Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The technique of cerebral angiography emanates from before the advent of noninvasive imaging, yet this approach serves a unique role in the management of patients with cerebrovascular disorders.
The risks and benefits of cerebral angiography include morbidity due to vascular injury with the advantage of exquisite spatial and temporal resolution to diagnose and treat numerous vascular lesions.
Common indications include ischemic or hemorrhagic stroke, as well as underlying or causative vascular lesions.
Interpretation of cerebral angiography is based on recognition of normal anatomy, collateral flow patterns, and pathologic findings.
Cerebral angiography may be particularly useful in the setting of acute stroke in the young adult or in other potentially elusive diagnoses, such as vasculitis.
Cerebral angiography may simultaneously allow for accurate diagnosis and tailored therapies, such as endovascular revascularization for acute stroke or angioplasty/stenting.
Limitations of cerebral angiography exist, including invasive nature of the procedure, operator-dependent image quality, limited generalizability, and cost.
Peri-procedural patient care, including monitoring for complications, are unique to this diagnostic modality.
After Egas Moniz invented cerebral angiography in 1927, it became the method of choice for the diagnosis of brain lesions , until the development of computed tomography (CT) and, years later, magnetic resonance imaging (MRI) limited the indication for catheter angiography to visualize cerebral vessels. CT angiography (CTA) and magnetic resonance angiography (MRA) have demonstrated excellent performance in noninvasively imaging large cervicocerebral vessels, further reducing the need for invasive angiography. Other noninvasive imaging techniques, such as duplex ultrasonography and transcranial Doppler ultrasonography, provide hemodynamic information that further limits the indications for cerebral angiography. Because of its exquisite spatial and temporal resolution that enables visualization of the smallest vascular lesions and flow patterns, cerebral angiography is still the reference standard for imaging the cervical and cerebral circulation. Moreover, the dramatic expansion of percutaneous treatments for ischemic and hemorrhagic cerebrovascular disease has brought a resurgence in catheter angiography as a necessary prelude and guide to endovascular interventions. ,
Cerebral angiography is increasingly performed by a diverse range of specialists, and this diversity has prompted the publication of qualification requirements for diagnostic and interventional procedures. With miniaturization and other advances in catheter technology and the development of non-ionic contrast agents, cerebral angiography is now, usually, a straightforward procedure that takes 30–60 minutes to complete. Cerebral angiography is painless and can generally be performed with the use of local anesthesia administered at the groin and no or mild sedation. General anesthesia is required only in patients unable to cooperate or in children. With the use of a closure device such as a collagen plug or percutaneous arterial suture for hemostasis, patients may be ambulatory within 1 hour after the procedure is completed, and anticoagulation need not be halted before the procedure. However, closure devices are only used in a select population of patients. Without closure devices, the patient can usually ambulate within 6–8 hours.
Vascular access is most often obtained via the common femoral artery and, rarely, via the brachial or axillary artery. In the past two decades, a shift to accessing the radial artery by the interventional cardiology field is noted. Multiple large trials, including the Radial versus Femoral Access for Coronary Intervention (RIVAL) and Radial Versus Femoral Randomized Investigation in ST Elevation Acute Coronary Syndrome (RIFLE), reported decreased access site morbidity compared to femoral access. In the neurointerventional field, a few groups are transitioning to radial access and reporting favorable safety and efficacy. After arterial puncture, an arterial sheath is placed into the artery. A sheath is a short catheter with a diaphragm at its exterior end that allows the passage and manipulation of additional smaller catheters without damaging the accessed artery. Catheterization of the aortic arch and further selective catheterizations are performed with the combined use of a catheter and a guidewire. A plethora of catheters and guidewires is available. Selection of the catheters and guidewires used in a particular patient is based on the patient’s vascular anatomy, the diagnostic question to be answered by the procedure, and the preferences of the operator. A hockey-stick-shaped catheter and a guidewire with a simple 45-degree curve are commonly used, both with hydrophilic coating. For difficult anatomy, other catheter shapes are available. The catheter is continuously flushed with heparinized saline to prevent thrombus formation.
The precise vessels that are catheterized depend on the indication for the procedure. Sometimes, catheterizing only one vessel is all that is required, for instance, for immediate follow-up evaluation after aneurysm clipping. At other times, the bilateral vertebral arteries, bilateral external and internal carotid arteries, and bilateral common carotid arteries must all be catheterized. In many instances, catheterization and angiography of the aortic arch and three to four vessels are necessary. The injection variables employed may alter contrast bolus delivery and have considerable effects on the opacification of downstream arteries, parenchyma, and veins. Standardized technique is therefore important for the comparison of angiographic results.
At the very end of the procedure, the accessed artery sheath is removed and manual compression is applied at the access site until hemostasis is obtained. Arteriotomy closing devices are now routinely used. These are essentially tools that percutaneously place a suture into, or a plug at, the puncture site to obtain immediate hemostasis. The indications for the use of a percutaneous closure device vary among institutions.
The risks associated with cerebral angiography have declined over the years. Increasing procedural safety is caused by many factors, including nonionic contrast agents, new catheters and guidewires that have safer designs and are made of less thrombogenic materials, and digital subtraction angiography. , ,
The risks associated with cerebral angiography are related to patient age and medical condition, the number of catheter exchanges, the total time for the procedure, and the load of contrast agent. , Contrast-induced neurotoxicity may elicit neurologic symptoms. Headaches or even transient encephalopathy during angiography have also been documented, although the underlying factors that trigger such events remain unclear. Interestingly, some data suggest that the performance of cerebral angiography may directly affect the tone of the vasculature, and vasodilation is the condition most often noted. Increased use of angiography as part of therapeutic interventions and the introduction of novel acquisition techniques that may increase radiation exposure also warrant consideration in the future. Cerebral angiography is more risky in the elderly and in patients with particular conditions that predispose to thrombus formation on the catheter (hypercoagulable states) and to vessel dissection and rupture (e.g., Marfan disease). In the setting of acute ischemic stroke, it has also been suggested that microcatheter injections may predispose to hemorrhagic transformation.
Many studies have been performed to quantify the risks of cerebral angiography. Hankey et al. reviewed several series and found the general risk for permanent neurologic sequelae after cerebral angiography to be 1%. The mortality rate was less than 0.1%. The overall risk for a neurologic complication was approximately 4%. In a prospective study by Dion et al., of 1002 cerebral angiography procedures, the rate of permanent neurologic deficit was 0.1% and that of transient deficits was 1.2%. Other studies cite a persistent neurologic deficit rate of 0.3%–0.5% and a total neurologic complication rate of 0.5%–2%. The most recent data suggest angiography complication rates that may be exceedingly low. , , Kaufmann et al. reported neurologic complications in 2.63%, including 0.14% with strokes and permanent disability, and a mortality rate of 0.06% in a consecutive series of 19,826 procedures. Fifi et al. reported a complication rate of 0.30% across 3636 diagnostic cerebral angiograms over a 6-year period. Recent studies have also considered operator training or specialty as a factor in complication rates.
Among the potential neurologic complications of cerebral angiography are embolic events and vascular dissections. Transcranial Doppler ultrasonography studies monitoring for microembolic events during diagnostic angiography have shown that, although nonpathogenic air emboli introduced at the time of contrast injection are common, formed element emboli are uncommon. Symptomatic air embolism during angiography may occur in 0.08% of procedures. Carotid or vertebral artery dissections secondary to catheterization depend on operator experience and are less frequent (0.4%) with the newer, safer catheter designs. Although neurologically symptomatic complications from cerebral angiography are rare, clinically silent “complications,” diagnosed on diffusion-weighted MRI, are more common, being found in 26% of diagnostic angiography procedures. Covert MRI diffusion lesions are associated with the presence of multiple vascular risk factors, a greater difficulty in probing vessels, the amount of contrast medium required, longer fluoroscopy time, and the use of multiple catheters.
One particular concern of cerebral angiography in the setting of recently ruptured cerebral aneurysms is the risk of precipitating rebleeding by injecting contrast agent with an increased pressure head. This risk is extremely low. In a meta-analysis of 415 patients with acute subarachnoid hemorrhage, Cloft et al. found no cases of rebleeding during angiography.
Nonneurologic complications consist of local complications (i.e., hematoma, pseudoaneurysm, and infection ), renal failure, contrast agent allergies, and arterial occlusions. With the more common use of arteriotomy closure devices, distal embolization of the foreign body to the accessed artery can be rarely seen. Contrast or even heparin during angiography may also cause transient reductions in serum calcium values, possibly due to chelation. In a cooperative study among leading interventional radiologic societies, non-neurologic major complication rates were as follows: renal failure, 0%–0.15%; arterial occlusion requiring surgical intervention, 0%–0.4%; arteriovenous fistula or pseudoaneurysm, 0.01%–0.22%; and hematoma requiring transfusion or evacuation, 0.26%–1.5%. Recent use of percutaneous closure devices has demonstrated low rates of complications, although the use of concomitant antithrombotic agents may increase events.
When an allergy to iodine contrast medium is known before procedure initiation, premedication with steroids and antihistamines is usually sufficient to prevent any adverse reactions. In patients with previous severe allergy, such as shock, in whom iodine is contraindicated even with premedication, gadolinium-based contrast agents, which are radiopaque as well as paramagnetic, may be used.
To recognize and understand vascular disease, one must first be acquainted with the normal angioarchitecture of the cerebrovascular system and its variants. The three phases of an angiographic imaging sequence—arterial, capillary, and venous—are shown in Figs. 49.1–49.3 . Each phase has its particular sensitivity for the different pathologic entities. One should be able to recognize common variants, such as persistent fetal anomalies, vascular fenestrations ( Fig. 49.4 ), and collateral pathways.
