Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This review will trace the general mechanisms of renal cell injury before focusing on selected specific nephrotoxins that demonstrate the key principles of injury. Potentially, all of the cellular events involved in injury leave molecular footprints, which can be used as biomarkers in urine or plasma. Event specific biomarkers may allow mapping the time course of injury and the recruitment of secondary injury pathways. We will review the development and use of biomarkers in the detection of nephrotoxic injury. The selected nephrotoxins represent those agents where the key pathways of injury have been extensively investigated.
Keywords
nephrotoxicity, acute kidney injury, cellular injury, apoptosis, biomarkers, cyclosporine, aminoglycosides, cisplatin, acetaminophen, lithium
Nephrotoxins produce acute or chronic kidney failure or functional injury, usually as a result of specific injury to tubular epithelial cells. While these toxins usually produce localized and specific injury to target cells in the kidney by specific mechanisms, many of the final common pathways of cytotoxic injury are shared with ischemia-reperfusion injury. As the latter have been studied more extensively, these are included as part of this review. The common cellular pathways involved lead to cellular adaptation, sublethal or lethal injury, and pathways for regeneration and repair. These events are not confined to the target cells but also involve other cells in the kidney, which may not necessarily reveal apparent injury but which invoke a stress response and/or inflammatory and vascular responses. The role of direct and indirect injury in mediating the phenotypic response to injury is illustrated schematically in Figure 85.1 . The parallel response in the cells that are not directly targeted, such as cytokine or growth factor release or release of inflammatory mediators and recruitment of further inflammation together with the response of target cells, are factors that determine the final outcome of toxic injury. The accompanying vascular response may well be pivotal. Thus, the pathophysiology of acute kidney injury includes a complex interplay among specific cellular injury, inflammation, and altered renal hemodynamics. This review will trace the general mechanisms of renal cell injury before focusing on selected specific nephrotoxins that demonstrate these principles of injury. Potentially, all of the cellular events involved in injury leave molecular footprints, which can be used as biomarkers in urine or plasma, Although such biomarkers have been used largely for diagnostic and predicitive purposes, it is through these biomarkers that we may be able to map such events non-invasively. Event specific biomarkers may allow mapping the time course of injury and the recruitment of secondary injury pathways. We will review the development and use of biomarkers in the detection of nephrotoxic injury.
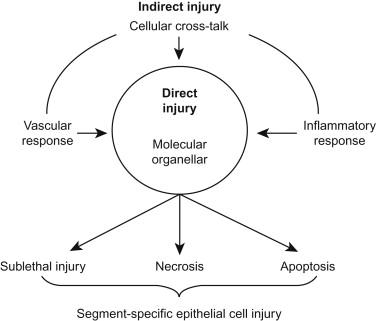
Renal susceptibility to toxic injury emanates from a combination of nonspecific and specific factors. The major nonspecific factor is high blood flow with the kidney receiving 20–25% of the cardiac output, thus ensuring high delivery rates of circulating toxins. The general principles governing the susceptibility of a particular nephron segment to nephrotoxic or ischemic injury include segmental transport functions, metabolic activity, metabolic reserve, filtered load, and local blood and substrate supply including oxygen. The concentration of a drug or its metabolites will contribute to toxicity, and this will be modified by local tubular reabsorption, secretion, and metabolism, as well as by the local reabsorption of water. Intracellular activating mechanisms and organelle distribution will contribute further to toxicity. Because of the heterogeneity of these factors along the nephron, it is not surprising that the pattern of injury differs among nephrotoxins. Toxin delivery to a target site is a sine qua non for toxicity. Protein binding of the potential nephrotoxin will dictate whether the molecule is freely filtered at the glomerulus, dictating early exposure to the luminal aspect of proximal tubular cells or, if highly protein bound, to basolateral transport systems anywhere along the nephron. Thus, there is good correlation between the morphological and functional attributes of each nephron segment and the potential development of nephrotoxicity, especially in the proximal tubule where the greater proportion of specific drug toxicity occurs. Survival after toxicant exposure is also affected by the cycle status of the cell and the extent to which activation of pro- versus anti-apoptotic pathways and pro- versus anti-inflammatory pathways occurs; these factors collectively represent the “stress response” of the cells and are affected by the metabolic state of the cell. The anti-apoptotic and -inflammatory factors may be considered as a type of functional cytoprotective reserve (akin to antioxidant status in the face of oxidant stress), which might account for the differential susceptibility of particular tubular cells.
