Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
It is well known that preterm birth is associated with increased risks of brain injury and impaired brain development resulting in impairments in motor, cognitive, and behavioral function. However, the focus has traditionally been on brain injury patterns in the cerebrum, such as intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL). In recent years, it has become evident that the cerebellum is another major target for injury and developmental impairment associated with prematurity. Such primary injuries can include cerebellar hemorrhage or ischemia, with long-term outcomes dependent on the size and location of the lesions. Secondary impairments in cerebellar development are more prevalent and may be associated with a number of clinical risk factors in the early postnatal period ( Table 129.1 ). Thus, to comprehensively understand brain injury after preterm birth and optimize long-term outcomes, it is important to not only consider supratentorial brain injury, such as IVH and PVL, but also injury to the infratentorial cerebellar structure.
| Classification | Mechanism |
|---|---|
| Preterm cerebellar injury | Large cerebellar hemorrhage (>4 mm diameter) |
| Small cerebellar hemorrhage (<4 mm diameter) | |
| Cerebellar infarct | |
| Cerebellar hypoplasia of prematurity (CHOP) | Cerebral injury (e.g., periventricular hemorrhagic infarction, periventricular leukomalacia) |
| Intraventricular hemorrhage | |
| Postnatal glucocorticoid exposure | |
| Postnatal opioid exposure and pain | |
| Cardiorespiratory instability | |
| Nutrition and growth | |
| Socioeconomic factors |
The human cerebellum is formed by the anterior, posterior, and the flocculonodular lobes and shows a surface with fine parallel folia/gyri along the sagittal axis. Functional structures of the cerebellum structures include (1) the medial section, the spinocerebellum with the vermis; (2) the lateral sections, the cerebrocerebellum; and (3) the most posterior section, the vestibulocerebellum (flocculonodular lobe). The spinocerebellum is involved in direction and rate of intended movements, the cerebrocerebellum plans and modifies motor output to muscles, and the vestibulocerebellum regulates balance, posture, and eye movements. Although the cerebellum is traditionally thought to coordinate movements, it is now generally accepted to also play a role in higher-order functions like cognition ; however, the specific cerebellar structures involved are unclear. ,
Anatomically, the cerebellum consists of the cortex, white matter, deep cerebellar nuclei, and the fourth ventricle. The cortex represents most of the volume and can be divided in the most medial granule layer, the intermediate Purkinje layer, and the outer molecular layer ( Fig. 129.1 ). The granule layer is packed with granule (the most abundant neuronal cell type in the brain), Golgi, Lugaro, and unipolar brush cells. The Purkinje layer is a thin layer of Purkinje cells and Bergmann glia cells. The molecular layer contains the dendritic trees of the Purkinje cells, as well as the stellate and basket interneurons. The Purkinje and granule cells are the two major neuronal cell types in the cerebellar circuit. Underneath the cortex lies the cerebellar white matter, which also contains gray matter deep nuclei structures. The cerebrospinal fluid–filled fourth ventricle is located between the brain stem and cerebellum.