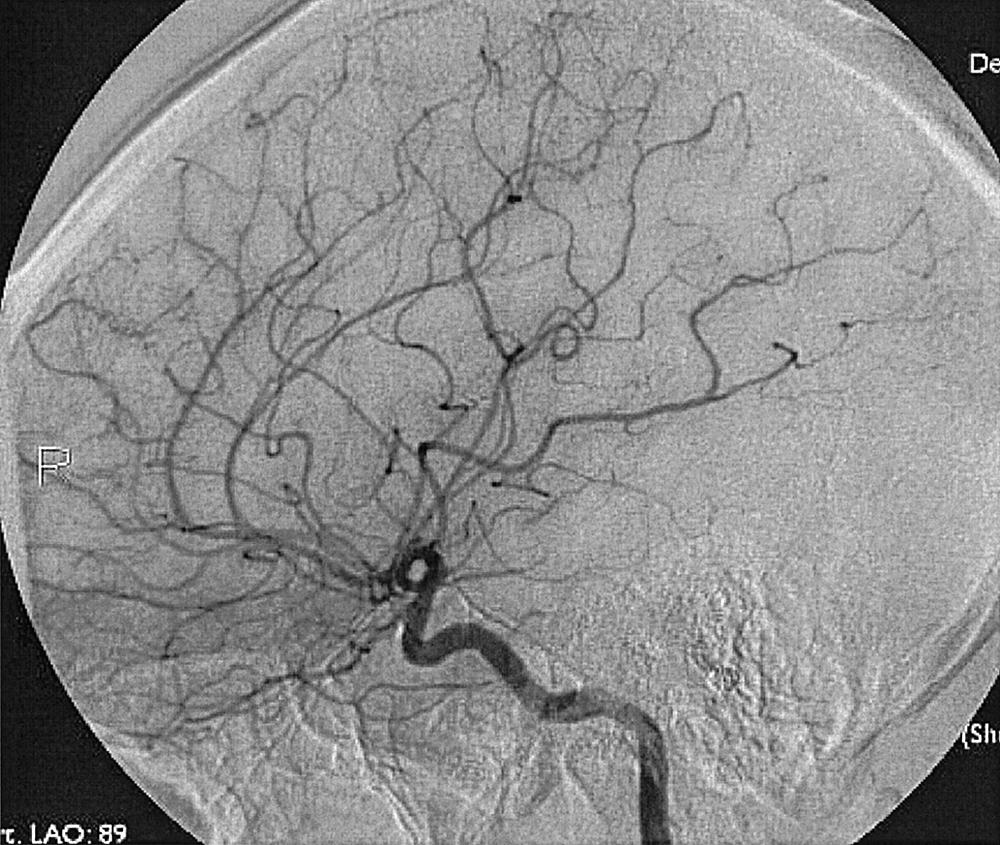
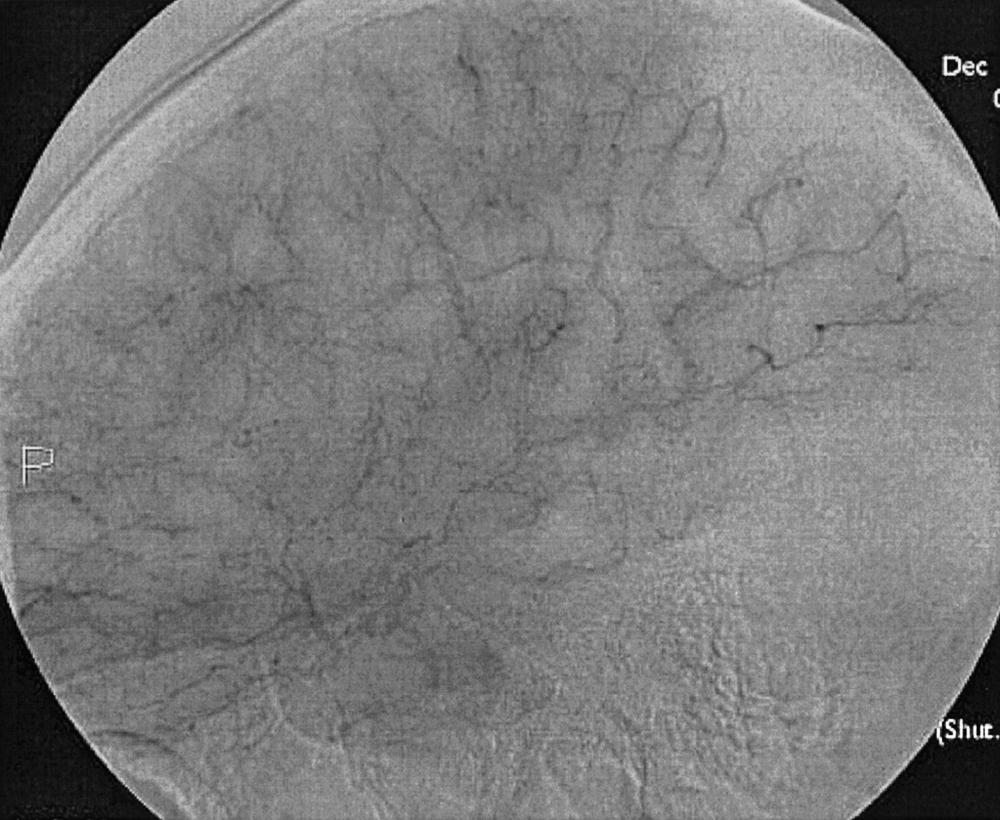
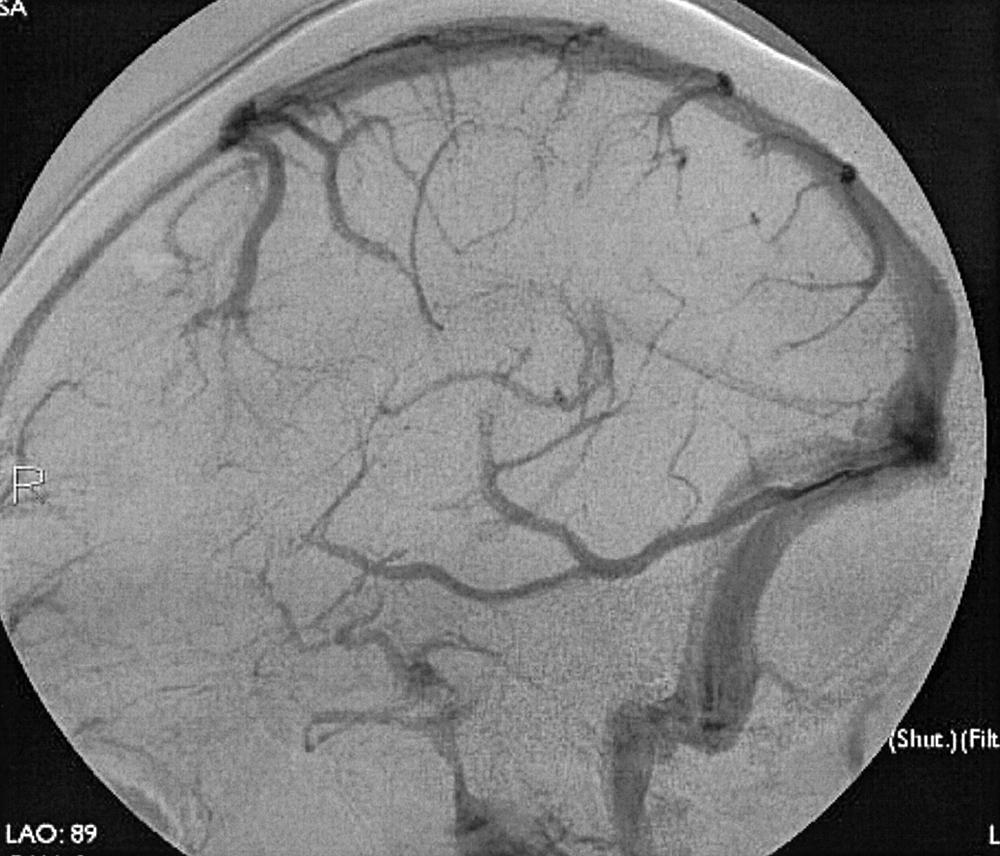
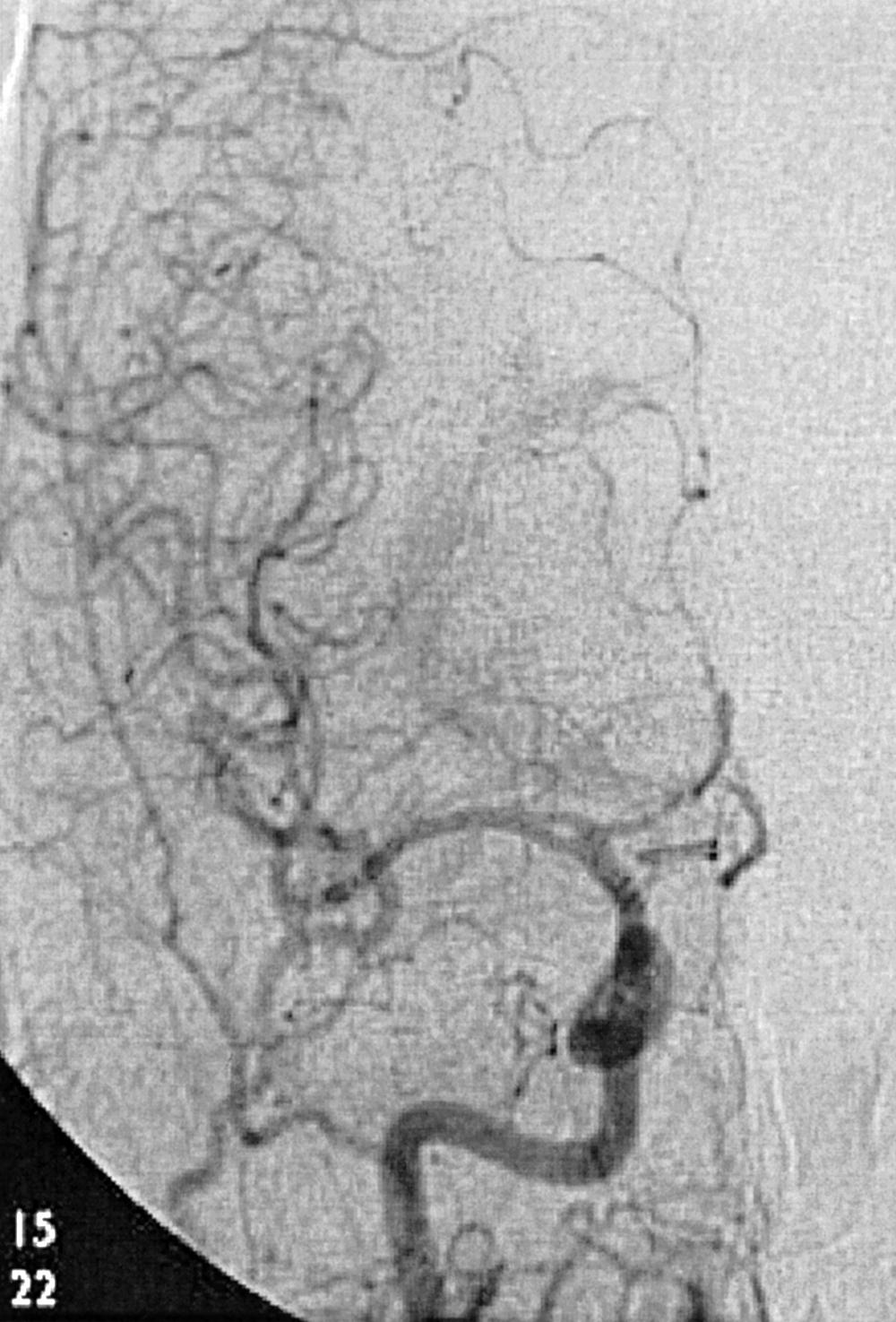
Intracranial hemorrhage may be epidural, subdural, subarachnoid, or intraparenchymal.
