BTA is commercially available as a desiccated powder, which must be reconstituted before administration. Manufacturer guidelines recommend reconstitution with preservative-free saline rather than preserved saline, which contains benzyl alcohol, a bacteriostatic agent. , Although a seemingly innocuous difference, it has important applications for the shelf life. To minimize the risk of bacterial contamination, use of preservative-free saline necessitates the use of the entire quantity in a single day, rather than any excess for future use, which has economic implications for the manufacturer versus the provider.
However, there are several studies suggesting that same-day reconstitution is not the only method of effective administration. Several studies suggest BTA remains stable and equally efficacious with preservative-containing normal saline up to 5 weeks later. Other studies suggest that the contamination risk to patients is actually minimal. In one such study, 127 consecutive reconstituted vials were used to treat one to three patients for an average of four to five procedures, each within the course of the week. Each vial was refrigerated then reaccessed by different personnel using individual, single-use sterile syringes to draw aliquots for each patient. At the end of the study, each vial was cultured, and no microbial growth was detected in any of the 127 vials. Moreover, some studies assert preservative-containing normal saline is less painful to patients. In a prospective randomized control trial of 15 patients, Alam et al. found that 100% of patients reported less pain on their hemiface that was injected with preservative-containing reconstituted BTA versus the side with preservative-free BTA; the reported pain was on average 54% less. These results have been corroborated by other randomized, controlled trials in subsequent years.
Recognizing this data, the American Society for Dermatologic Surgery set forth guidelines in 2015 to specifically address the discrepancy between clinical data and suggested manufacturer’s guidelines. Consensus recommendations had two key findings for facial aesthetic use of BTA: (1) the same vial may be refrigerated or refrozen at least 4 weeks before efficacy is compromised and (2) a single vial can be used to treat multiple patients, assuming appropriate technique is used.
The manner of reconstitution may also impact the efficacy of BTA. Swirling without inversion can potentially lead to loss of potential product. Overly vigorous mixing can yield this result as well. In a study by Dressler et al., the impact of mechanical stress on BTA reconstitution was investigated. Ten vials of 100 units Botox were reconstituted with 2 mL normal saline using either a gentle or an aggressive reconstitution method. Potency was then analyzed using the time to diaphragm paralysis in a mouse model; the result was a 42% loss in potency, suggesting that mechanical stress may partially inactivate reconstituted BTA.
The volume used for reconstitution is yet another area under debate. In management of spastic conditions, large volume dilution appears advantageous. Animal models suggest that higher volume of a low concentration may better relax skeletal muscles, likely since the greater volumes allow for more distribution of the product within the target muscle. , In a randomized, control trial of children with spastic hemiparesis resulting in elbow flexion contractures, children’s biceps were injected with either a more dilute (5 mL per 100 units) or more concentrated solution of BTA (1 mL per 100 units). Children who received the higher volume, dilute BTA injection demonstrated a greater improvement in spasticity and range of motion.
In aesthetic applications, most clinical studies do not show a relationship between dilution and efficacy, even with up to a fivefold dilution. Other studies similarly show no difference in terms of efficacy, but instead suggest that dilution may be impacting other clinical factors, including patient pain and the degree of product spread. In one study, Hankins et al. injected the glabellar wrinkles of 46 patients with Botox at different concentrations ranging from 10 to 100 U/mL and followed the patients on a monthly basis. They found no difference in efficacy but noted larger volumes were associated with greater patient discomfort. In a prospective, randomized control trial, Hsu et al. injected the crow’s feet of 10 volunteers with 5 units of Botox, diluted to 100 U/mL on one side and diluted fivefold to 20 U/mL on the contralateral side. They found the area affected by BTA to be 50% greater in 9 of the 10 test subjects in the more dilute group. In another randomized control study by Carruthers et al., 89 women were randomized to receive glabellar Botox, diluted to either 100, 22.2, 20, or 10 U/mL. They found no statistically significant difference between dilution and response, although the six cases of eyebrow ptosis were all reported in the more dilute concentrations. Lastly, Abbasi et al. injected two concentrations of Dysport (6 units in 0.1 mL or 6 units in 0.3 mL) into the first prominent horizontal crease 2.5 to 3 cm above the orbital rim. They found that the more dilute BTA had a significantly greater area of effect compared to the more concentrated BTA (794.1 mm 2 vs. 476.6 mm 2 ), although the study was limited because of the marked interpatient variation.