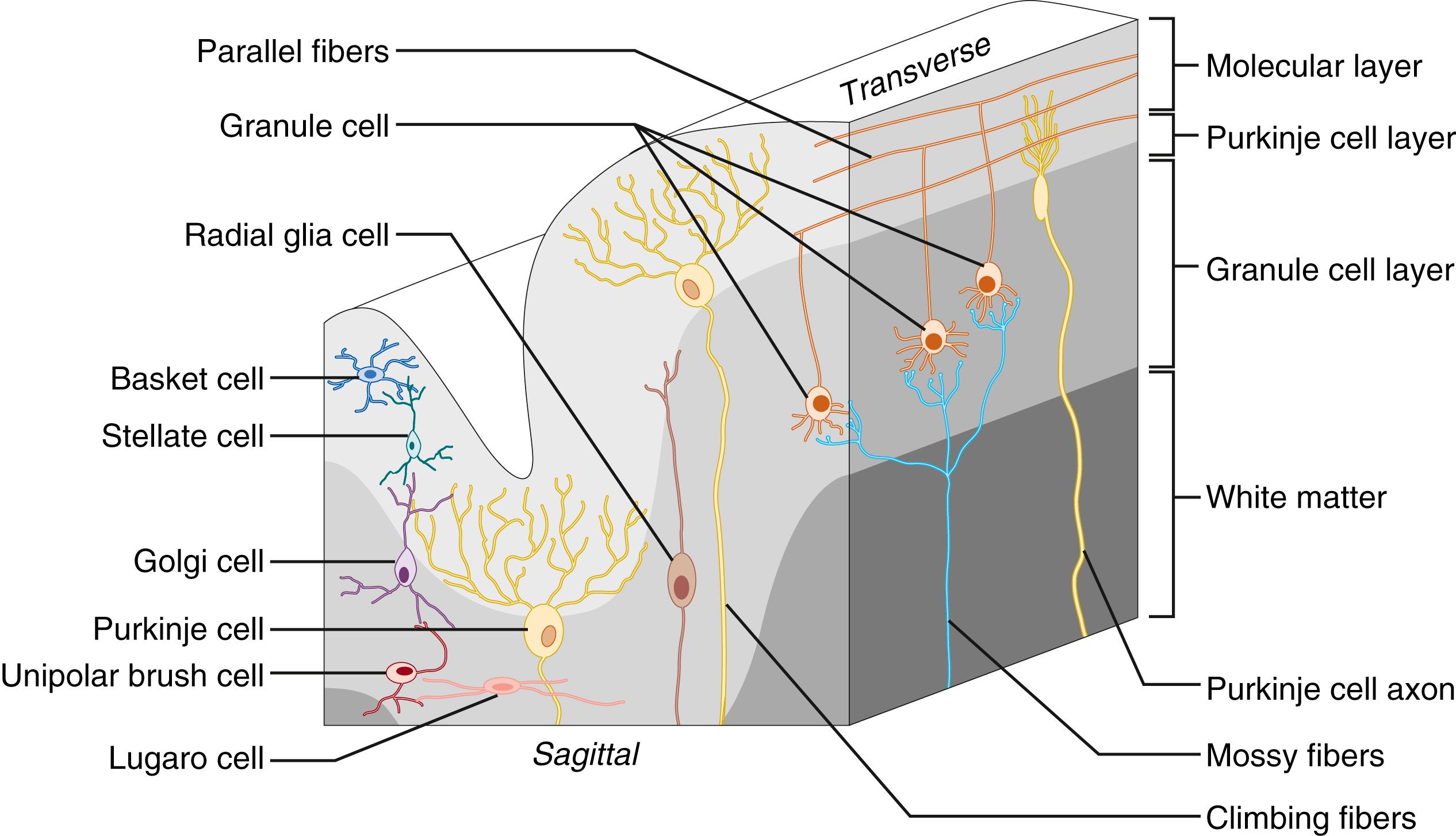
The cerebellum receives information from the motor cortex, the proprioceptors, vestibular organs, and other brain stem nuclei. Information enters the cerebellum via the mossy fibers, which project onto the granule cells and the deep cerebellar nuclei, or via the climbing fibers, which project onto the Purkinje cells (see Fig. 129.1 ). All climbing fibers originate from the inferior olivary nucleus, and the mossy fibers originate from multiple sources. Before entering the cerebellar cortex, climbing fibers also give off collaterals to the deep nuclei. Next to the input from one climbing fiber, the Purkinje cells receive input from numerous granule cells via the parallel fibers onto their distal dendrites. The Purkinje cells in turn innervate other parts of the cerebellar cortex, including other Purkinje, Golgi, Lugaro, and basket cells, and project onto the deep cerebellar nuclei. Four deep nuclei reside in the cerebellar white matter and are from lateral to medial: dentate, emboliform, globose, and fastigii. The dentate nucleus belongs to and communicates only with the lateral cerebrocerebellum. The other three deep nuclei belong to the spinocerebellum and communicate with different regions of the cerebellum and cerebrum. The deep nuclei integrate the inhibitory (gamma-aminobutyric acid [GABA]-ergic) input from the Purkinje cells and the excitatory input (glutamatergic) from the mossy and climbing fibers. Next to the vestibular nuclei, the deep cerebellar nuclei give the sole output of the cerebellum.