Epidural hemorrhage is usually secondary to head trauma. The vessels coursing in the epidural space, most notably the middle meningeal artery, are torn and arterial bleeding results. These hemorrhages are often associated with skull fractures. Because epidural hemorrhages are usually of traumatic origin, correlation with the clinical history as well as noninvasive imaging almost invariably identifies the cause, obviating the need for angiographic evaluation.
Subdural hemorrhage (SDH) is secondary to bleeding from the cortical bridging veins that drain into the superior sagittal sinus or other dural sinuses. The characteristic angiographic appearance is a lentiform avascular collection outlined by displaced cortical vessels. The cortical bridging veins are vulnerable to venous hypertension or tearing in the subdural space secondary to trauma. Venous hypertension can be caused by a variety of reasons, including sinus thrombosis and duralarteriovenous fistulas ( Fig. 49.5 ). Uncommonly, a subdural hematoma may result when an intracerebral aneurysm that previously hemorrhaged creates adhesions that direct subsequent hemorrhaging into the subdural compartment. In this situation, an intraparenchymal focus of hemorrhage is also often present, detection of which should raise the suspicion for aneurysmal bleeding. In a subdural hemorrhage, if a duralarteriovenous fistula or aneurysm is suspected, cerebral angiography should be performed; however, if trauma or significant brain parenchyma atrophy is present in an older patient, angiography should be reserved.
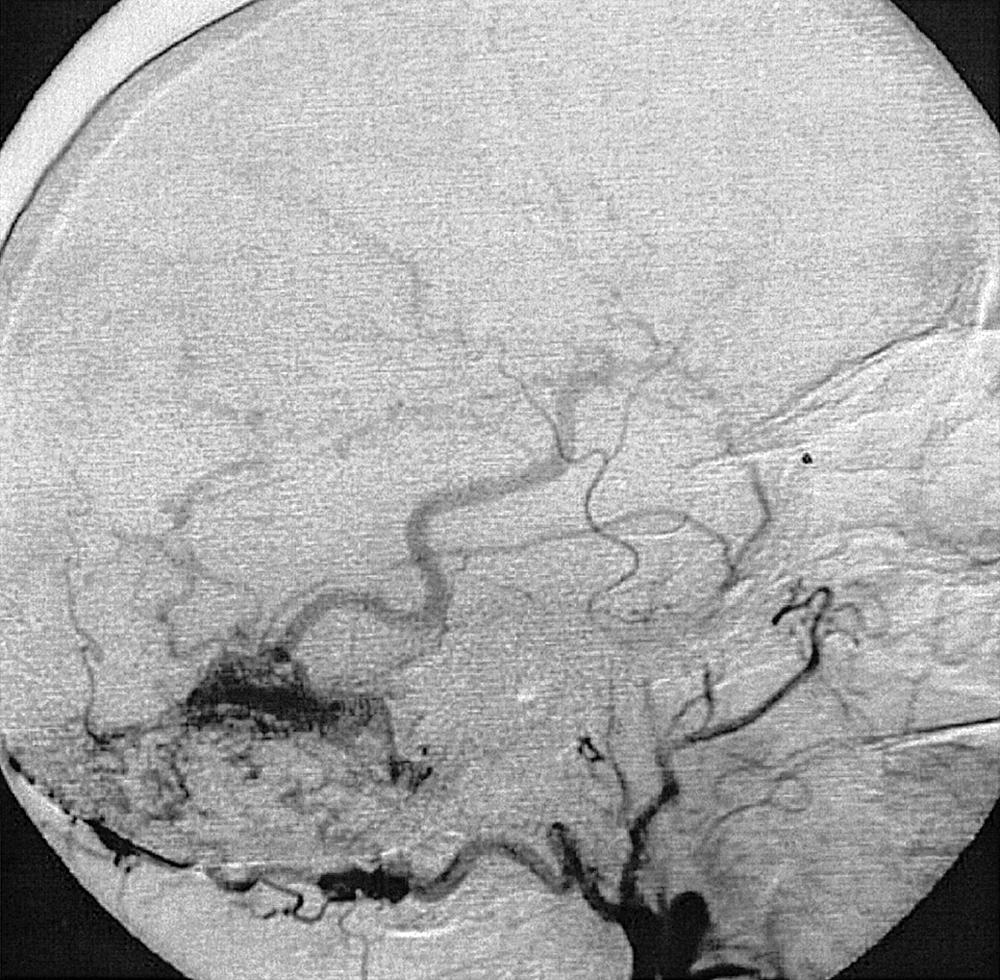
Subarachnoid hemorrhage (SAH) has an incidence of 6–8 per 100,000 person-years, and studies suggest that 2%–6% of the population have an intracranial aneurysm. In 85% of SAHs, the cause is a ruptured aneurysm, usually a berry aneurysm. Among the other causes of SAH are trauma (in which case a parenchyma focus of contusion or hemorrhage is often seen on imaging), intraparenchymalhemorrhage extending into the ventricular system, cerebral or cervical arteriovenous malformation (AVM) or fistula, venous thrombosis, mycotic aneurysm, non-aneurysmal perimesencephalic hemorrhage, vasculitis, and dissections; some SAHs are cryptogenic.
In less than 5% of AVMs that bleed, the hemorrhage is confined to the subarachnoid space, and no intraparenchymal blood is present. Generally, if the findings of angiography performed in the early phase of a nontraumatic SAH are normal, the procedure should be repeated after 1 week. There are several explanations why an aneurysm may not be detected on angiography; among these are parenchymal hematoma compressing the aneurysm; thrombus in the aneurysm, its neck, or both; perianeurysmal vasospasm; and suboptimal views of the region harboring the aneurysm. The diagnostic yield of a second cerebral angiogram in eight different series was 17%. If findings on the second angiogram are also normal, a third angiographic procedure performed several months later can be of benefit. In one series, aneurysm was detected by a third angiogram in 1 of 14 patients. For a patient in whom a CT scan shows a truly perimesencephalic hemorrhage pattern, the probability that cerebral angiography will reveal a vertebrobasilar aneurysm is 4%. Thus, the diagnosis of perimesencephalic hemorrhage requires normal findings on a cerebral angiogram. In a truly perimesencephalic pattern of SAH, a second angiographic procedure is not necessary if the findings of the first angiogram are normal.
Berry or saccular aneurysms are the most prevalent intracranial aneurysms. They are localized mostly in the anterior circulation (90%) as follows: anterior communicating artery, 40%; middle cerebral artery bifurcation, 30%; intracranial internal carotid artery, 20%; and posterior circulation, 10%. Other cerebral aneurysms are fusiform, traumatic (pseudoaneurysm), mycotic, or dissecting. A selection of aneurysms is illustrated in Figs. 49.6–49.18 . Cerebral aneurysms have been associated with autosomal-dominant polycystic kidney disease and Marfan syndrome.