There is no consensus opinion about the proper dilution of BTA for aesthetic purposes. Even an expert panel published in 2016 by the Global Aesthetics Consensus Group offered no clear guidelines. Instead, they acknowledged the difference in opinions, mentioning some panelists use more dilute concentrations when trying to achieve greater product spread while others feel it is associated with greater pain.
Minimizing patient discomfort is an essential to patient satisfaction. To minimize pain, multiple authors have tried to optimize administration techniques. Some authors have advocated for use of topical anesthetic cream (EMLA) to reduce patient discomfort. , Others have advocated for use of ice or ethyl chloride to cool the area, stating that this pretreatment can improve pain scores. In one prospective study of 40 patients receiving periocular BTA for blepharospasm or wrinkle reduction, the impact of ice versus EMLA was directly compared. Patients had half their faces pretreated with EMLA, ice, or both on separate sides. Reported pain scores on a scale of 1 to 10 were significantly lower with EMLA (3.25) or with ice (3.0) than the control (5.85). In patients who received both EMLA and skin cooling, 56.2% (9/16) preferred the experience of EMLA. Not every study has shown this positive effect, however. In a randomized control trial of 22 patients by Chorney et al., unilateral vibration, unilateral ice, and ice and vibration on either side were compared, finding no statistically significant difference in reported pain scores. Furthermore, ice may impact the efficacy and spread of the toxin itself, especially since protein efficacy and diffusion is impacted by temperature. Pirazzi et al. looked at the time of botulinum toxin to translocate across the plasma membrane in neurons in a rat model. At physiologic temperatures, this occurred in minutes; at 20 °C, the toxin could not translocate and enter neurons. This suggests that further studies are needed to determine what, if any, impact cooling before or after treatment may have on the clinical outcome of the toxin.
There is no recommended syringe type or needle to use with administration. In general, higher gauge needles should be used whenever possible to minimize discomfort. There is no consensus on syringe type, but a study by Foglietti et al. attempted to quantify how much BTA product was being wasted when using different types of syringes. The group compared 1-mL tuberculin Luer slip (TB) syringes with detachable 25-gauge needles to 1-mL ultrafine insulin syringes with 31-gauge needles. TB syringes had 0.05 mL product or the equivalent of 2 units leftover, which the authors estimate could cost a busy injectable clinic up to $155,500 in lost product over the course of a year.
Product may be injected either subcutaneously or intramuscularly. In one split-face comparison by Godin et al., 19 patients underwent BTA for forehead rhytids and were randomized to either intramuscular or subcutaneous injection. Both groups demonstrated similar efficacy as determined by eyebrow position and satisfaction surveys, but with less pain reported by the subcutaneous group. In addition, intradermal injections may lower sebum production, which can result in patient-reported improvement of skin oiliness and pores. , Some evidence also suggests it may increase collagen production, promoting improved skin quality. In a recent split-face randomized pilot study of 10 women by Sapra et al., no significant reduction was seen in terms of pore size or sebum production, but women with intradermal injections had significant improvement in skin texture and a relative midface lift at the end of 16 weeks. Although larger studies are needed, these preliminary results suggest that intradermal aesthetic injection may provide added benefits beyond selective paralysis.