Development of the human cerebellum starts during early embryonic development and continues into postnatal life. , Using magnetic resonance imaging (MRI), the gross anatomic changes of the cerebellum during development have been monitored. The cerebellar primordium is formed at the border of the mid- and hindbrain and can be identified as thickenings on the lateral sites of the alar plate facing the fourth ventricle, as early as gestational week 6. Around gestational weeks 8 and 9, the vermis starts to fuse, and after gestational week 12, the vermis and cerebellar hemispheres begin to grow rapidly. The formation of the white matter of the cerebellum becomes apparent between gestational weeks 32 and 37. Growth of the cerebellum, however, peaks after birth, which most likely can be explained by the growth of the cerebellar cortex. Growth of the cerebellum continues until at least 2 years postnatally in humans.
Anatomic and histologic studies in humans and fate-map studies in mouse models have yielded insight into the development of the cerebellar primordium. Two germinative neuroepithelia—the ventricular zone and the rhombic lip, which become anatomically apparent around gestational day 57 —generate the different cerebellar cell types.
The ventricular zone lies adjacent to the fourth ventricle and gives rise to cells of the deep cerebellar nuclei around gestational week 8 15 and to Purkinje cells during the fourth fetal month. At later stages, the ventricular zone produces Golgi cells that will reside in the granule layer and basket and stellate interneurons that will locate to the molecular layer.
Of these cell types, the development of the Purkinje cells is best understood. The Purkinje cells migrate along the fibers of the radial glial cells, past the deep nuclei. They create a scaffold for the granule cells to form the final layer of granule cells, the internal granule cell layer (IGL). Between gestational weeks 12 and 20, the Purkinje precursor cell population drastically reduces in number. This coincides with the radial migration of granule cell precursors and with the formation of a single layer of Purkinje cells. During late gestational stages and early neonatal life, the Purkinje cells create their final characteristic morphology, a flattened dendritic tree in a perpendicular plane to the folia. Shortly after reaching their maturity, the Purkinje cells receive a layer of myelin around their axons.