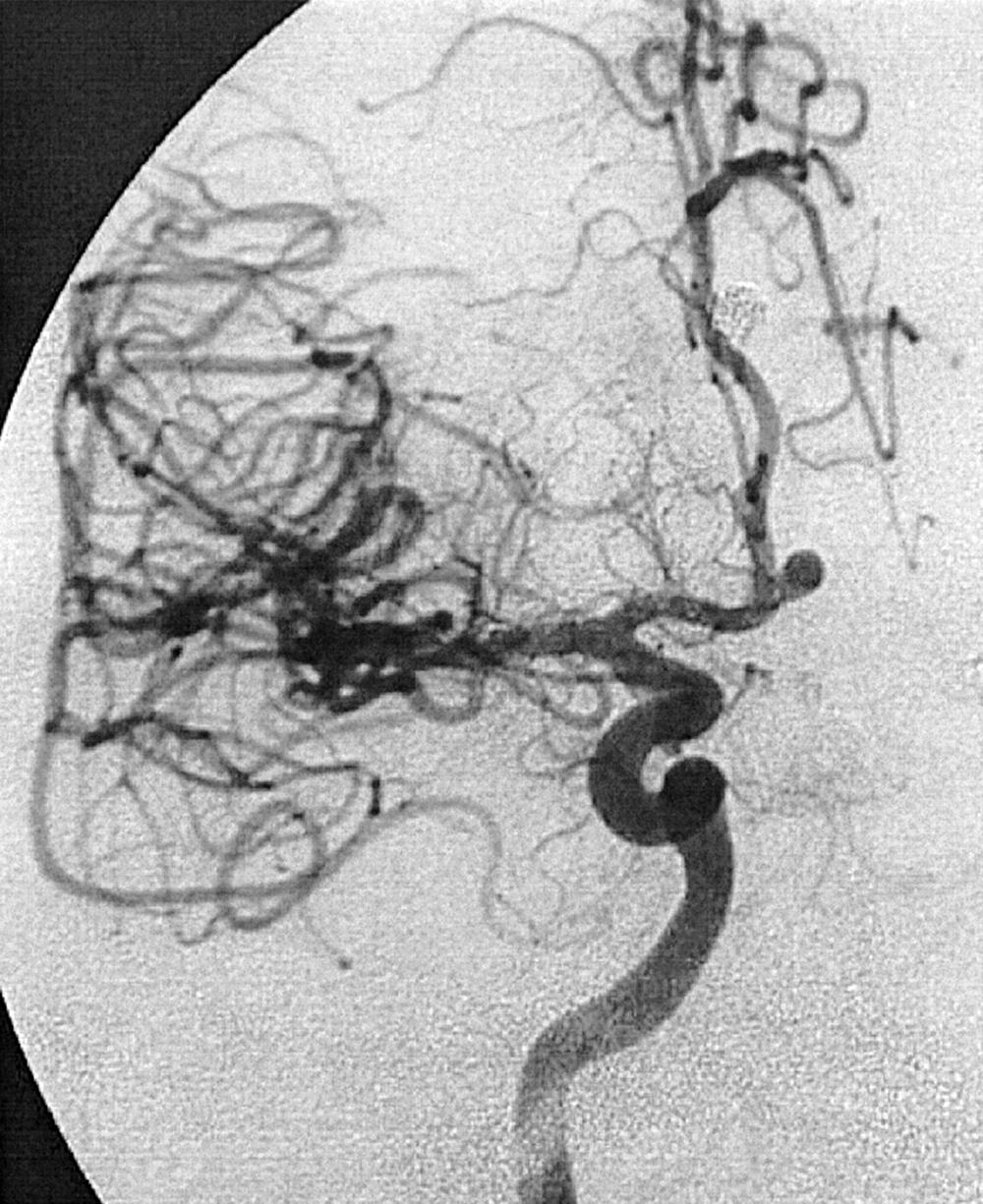
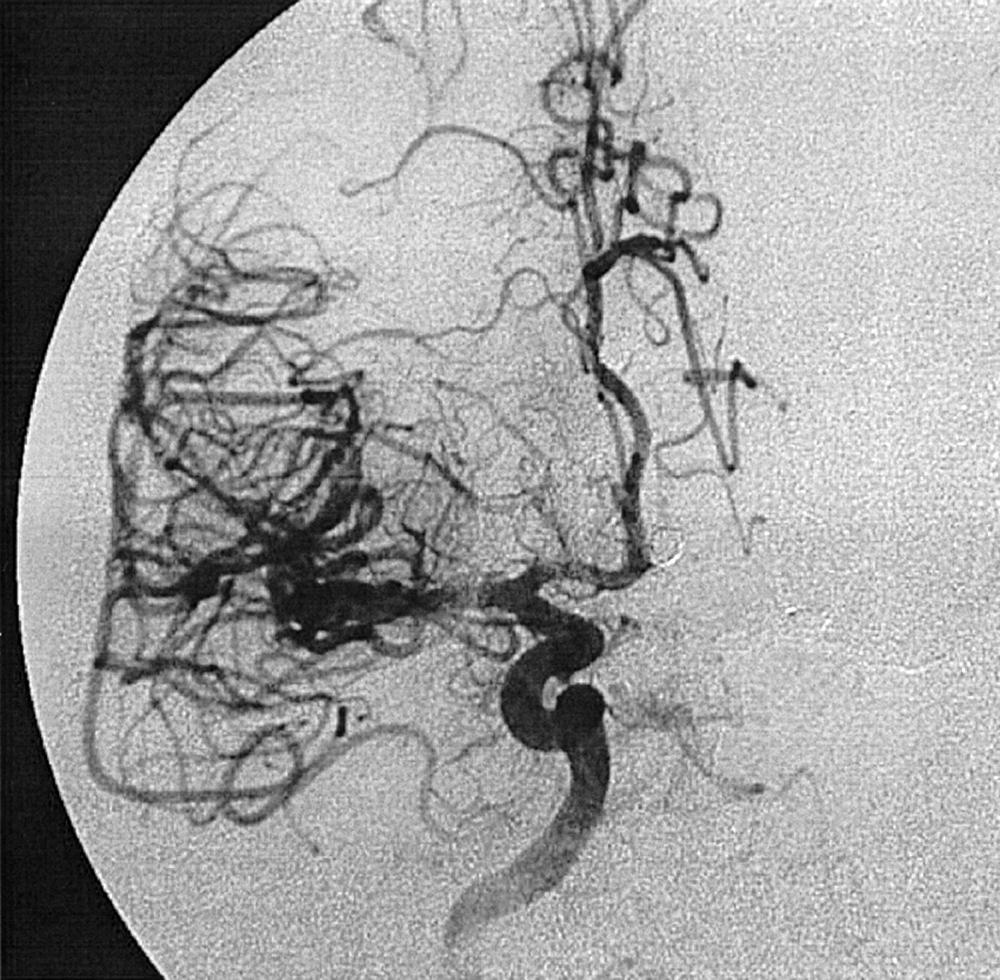
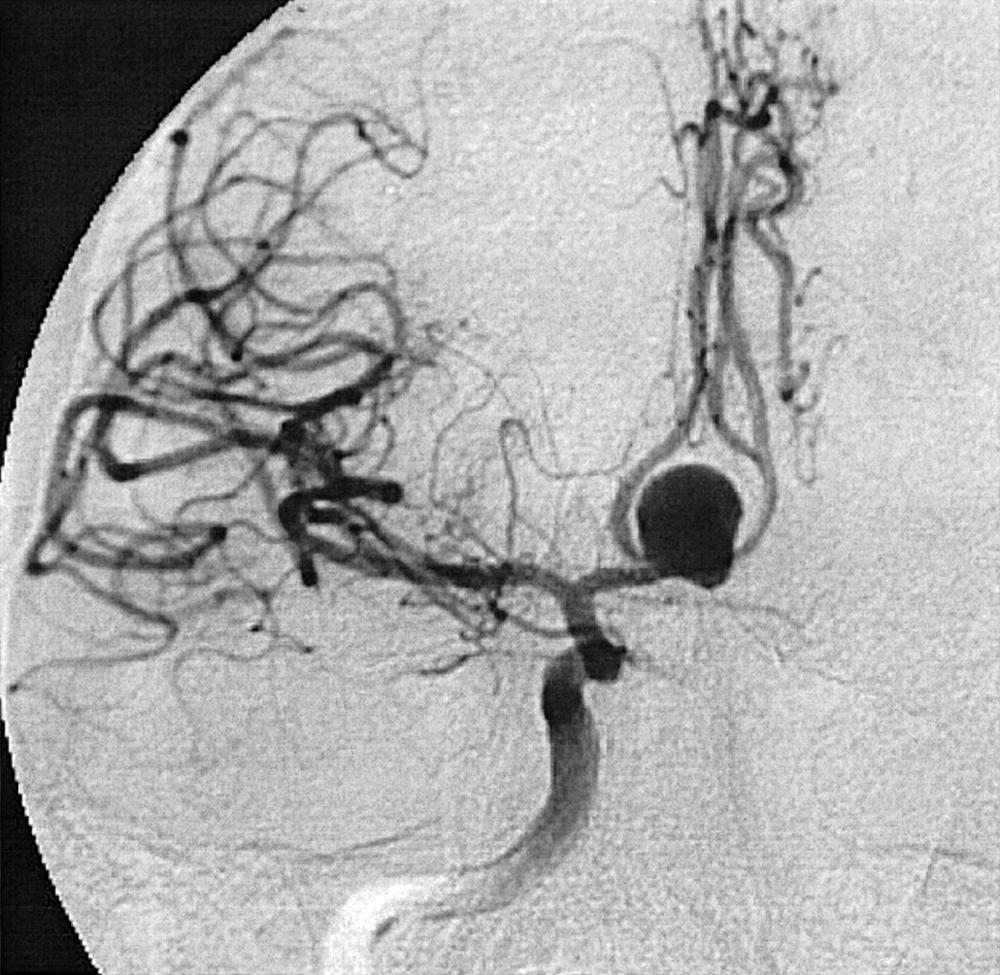
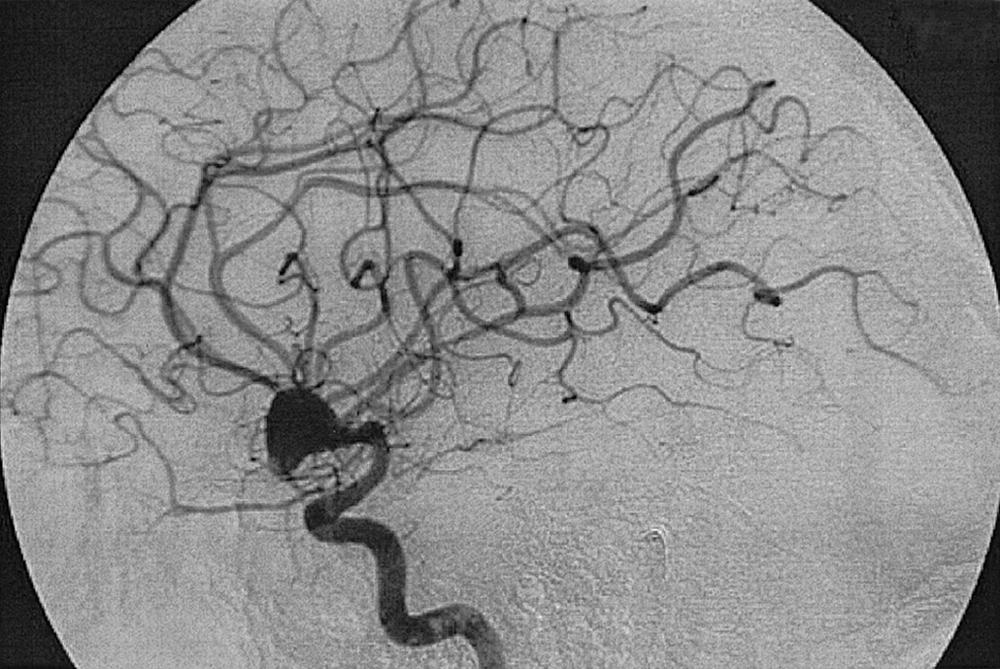
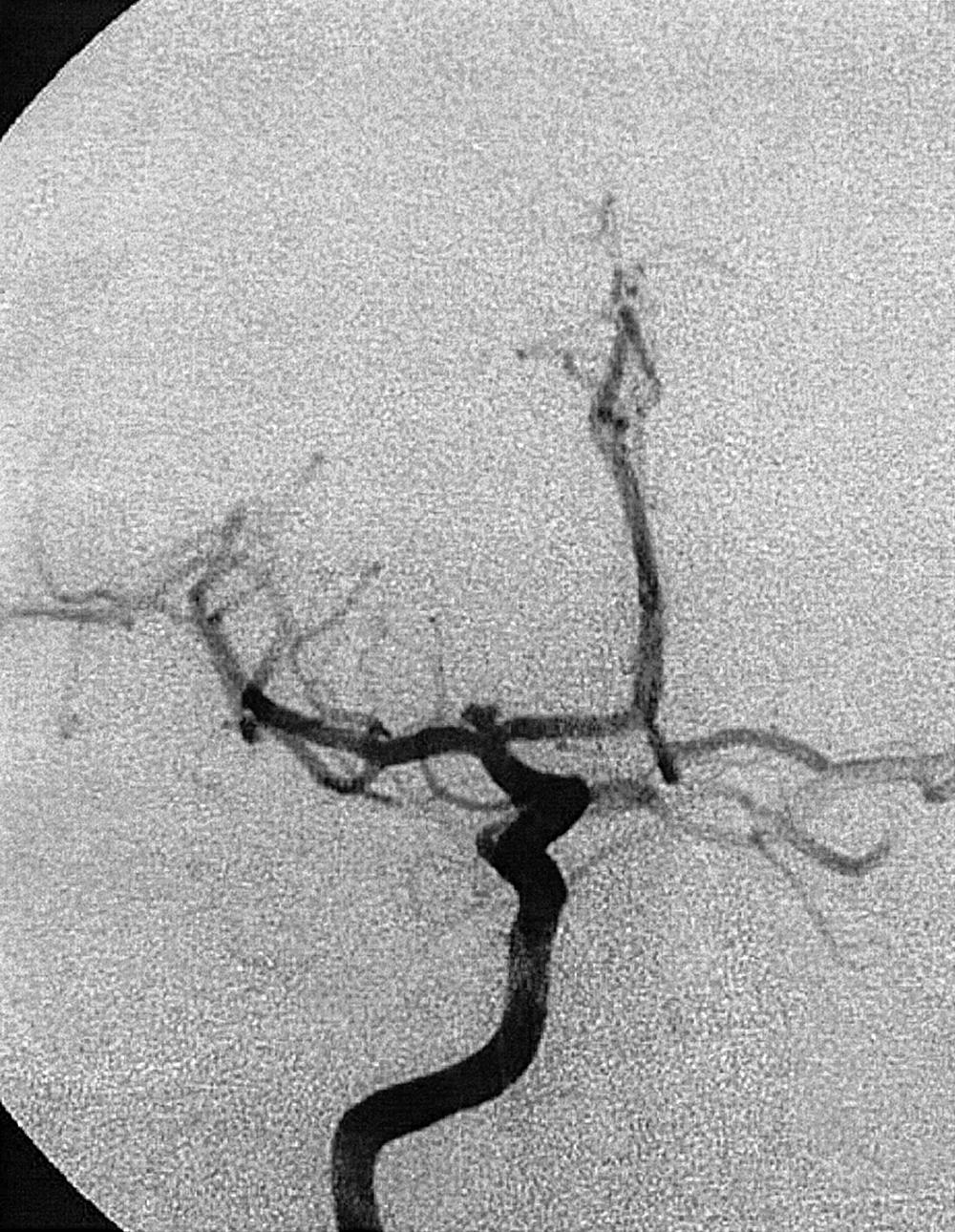
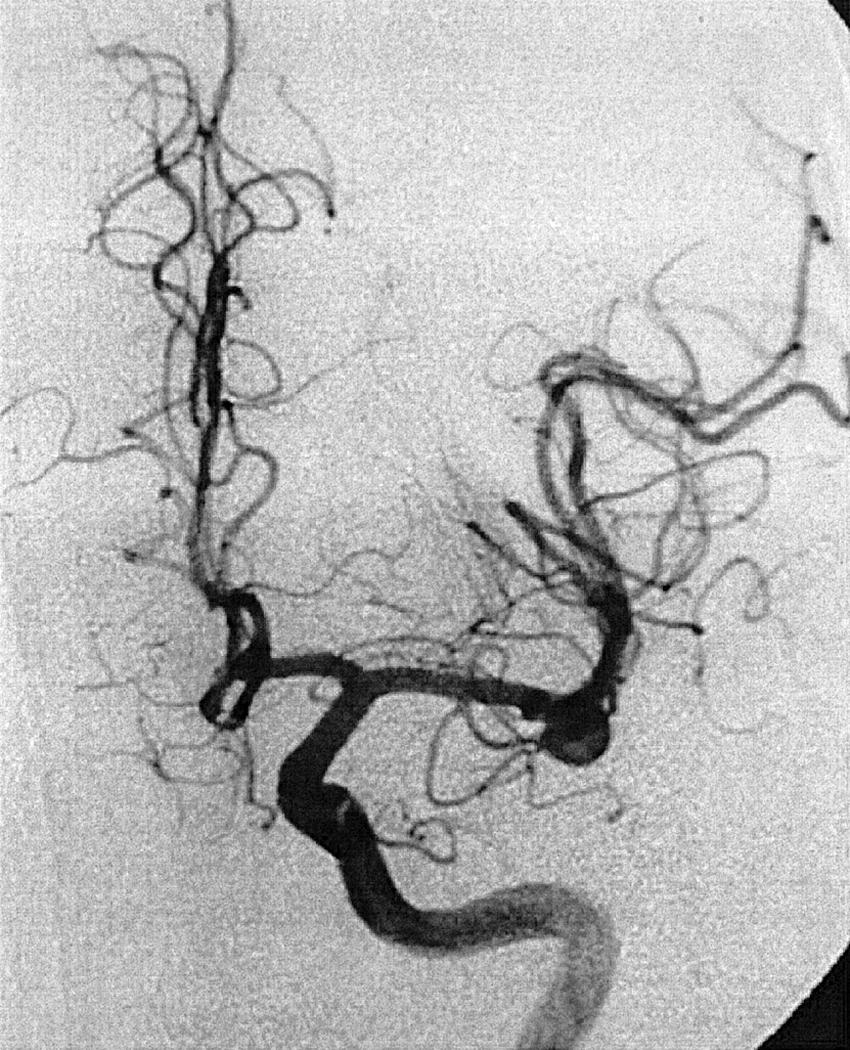
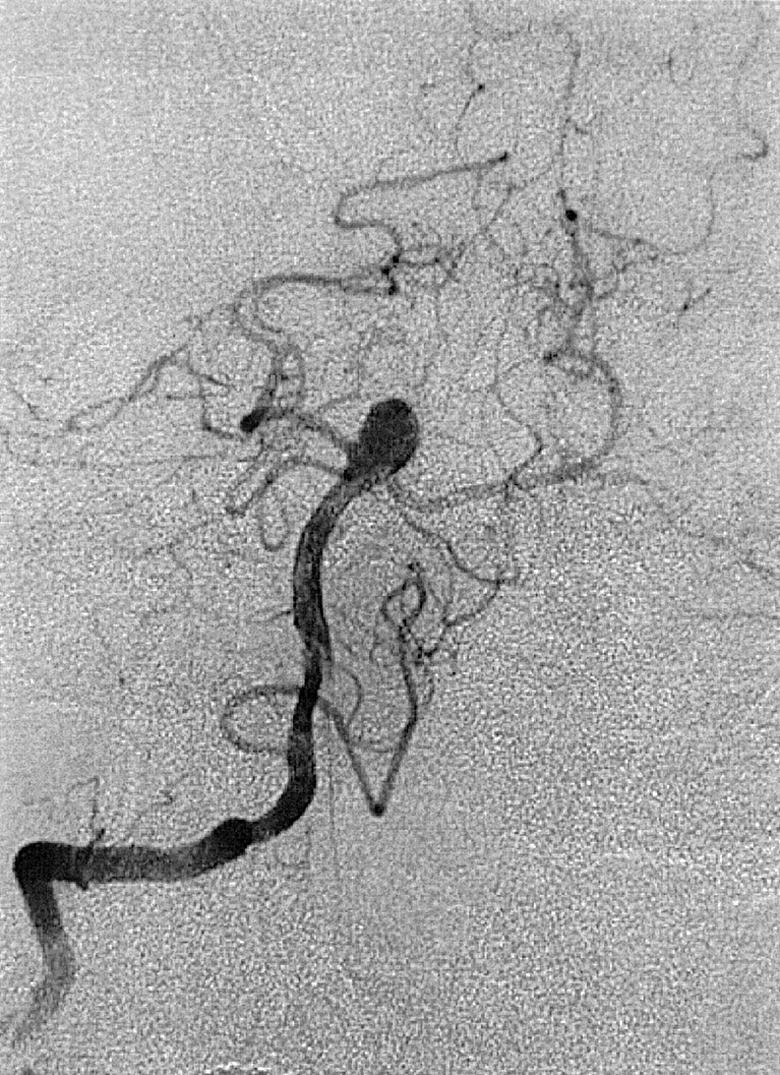
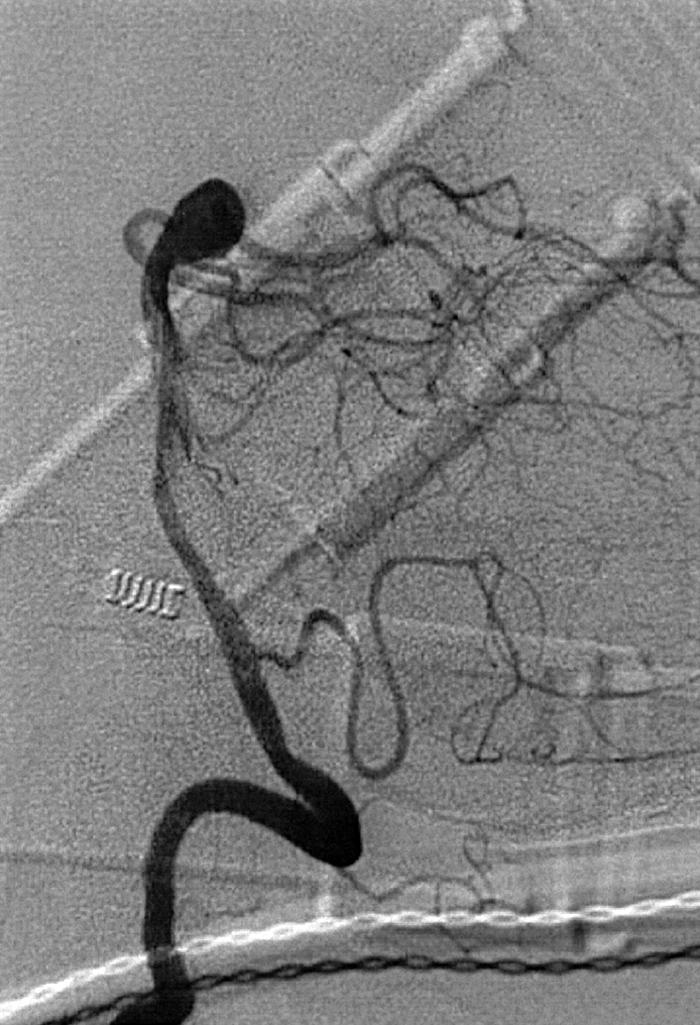
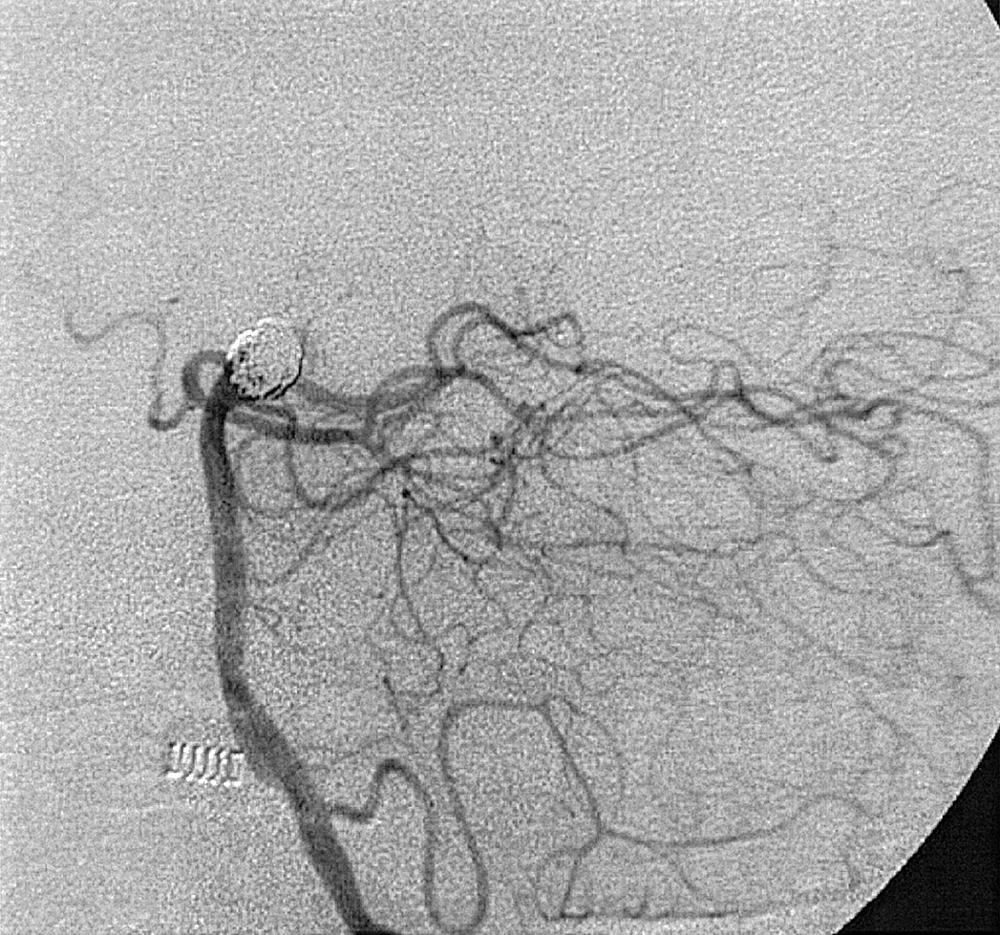
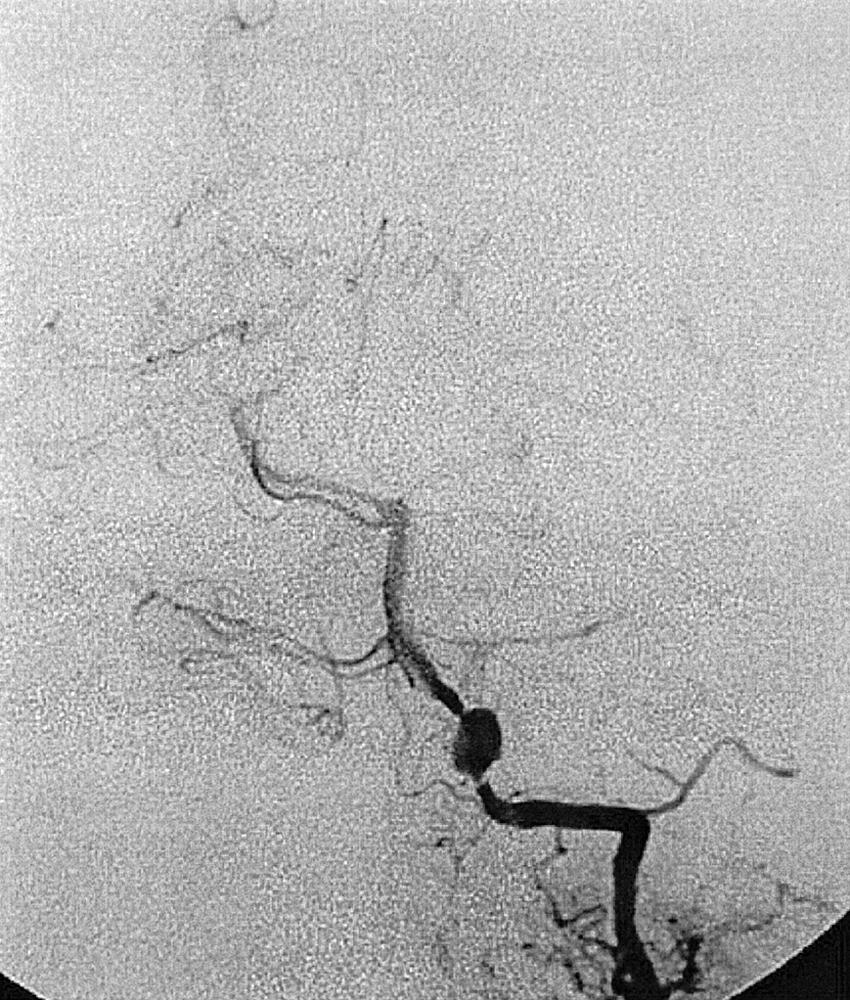
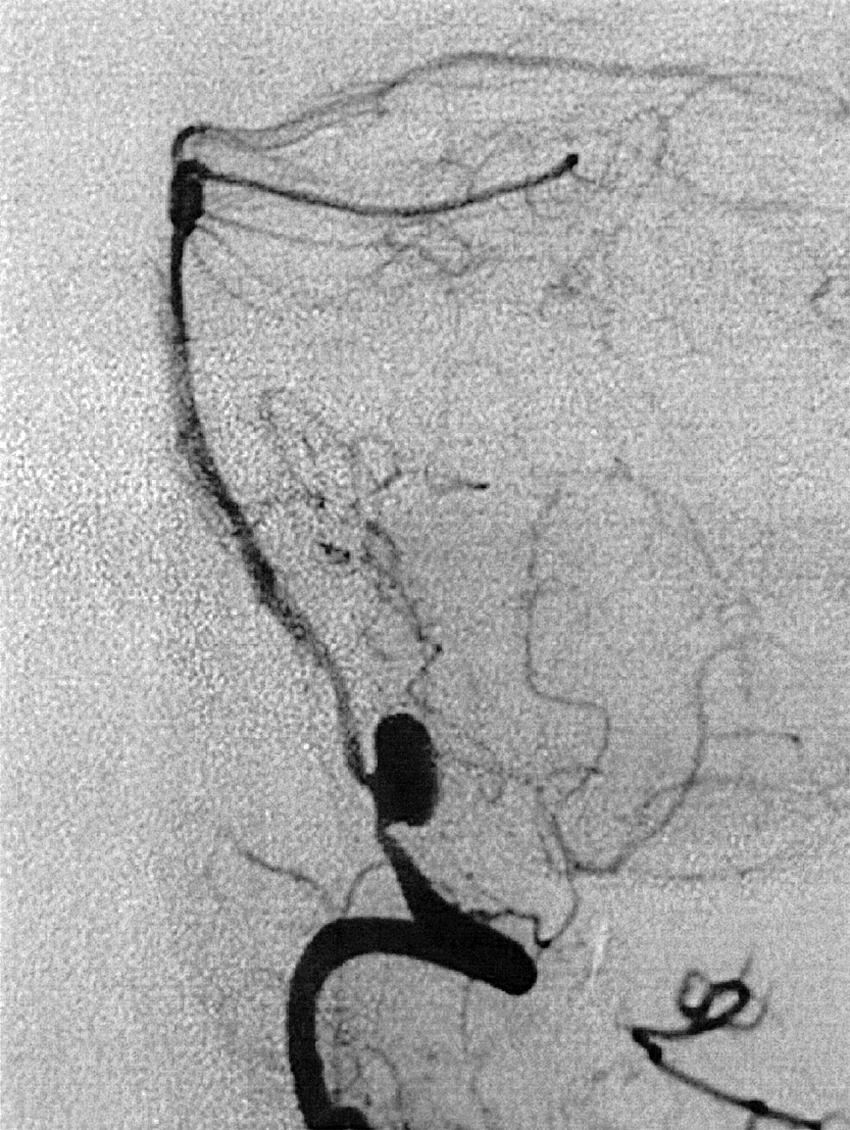
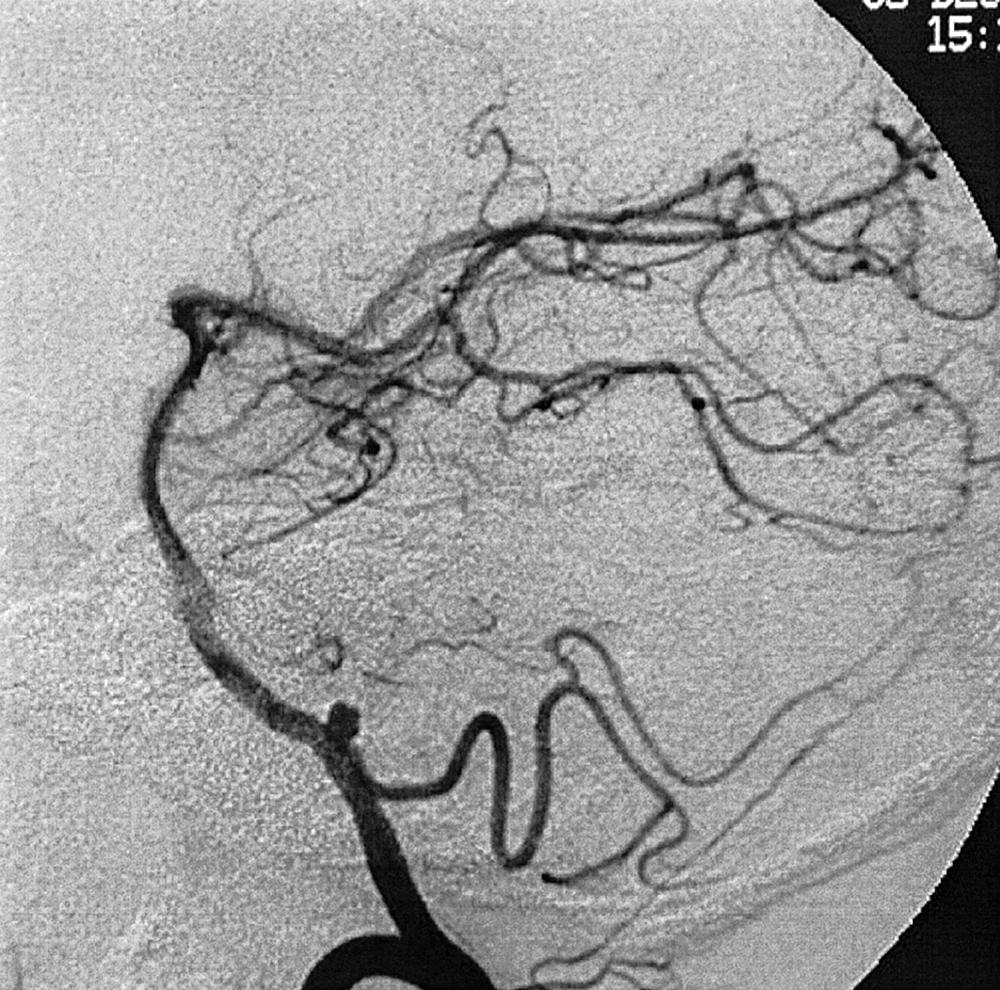
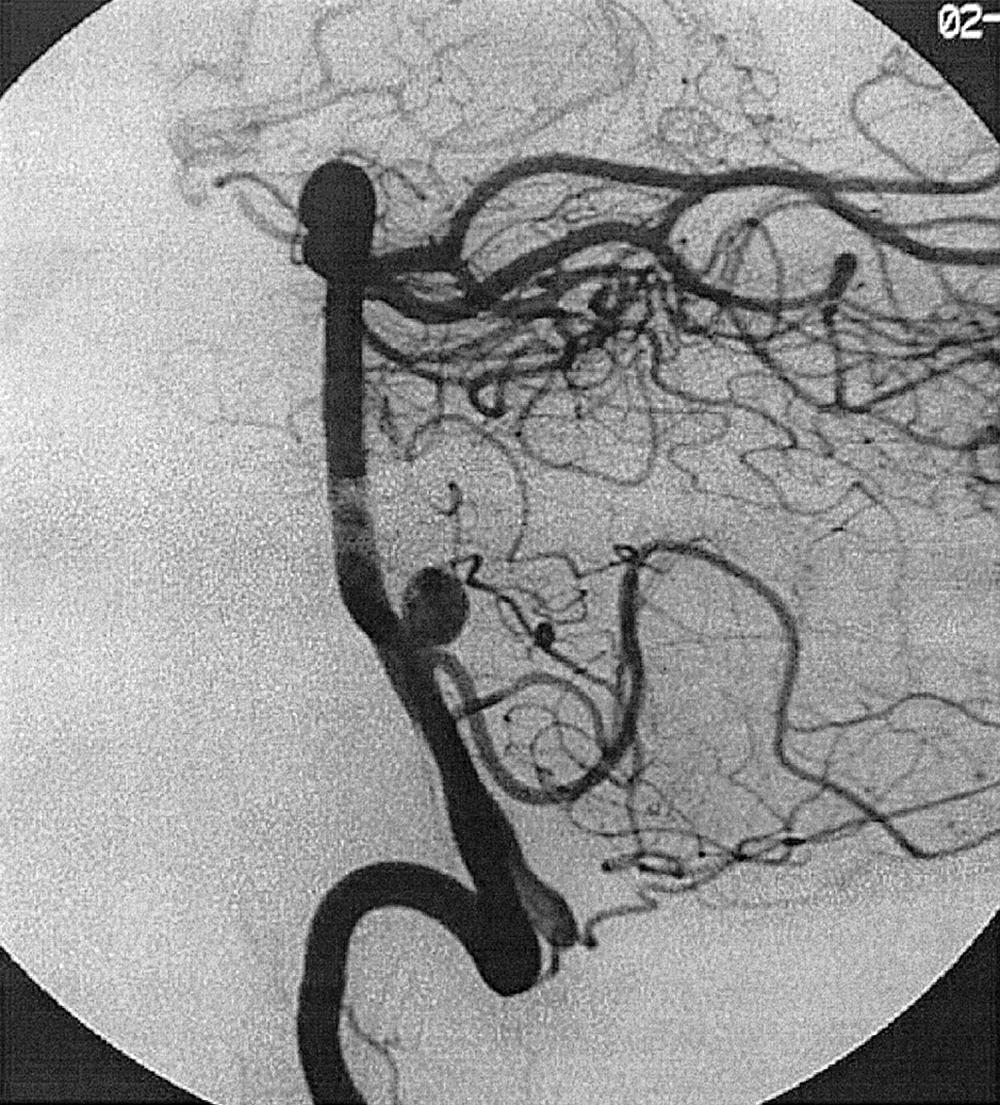
