Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Access to the venous circulation is an almost universal requirement in hospitalised patients for intravenous fluid, medicine or blood products administration. This is most commonly achieved with an indwelling peripheral intravenous cannula, but central venous access may be required for central venous pressure monitoring, parenteral nutrition, haemodialysis (HD), haemofiltration, the administration of cytotoxic drugs or long-term antibiotics.
For acute HD, central venous catheters (CVCs) are the mainstay of access and provide high dialysis flows (>300 mL/min) but have high complication rates and are less suitable for chronic use. For long-term HD, an arteriovenous fistula (AVF) or graft (AVG) can provide sufficient flow (>300 mL/min) to allow dialysis via two needles inserted into the fistula or graft itself.
In addition to central venous pressure monitoring, CVCs can be used to infuse large volumes of irritant solutions, such as antibiotics, blood products, parenteral nutrition and chemotherapeutic agents, particularly if required over long periods. In an emergency, CVCs allow the rapid administration of large volumes of fluid if peripheral access cannot be achieved. Depending on the indication and duration of access requirement, central catheters can also be inserted peripherally. Implantable injection ports, ‘portacaths’ (e.g., Bardport, Passport, Infuse-a-Port or MediPort), may be used for chemotherapy or long-term administration of other drugs. ,
CVCs are generally inserted under local anaesthetic through the internal jugular, subclavian or femoral veins, preferably using ultrasound guidance, by the Seldinger technique. If short-term access is required, a multi-lumen catheter is inserted into the internal jugular vein (IJV) so that the tip lies in the superior vena cava (SVC). For long-term access, a catheter with an attached Dacron cuff is placed in a subcutaneous tunnel (e.g., Hickman line) for fixation and to act as a barrier to infection.
Implantable access ports are usually inserted into the jugular or subclavian vein in the operating theatre and tunnelled so that the port lies over the anterior chest wall. The subclavian route is discouraged because of the higher incidence of subclavian vein stenosis, access thrombosis and complication rates such as pneumothoraces. They contain a diaphragm that may be accessed repeatedly using a special side-hole needle. Central vein access can also be achieved using a peripheral intravenous central catheter inserted in the antecubital or long saphenous vein.
Air embolus can be avoided by placing the patient in the Trendelenburg position during insertion.
![]() Accurate placement under ultrasound guidance will reduce the incidence of arterial puncture, haematoma, haemothorax and pneumothorax.
Accurate placement under ultrasound guidance will reduce the incidence of arterial puncture, haematoma, haemothorax and pneumothorax.
The long-term complications including infection and thrombosis are dealt later in this chapter.
Chronic kidney disease (CKD) is an important long-term condition caused by damage to the kidneys. It is initially without any specific symptoms and is detected incidentally on a routine blood test. In the later stages of the disease, patients might develop a number of signs and symptoms including hypertension, hyperkalaemia, fluid overload and anaemia.
The stages of CKD are based on the measured or estimated glomerular filtration rate (GFR), a measure of kidney function. There are five stages including stage 1 where kidney function is normal ( Table 16.1 ). The stages of CKD are useful tool for physicians and aid in describing patient’s renal failure and in planning management.
| Stage | Glomerular filtration rate | Description |
|---|---|---|
| 1 | >90 | Normal renal function |
| 2 | 60–90 | Mildly reduced renal function |
| 3 | 30–60 | Moderately reduced renal function |
| 4 | 15–30 | Severely reduced renal function |
| 5 | <15 | End-stage renal failure |
Renal replacement therapy (RRT) is provided to patients in end-stage renal disease (ESRD). It both prolongs survival and improves quality of life for these patients. RRT can take the form of HD, peritoneal dialysis (PD) or renal transplantation. In the rest of this chapter, we will focus on vascular access for HD.
About 75% of patients are known to have deteriorating renal function at least 90 days before dialysis is required so that permanent HD access can be created in advance. Unfortunately, this opportunity is frequently missed in UK practice, with only around half of patients starting HD with definitive access. Over 96% of patients start HD on a CVC if presenting late (<90 days), with 75% still using the same modality 3 months after onset of dialysis. Referral to a surgeon before commencing dialysis leads to 70% of patients having an AVF, whereas 90% will start dialysis on a CVC if not seen by the surgical team before the onset of dialysis. Urgent dialysis is required for hyperkalaemia, severe metabolic acidosis, uraemic symptoms or fluid overload.
For patients presenting as an emergency with ESRD, HD can start using a double-lumen CVC whilst awaiting a permanent access. However, CVCs have a high risk of infection, lead to central venous stenosis or thrombosis, compromising further access in the upper limbs, and have a higher morbidity and mortality than AVFs. Therefore their use should be short term in the majority of patients. In addition, patients who start HD on a CVC have an overall reduced life expectancy than those who start on an AVF.