The rhombic lip gives rise to the granule cells, the pontine nuclei, the inferior olive, and the unipolar brush cells. , , The cerebellar granule cell precursors (CGNPs) are generated in the upper rhombic lip. The proliferating CGNPs migrate in the outer region of the cerebellum, creating the external granule cell layer (EGL), , which can be identified around gestational day 51. Once the EGL is formed, CGNPs migrate radially along Bergmann glia past the Purkinje layer into the cerebellar primordium to form the IGL. The CGNPs in the superficial EGL are small and round, whereas CGNPs in the deeper layers show radially oriented bipolar cells ready to migrate. The IGL starts to shape around gestational week 15 and shows a clear layer by gestational week 30, but it continues to form in early postnatal life.
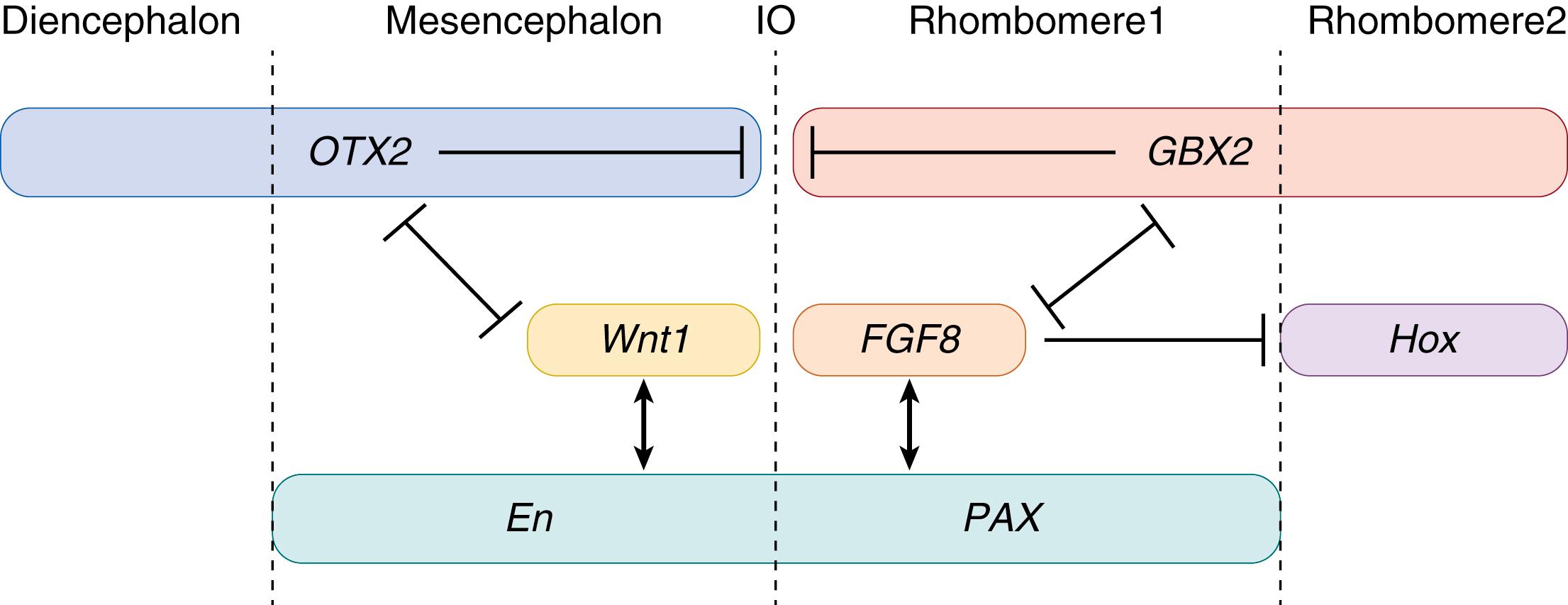
Genetic studies in the fruit fly, Drosophila, as well as chick, mouse, and human embryos, have informed our knowledge base of the regulatory genes involved in the development of the cerebellum. Although human development shows differences, many mechanisms are highly conserved between species. The specification of the mesencephalic and metencephalic structures, giving rise to the cerebellum, is regulated by their boundary, the isthmus organizer (IO). , The formation of the IO, as well as early cerebellar structures, is under the influence of fibroblast growth factors (FGFs), Wnt and bone morphogenic proteins (BMPs), , and transcription factors, including Engrailed1/2 (En), PAX2/5/8, OTX2, and GBX2 ( Fig. 129.2 ). Loss-of-function mutations in the genes encoding these signaling pathways and transcription factors result in abnormal cerebellar development. , Wnt1 and FGF8 are critical early regulators of IO formation. Wnt1, expressed anterior of the IO, is a secreted glycoprotein and plays an important role in patterning of the mesencephalon and the most posterior rhombomere, r1. FGF8, expressed posterior of the IO, patterns the hindbrain by limiting Hox gene expression. R1, the only hindbrain segment structure that does not express Hox, gives rise to the cerebellum, and inactivation of FGF8 leads to the disappearance of the cerebellum. Hence FGF8 is a key regulator in the inductive activity of the IO. Next to Wnt1 and FGF8, many other factors are involved in the development of the cerebellum. GBX2 and OTX2 are expressed very early in the central nervous system (CNS) and play a role in the positioning of the IO (see Fig. 129.2 ). PAX2 is necessary for regulating FGF8 expression. Additionally, the En genes are involved in maintenance of Wnt1 and FGF8 expression and have important functions in patterning of mid- and hindbrain regions. , Transcriptional regulation of these and other genes involved in the human IO needs to be confirmed, in which recent human stem cell technologies show great prospects.