Postinjection care is another widespread component believed to optimize the patient’s results. However, there is minimal evidence to support many protocols. Some advocate patients should keep their head elevated to minimize unwanted spread of the product, despite the lack of evidence that position changes can lead to lid ptosis. Others claim increased muscle use postinjection may facilitate toxin spread. This is supported by a randomized control trial by Wei et al., where 98 patients were injected with BTA into their masseters and the subsequent duration of muscle atrophy was measured; they found that in patients asked to strengthen their chewing effort, postinjection had a prolonged period of atrophy, suggesting more effective spread and deactivation of the muscle. There is limited information about the potential impact of postinjection massage but many caution against it, fearing it could facilitate undesired spread into adjacent facial mimetic muscles.
BTA treatment selectively weakens facial mimetic muscles, reducing rhytids that occur as a result of animation (see Figure 1 ). Some rhytids are static and will only respond to skin resurfacing procedures. For most facial rhytids, however, animation makes them worse to some degree; this degree determines the relative efficacy of BTA treatment. Subsequently, comparison of the patient’s static and dynamic rhytids is key to determining which areas would benefit from treatment. An understanding of facial anatomy is paramount for maximizing outcomes while minimizing risk of complications (see Figures 2–4 ).
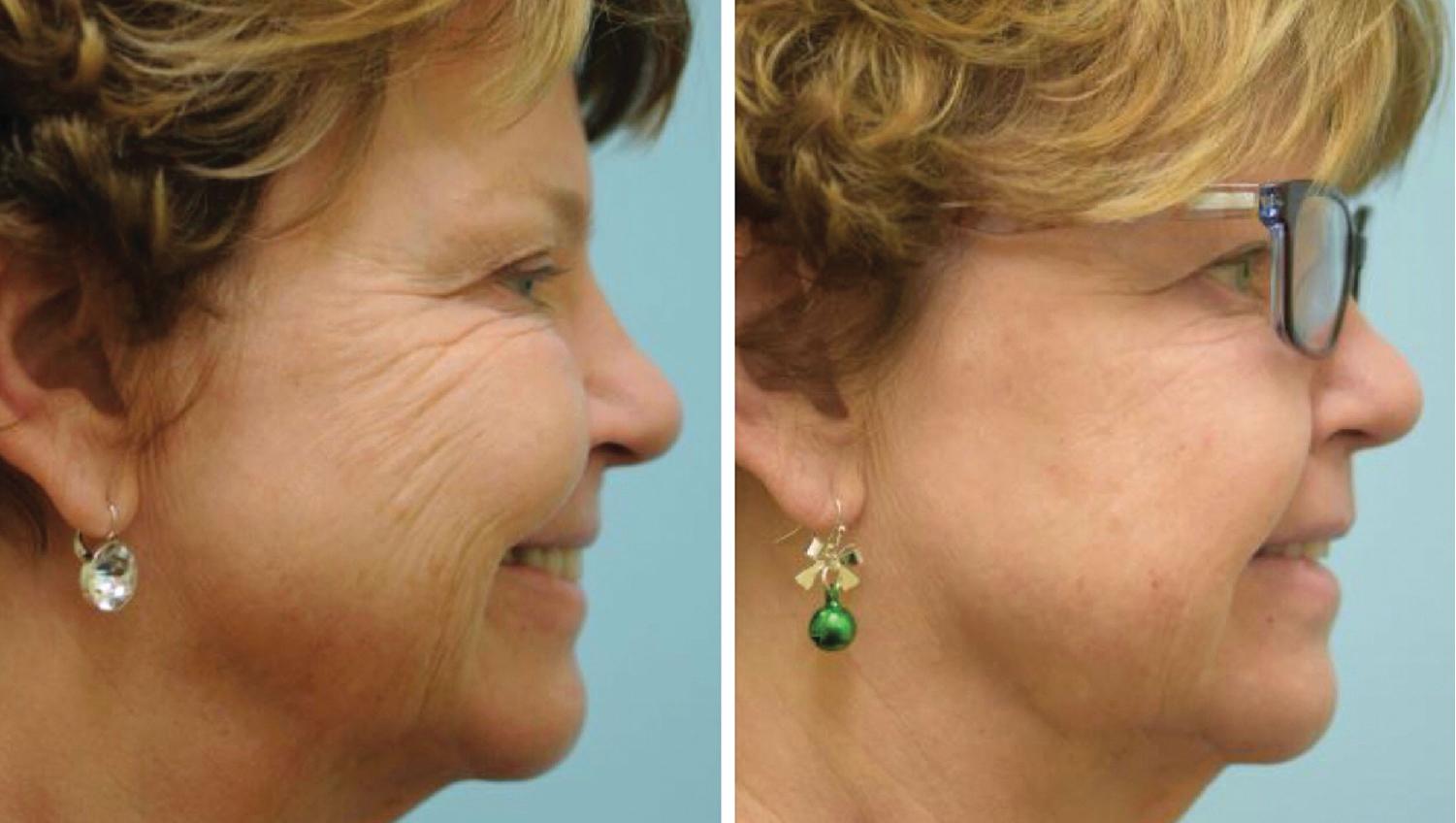
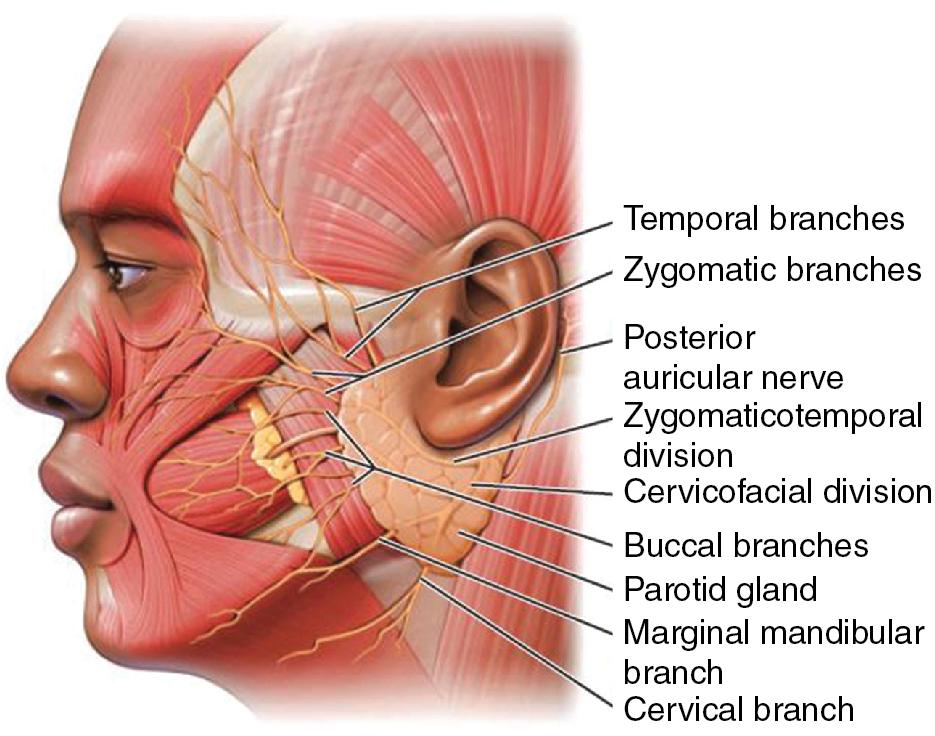
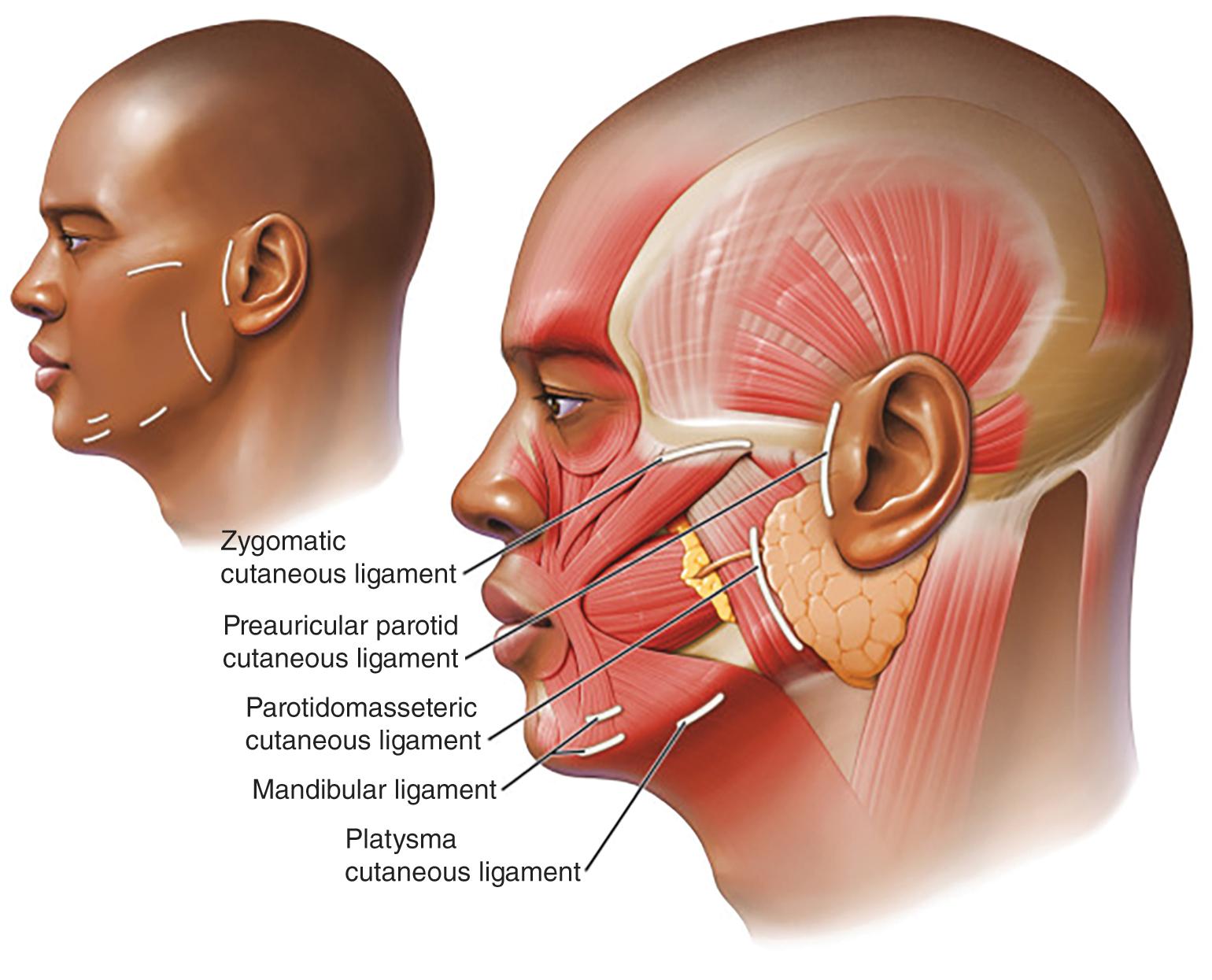
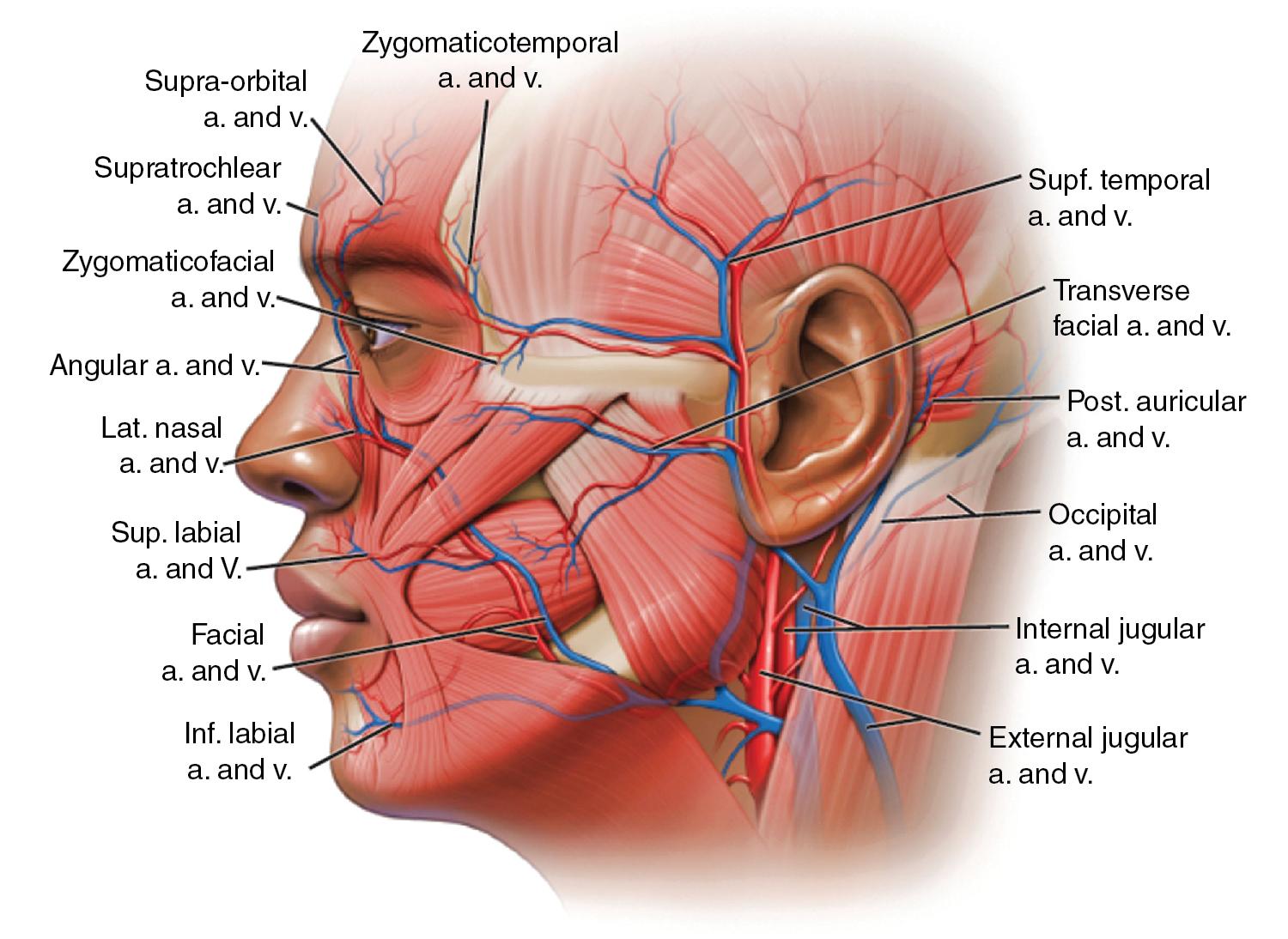
Currently, all four formulations of BTA are approved by the FDA only for treatment of glabellar region in patients under 65 years; Botox is also approved for treatment of lateral periorbital lines and forehead lines. Despite these relatively narrow indications, BTA is routinely used off-label with good success in a variety of diverse applications in patients of all ages.
BTA is a class C medication for pregnant and breastfeeding patients. It should be avoided in patients with neuromuscular diseases (such as myasthenia gravis or Eaton-Lambert syndrome). In addition, BTA should be avoided in certain patients on medications, which could potentiate the toxin’s effects (such as aminoglycosides or calcium channel blockers) or those with allergies to its components (including albumin or cow’s milk protein). ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here