![]() Temporary (non-tunnelled) catheters are used in patients who require short-term dialysis for transient renal failure or who present acutely with ESRD. They are also indicated after failure of a permanent access, whilst awaiting maturation of a new AVF or insertion of an AVG. Tunnelled catheters are preferred if dialysis is required for more than 2 weeks or for permanent access when the creation of an AVF or AVG is contraindicated or technically impossible.
Temporary (non-tunnelled) catheters are used in patients who require short-term dialysis for transient renal failure or who present acutely with ESRD. They are also indicated after failure of a permanent access, whilst awaiting maturation of a new AVF or insertion of an AVG. Tunnelled catheters are preferred if dialysis is required for more than 2 weeks or for permanent access when the creation of an AVF or AVG is contraindicated or technically impossible.
The subcutaneous tunnel may reduce the rate of infection, but this has not been proven in a randomised trial.
Temporary femoral vein catheters are useful for acute dialysis but have a higher rate of infection than internal jugular CVCs and should be replaced by a tunnelled (preferably jugular) venous catheter at the earliest opportunity. A median survival of 166 days has been reported for tunnelled femoral CVCs.
The right IJV is preferred as this provides the most direct route to the SVC and right atrium (RA). The left IJV has a greater complication rate because the catheter has to traverse two 90-degree bends to reach the RA. As discussed earlier in the chapter with regard to CVCs, the subclavian route is discouraged because of the high incidence of subclavian vein stenosis and thrombosis that may compromise future access in the ipsilateral arm. When other routes have been exhausted, tunnelled catheters can be placed in the femoral vein or even the inferior vena cava (IVC) via a transhepatic or translumbar approach.
The catheter tip is usually placed at the SVC/RA junction. Atrial placement minimises recirculation and reduces the risk of migration on standing, but may cause arrhythmias by stimulation of the sinoatrial node.
![]() The preferred site for a CVC is the right IJV. CVCs should be inserted under fluoroscopic or ultrasound guidance, without which there is a high malposition rate of 29%.
The preferred site for a CVC is the right IJV. CVCs should be inserted under fluoroscopic or ultrasound guidance, without which there is a high malposition rate of 29%.
The complications related to catheter insertion are the same as for other CVCs described earlier and can be reduced by ultrasound guidance and a micropuncture technique.
Catheter dysfunction occurs when an adequate extracorporeal blood flow of 300 mL/min cannot be achieved and therefore adequate HD is not achieved. Early dysfunction is usually caused by malposition or kinking and is corrected by repositioning. Later dysfunction is primarily caused by thrombosis or fibrin sheath formation. Rarely, tip migration demands repositioning with a snare or exchanging over a wire.
Catheter patency can be maintained between dialysis sessions using a catheter-locking solution. The standard procedure has been heparin instillation (1000–10 000 U/mL) into the catheter lumen in a volume sufficient to fill to the lumen tip. There is a risk of heparin loss because of diffusion into the bloodstream and unintentional systemic anticoagulation. Low-dose heparin (1000–5000 U/mL) seems as effective as high-dose. Trisodium citrate, which also has antibacterial properties, is also an effective catheter lock and has been shown to be comparable to heparin lock in a recently published randomised clinical trial.
A recent randomised clinical trial compared 270 patients on HD who had a CVC using a locking regime of heparin 5000 U/mL or a solution containing trimethoprim 5 mg/mL, ethanol 25% and Ca-EDTA 3%. The rate of central line associated bloodstream infection in the trimethoprim, ethanol and Ca-EDTA lock solution group was significantly reduced compared to the patients assigned to heparin and the rates of catheter removal did not differ. It is important to avoid the use of dedicated dialysis catheters for other uses as the lock solution can cause significant systemic coagulopathy.
Catheter thrombosis is the most common cause of poor long-term function and loss of access. Prophylactic warfarin can be effective at reducing thrombosis. There is a risk of bleeding and a need for regular monitoring with its use. The ideal target international normalised ratio range has not been established with conflicting evidence.
Catheter malfunction caused by thrombus can be treated by lytic agents such as recombinant tissue plasminogen activator (rTPA) or urokinase.
Urokinase has been withdrawn in the USA because of safety concerns.
Poor flow can be treated by a post-dialysis lock or intra-dialysis lytic infusion. Both seem to be effective methods but again there are no randomised trial data to guide clinical practice.
Lytic agents are also used for the treatment of catheter thrombosis. An instillation of rTPA 1 mg/mL for 30 minutes restored or maintained a flow rate of greater than 300 mL/min without line reversals in 36 of 50 (72%) patients, with a second instillation restoring patency for a further four patients (80%). The majority of patients required further thrombolysis or radiological intervention in the 4-month follow-up period.
The optimal dwell times for lytic agents have yet to be determined. rTPA infusions are effective even when there is an associated fibrin sheath.