The ventricular zone gives rise to its GABAergic derivatives in different waves. However, knowledge about genetic control of the generation of the different cerebellar cell types from the ventricular zone is limited. In mice, basic helix-loop-helix (bHLH) transcription factor, Ptf1a, is expressed during early embryonic stages and contributes to all GABAergic neurons in the cerebellum. However, when downstream target of Ptf1a, Neph3, is expressed in combination with E-cadherin, it specifically locates the Purkinje cells progenitors in the ventricular zone. Regional expression of proneural genes Ngn1, Ngn2, and Mash1 in the ventricular zone and the early stage cerebellar nuclei indicates selective involvement in the generation of the different GABAergic derivatives. PAX2 expression is specifically involved in the generation of GABAergic interneurons. PAX2-positive progenitors first give rise to the interneurons in the deep nuclei, then to the Golgi and Lugaro cells in the granule cell layer, and finally, to the basket and stellate interneurons in the molecular layer. More research is necessary to understand how the generation of the different cerebellar cell types in the ventricular zone is genetically regulated.
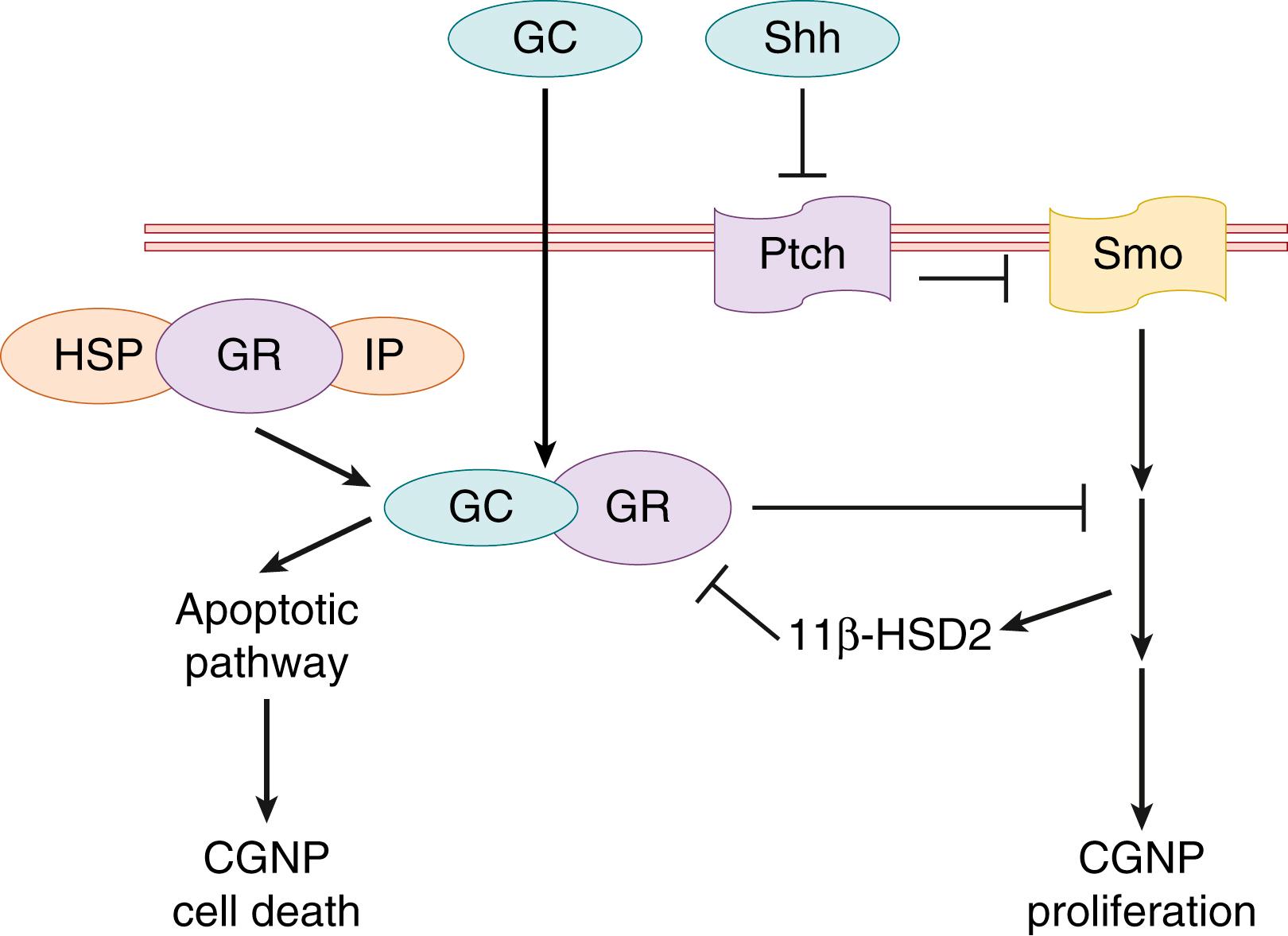
Under the influence of signals of the IO, the rhombic lip arises and expands. , Math1 (i.e., ATOH1 ), expressed in the germinal epithelium, is involved in the proper formation of the rhombic lip and in the generation of the CGNPs from this germinal layer. , Math1 mutations lead to disappearance of several cell types generated by the rhombic lip. When the proliferating CGNPs populate the EGL, they begin to express zinc finger proliferation 1 and 3 (Zic1 and Zic3). , , Defects in Zic1 and Zic3 lead to neural tube defects and aberrant generation of CGNPs. , When CGNPs are ready to migrate to form the EGL, they start to express mature markers, like tubulin-associated glycoprotein 1 (Tag1), and become dependent on Sonic hedgehog (Shh). Shh is the most important mitogenic factor for CGNPs. Shh binds the transmembrane receptor Patched (Ptch), which relieves the inhibition on transmembrane protein G-coupled receptor Smoothened (Smo; Fig. 129.3 ). Then Smo is free to accumulate in the cilia and to activate the expression of the downstream transcription factors, including N-Myc and G1 cyclins Gli1, Gli2, and Gli3, which results in a proliferative response of the CGNPs. Before Shh is active, it needs to be modified by cholesterol. Patients