Angiography should evaluate the location of the aneurysm, size of the neck and dome (dome–neck ratio), morphology, and relationship to the parent vessel, and should show whether the aneurysm incorporates the origin of other vessels. Multiple aneurysms are found in 20% of aneurysmal SAHs, and it may be difficult to identify which aneurysm bled and should be treated first. Three important features indicating the causative aneurysm are (1) the location of the aneurysm in relation to the SAH, (2) the aneurysm shape, because a ruptured aneurysm may be lobulated or have a teat, and (3) the aneurysm size. Active extravasation of contrast material from an aneurysm at the time of angiography is exceptional and indicates intraprocedural rupture. Three-dimensional rotational angiography may be more sensitive for aneurysm detection, yet may still result in a 4.2% rate of angiogram-negative SAH. , Angiography may also be used for later assessment of arterial status after definitive treatment of an aneurysm, and evidence shows very low complication rates.
Intraparenchymal hemorrhage can be secondary to a variety of pathologic entities, including hypertension (80%), amyloid angiopathy, trauma, hemorrhagic transformation of ischemic stroke, arterial dissection, tumor, sinus thrombosis, AVM, cavernous hemangioma, and saccular aneurysm. Approximately 20% of hemorrhages caused by intracranial aneurysms have an intraparenchymal component, especially if a mycotic aneurysm is the cause. If an AVM is suspected on MRI or CT, angiography is required to characterize the lesion, its venous drainage, arterial feeding arteries (feeders), and nidus. The characteristic angiographic appearance of an AVM consists of enlarged arterial feeders, a nidus, and early draining veins ( Figs. 49.19–49.22 ).