Tenecteplase is a new lytic agent with increased fibrin specificity, greater resistance to plasminogen activator inhibitor 1 and a relatively long half-life. A randomised study showed a 1-hour dwell of 2 mg of tenecteplase more effective than placebo in restoring flow in dysfunctional HD catheters. An extended dwell improves treatment success.
Mural thrombus is commonly seen in the SVC and RA with CVCs. If it compromises venous return, facial and arm oedema results, which is termed superior vena cava syndrome . Central vein thrombus can be identified by magnetic resonance, computed tomography (CT) or conventional venography. Infusion of a fibrinolytic agent can be successful, although organised thrombus may require angioplasty and stenting.
Fibrin sheaths cause up to 43% of catheter dysfunction. Contrast injection through the dialysis line may show a filling defect near the catheter tip or retrograde flow along the external surface of the catheter ( Fig. 16.1 ). This may be treated by infusing a fibrinolytic agent over 6 hours, mechanical stripping using a snare from the femoral vein, or catheter exchange over a guidewire.
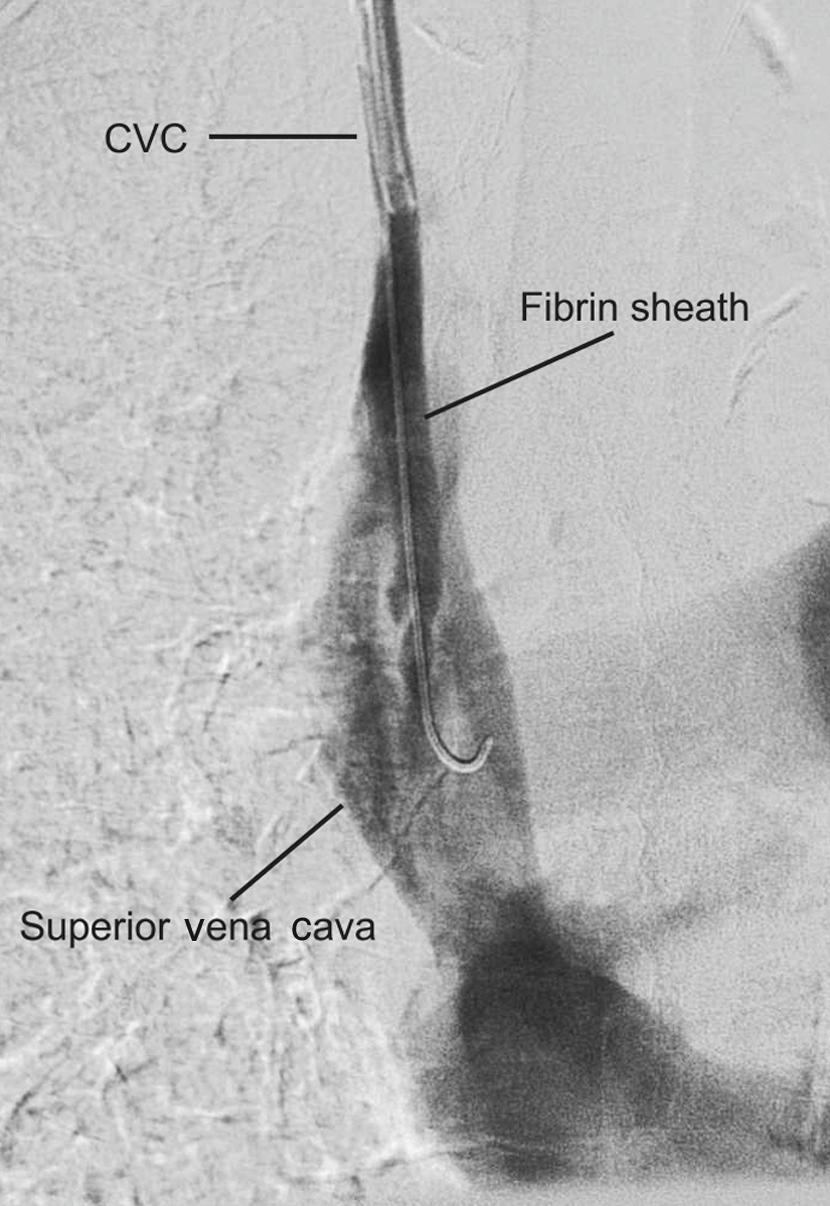
Stripping has a high technical success rate, but the fibrin sheath frequently recurs. In a randomised trial, there was no significant difference in additional patency between percutaneous stripping or urokinase. In another randomised trial, 4-month catheter patency was significantly better after catheter exchange than percutaneous stripping.
If the catheter is exchanged over a guidewire, the sheath must be mechanically disrupted with an angioplasty balloon, or the new catheter will be re-inserted down the existing sheath. There are no controlled trials comparing all three techniques.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here