with Smith-Lemli-Opitz syndrome, who have a reduced cholesterol metabolism, show a hypoplasmic cerebellum. In contrast, gain-of-function mutations in the Shh pathway (SAG) (e.g., loss of Ptch) are associated with sporadic medulloblastoma and familial basal cell nevus (also known as Gorlin ) syndrome. Mouse studies have shown that Shh is secreted by the Purkinje cells. In humans, however, there is an early stage of Shh signaling, before the Purkinje cells start to secrete Shh. Bergmann glia also show expression of Ptch and Gli1 and are affected by Shh signaling. , Although it is unclear how Shh signaling regulates Bergmann glia differentiation, their fibers are malformed when Gli2 is mutated. Overall, Shh has an important function in cerebellar development and growth. As such, it has become a focus of studies that seek to understand the molecular mechanisms affected by developmental injuries to the cerebellum in human preterm neonates.
Cerebellar injury, such as cerebellar hemorrhage, ischemia/infarction, and secondary cerebellar hypoplasia, are now commonly diagnosed in preterm infants , because of improved posterior fossa neuroimaging, including both cranial ultrasound (CUS) and MRI. Preterm infants have a high risk of developing cerebellar hemorrhage, resulting from damage to the vulnerable germinal matrix present in the fourth ventricle and the external granular layer covering the surface of the cerebellum. This is a highly vascularized region of the brain, with immature vascular walls prone to hemorrhage.
The ventral surface of the posterior lobe of the cerebellum is often involved, together with the deep cerebellar cortex or related cerebellar white matter. Postmortem analysis shows that cerebellar hemorrhage is often multifocal, bilateral, and variable in size in various ages of preterm infants, although this observation is based on a biased population of the most severely ill babies. Pathology in the deep cerebellar nuclei, such as dentate nuclei, as well as the inferior olivary, often occurs in association with cerebellar hemorrhage. Cerebellar hemorrhage in surviving preterm infants is usually unilateral and is often associated with supratentorial lesions, primarily IVH, suggesting they might share common pathogenetic mechanisms. Such hemorrhages are sometimes followed by cerebellar atrophy. In large cerebellar hemorrhages, a reduction in contralateral cerebral volume has been found, which might be because of impaired remote trans-synaptic trophic effects.