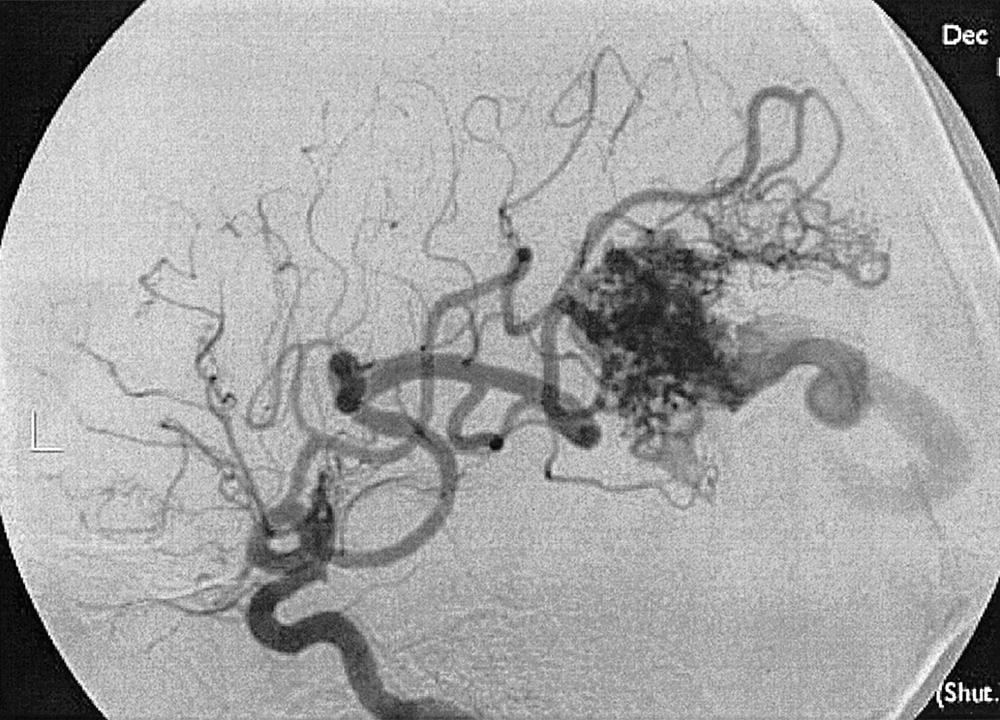
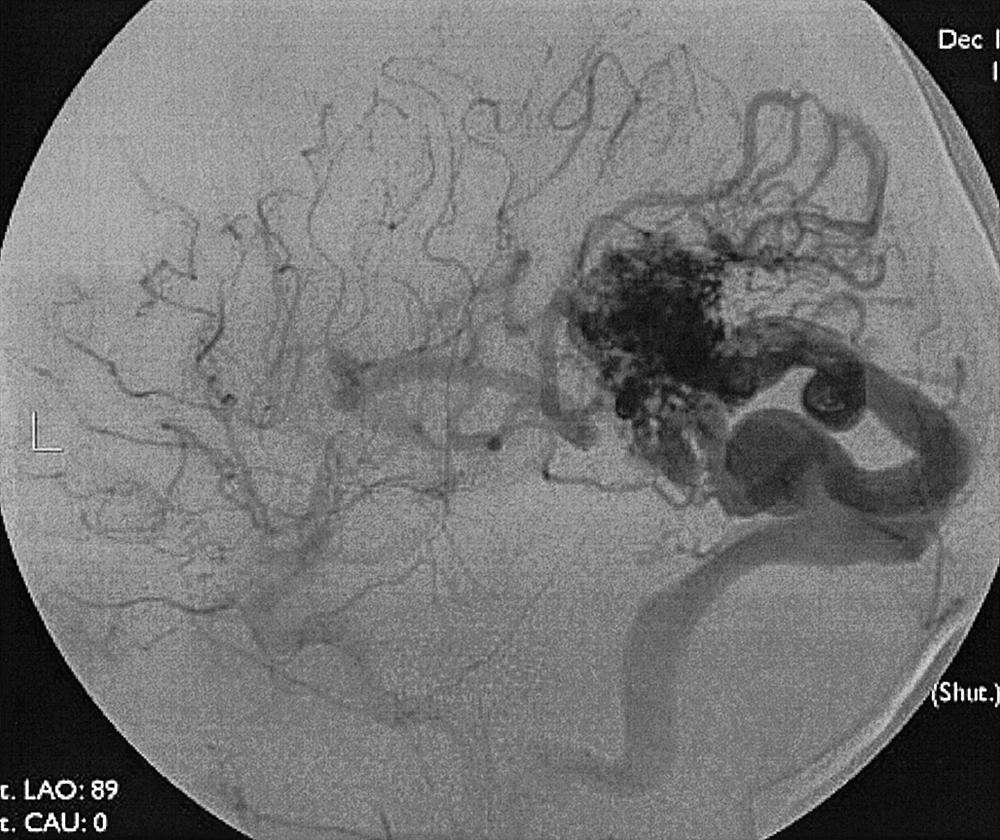
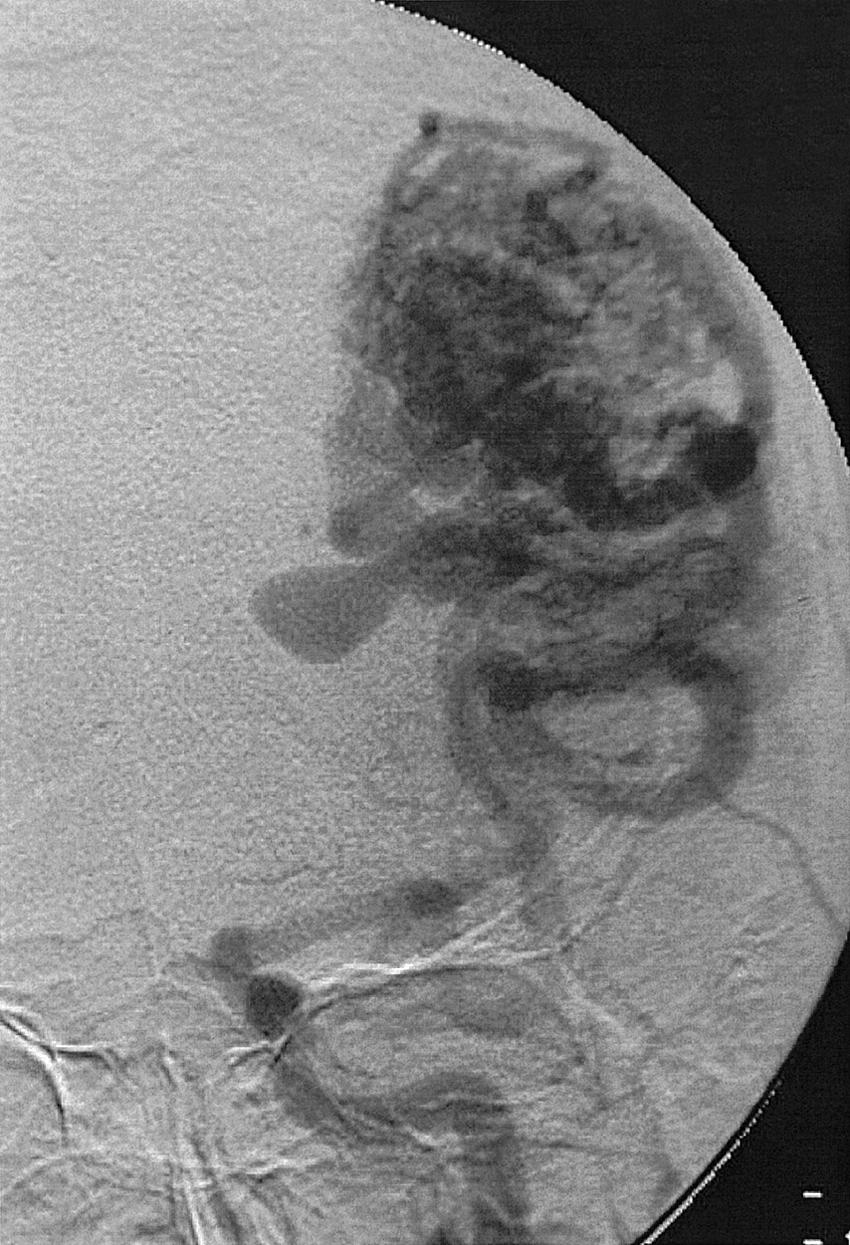
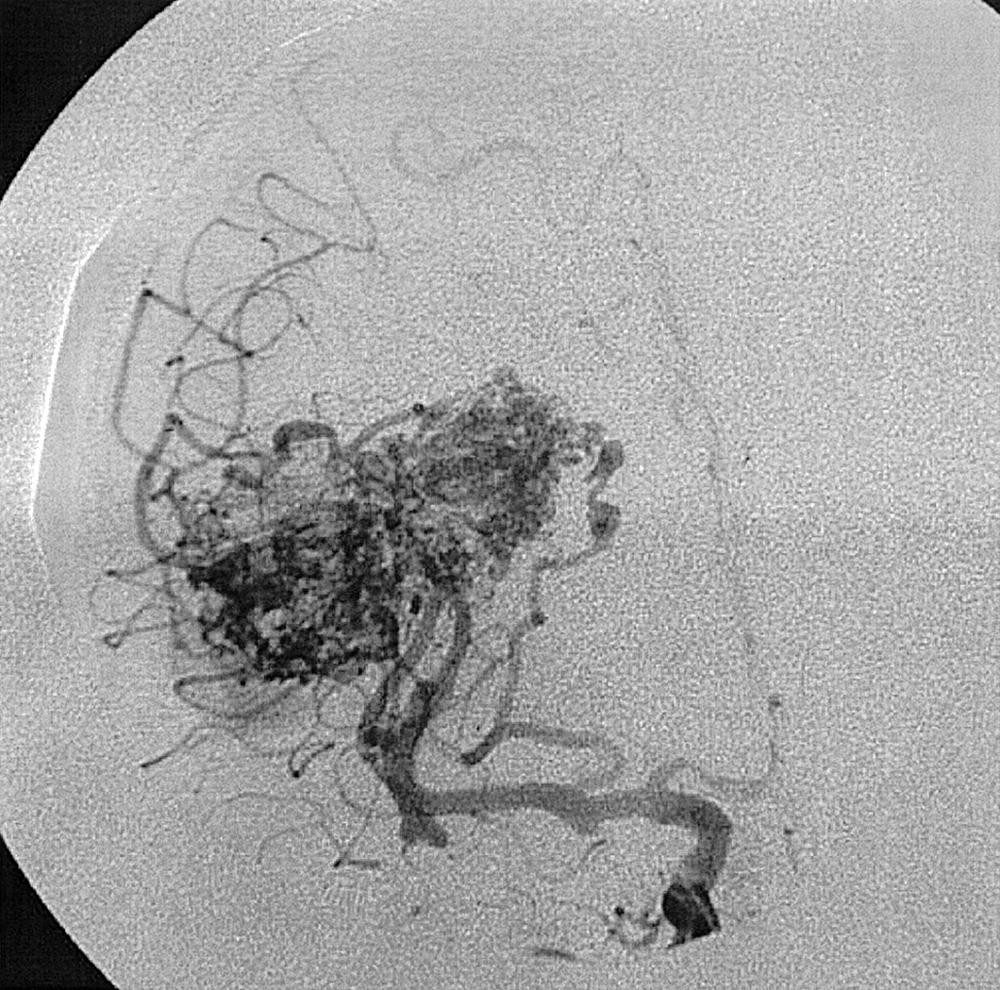
The association of AVMs and aneurysms on the arterial feeders is well known and is secondary to the high-flow state through the AVM (see Figs. 49.21 and 49.22 ). Furthermore, selection of optimal treatment modalities for AVMs requires knowledge of the detailed angioarchitecture of the lesion. For example, the presence of a lenticulostriate arterial supply to the nidus makes surgery more difficult (see Fig. 49.22 ).
In general, angiography should be considered in the evaluation of intracranial hemorrhage in (1) any patient younger than 70 years with a lobar hemorrhage and (2) any patient of any age with a deep hemorrhage who does not have a history of hypertension, or CT or MRI evidence of small-vessel arteriopathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here