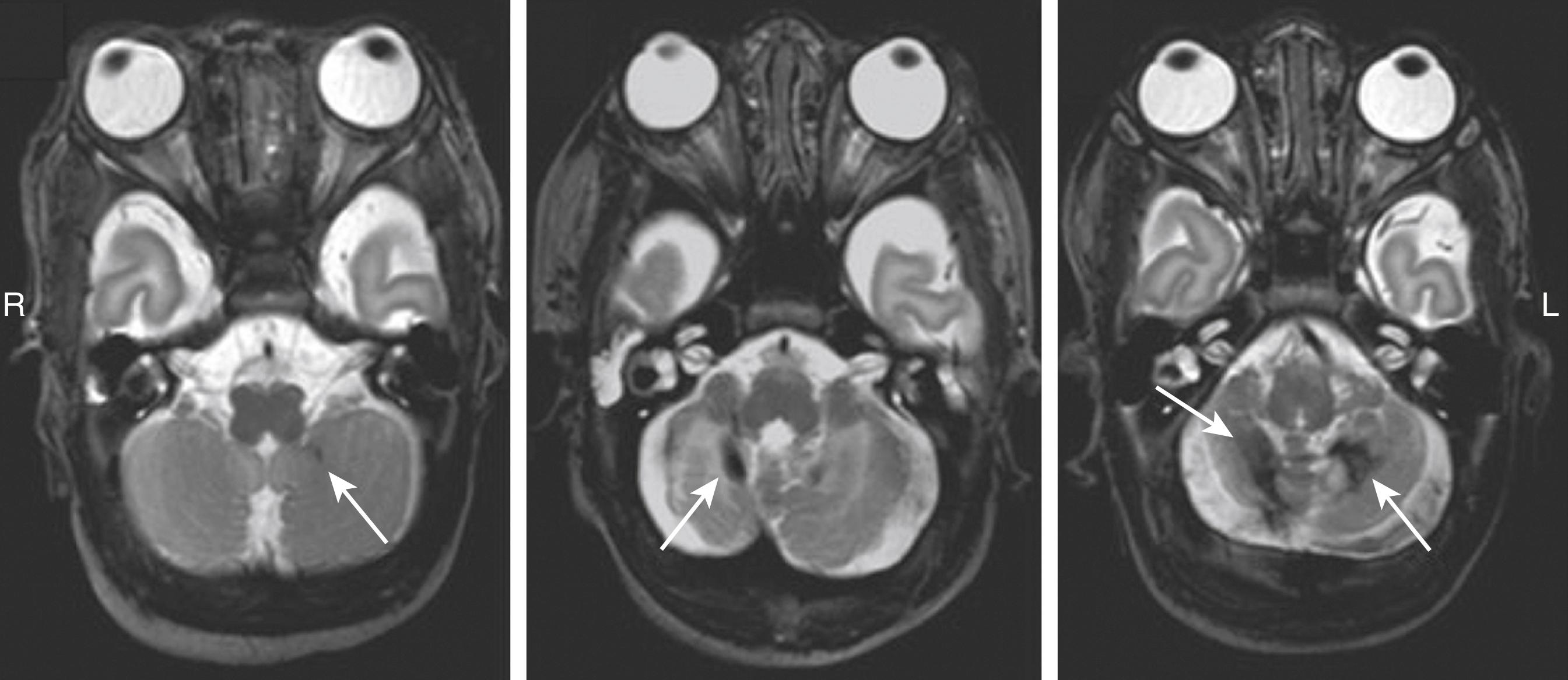
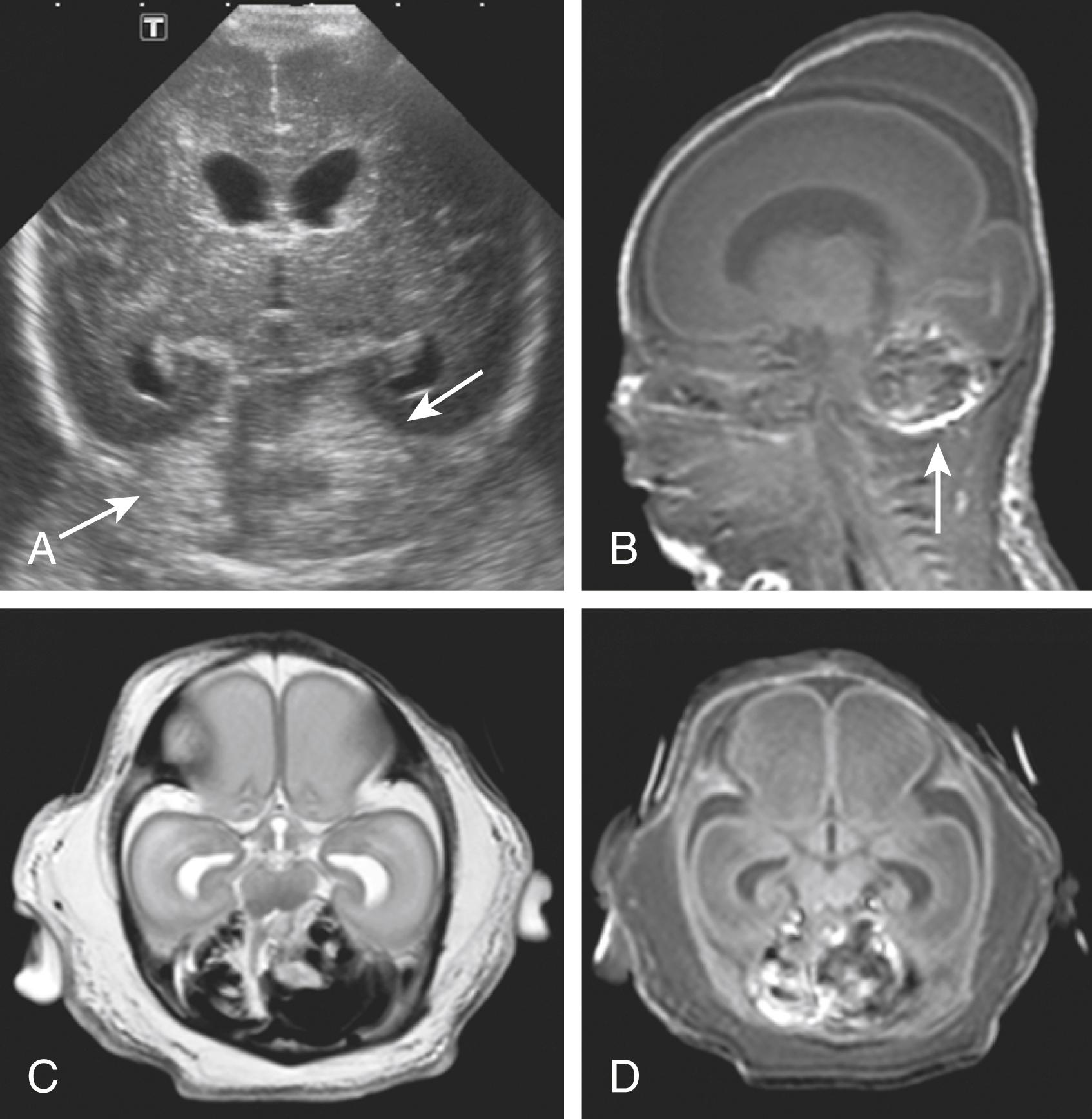
Cerebellar hemorrhage can be divided into different patterns, varying from a focal form, which is a milder form with small multiple punctate lesions or a larger hemorrhagic lesion in one cerebellar hemisphere, to a most severe form, which comprises large bilateral cerebellar hemorrhages ( Fig. 129.4 ). One study described six types of cerebellar hemorrhages using CUS and speculated on underlying pathogenetic mechanisms: (1) subarachnoid, due to extension of subarachnoid hemorrhage into the cerebellum; (2) small folial hemorrhages; (3) large lobar hemorrhages due to bleeding of the germinal matrix; (4) bilateral lobar involvement; (5) giant lobar hemorrhage involving the vermis, originating from bleeding of the subependymal germinal matrix or dissection of blood from the fourth ventricle into the vermis; and (6) contusional hemorrhage mainly related to birth trauma and occipital osteodiastasis. The vermis is involved in almost one third of patients. Large cerebellar hemorrhages or subarachnoid blood in the cisterna magna or underneath the tentorium may compress the fourth ventricle. This may cause ventricular dilatation by cerebral spinal fluid circulation disturbances ( Fig. 129.5 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here