Heterogeneity of renal blood flow (RBF) defines the vascular limits of substrate and toxin delivery. The outer cortex receives 80% of the total RBF, the inner cortex and outer medulla only 15%, the inner medulla 5%, and the papillary region 2%. Renal oxygenation is modified by diffusional shunting of oxygen from arterial to venous vessels running in close approximation. This is most apparent in the vasa recta, so that oxygen tension also falls off sharply at the corticomedullary junction, thereby producing the characteristic low oxygen tensions found in the renal medulla averaging approximately 10 mm Hg. However, preglomerular shunting of oxygen also contributes and reduces cortical oxygenation to below renal vein values. This arrangement is essential for maintenance of the medullary interstitial osmotic gradient generated by the countercurrent multiplier system that allows modulation of water reabsorption for the generation of concentrated or dilute urine. In a teleological sense, the arrangement also provides a selection advantage by creating a zone of borderline oxygenation in the kidney that may be ideal for sensing hypoxia by erythropoietin-producing interstitial cells in cortex. The disadvantage is that any significant reduction in RBF—for example, in shock—can easily lead to hypoxic damage, at least in outer medullary tissues, including “medullary rays” situated in the cortex and containing the S3 proximal tubule and TAL segments arising from glomeruli in the outer and mid-cortical layers. Although both proximal tubular S3 cells and medullary thick ascending limb cells are exposed to a similar risk of hypoxia, only proximal tubular cells undergo early cell death following equivalent hypoxic insults in vivo . This suggests that there are differences in susceptibility to injury between these segments, which may reflect differences in segmental cytoprotective mechanisms as discussed below.
Regulation of regional blood flow may be critical to localization of hypoxic and toxic injury. Autoregulation of cortical blood flow is highly efficient, whereas the extent of autoregulation of medullary blood flow remains controversial. Some studies indicate that medullary blood flow is not efficiently autoregulated, especially during volume expansion. Other studies show efficient autoregulation of medullary blood flow under other conditions. Such disparate observations may arise from different methods of estimating medullary blood flow. Modulation of cortical and medullary blood flow may be critical to the actions of several nephrotoxins, such as iodinated contrast and nonsteroidal anti-inflammatory drugs. Heterogeneity of nitric oxide synthase (NOS) distribution within the kidney influences these regional differences in renal perfusion, and contributes to cytotoxic injury. All three isoforms of NOS are present in the kidney. NO from eNOS is involved in regulation and maintenance of blood flow, and NO synthesized by nNOS in the macula densa is important in the modulation of tubuloglomerular feedback (TGF) and regulation of glomerular filtration rate (GFR). iNOS is present in the tubules of normal rat kidney, predominantly in the medullary thick ascending limb, but its involvement in regulation of kidney function under normal physiological conditions is unclear. Quantitative studies of NOS activity and NO production suggest that the renal medulla is the principal site for basal NO synthesis in the kidney, suggesting an important role of NO in regulation of medullary circulation.
There is increasing interest in the role of the endothelium in ischemia-reperfusion injury, which may provide some insights into the effects of nephrotoxic injury on the renal vasculature. Endothelial dysfunction and vasoconstrictor hypersensitivity are well-recognized sequelae of ischemic acute renal failure (ARF). After ischemia-reperfusion injury loss of GFR arises from a combination of a reduced transcapillary hydraulic pressure gradient (ΔP) during delayed graft function and back leak of the glomerular filtrate presumed secondary to both epithelial injury and tubular obstruction. The decrease in ΔP may arise from two mechanisms. The first is a reduction in perfusion pressure secondary to an increase in afferent arteriolar tone as a result of some combination of vasoconstrictor hypersensitivity, endothelial dysfunction impairing the generation of vasodilators, and increased TGF. The afferent arteriole provides the highest contribution to renal vascular resistance and is the key modulatory site for renal autoregulation. The second mechanism is a rise in intratubular pressure arising from actual or functional tubular obstruction. Recent studies confirming the continuing presence of TGF activity after ischemia-reperfusion injury support the postulated contribution from enhanced TGF secondary to reduced sodium and water reabsorption. Structural obstruction of the nephron is recognized in ischemic ARF, and functional (high-flow) obstruction causing intratubular pressure increases sufficient to stop filtration have been observed in toxic ARF after p-aminophenol. Endothelial cell dehiscence and microvascular obstruction may also contribute a significant component of this reduction in renal blood flow, as recent studies have observed not only endothelial cell dehiscence, microvascular aggregation, and obstruction, but even reversal of flow. These observations coupled with evidence of endothelial ICAM1 adhesion molecule upregulation and leukocyte adhesion in the vasa recta explain the outer medullary vascular congestion observed consistently after ischemic injury, usually described as the “no-reflow” phenomenon.
Iodinated contrast agents may be injurious through modulation of regional blood flow. One of the two principal explanations for genesis of acute renal failure by contrast is induction of vasoconstriction, either directly by high osmolar-contrast agents or by release of endothelin or adenosine or both. Medullary blood flow may be critical, since the potential for regional hypoxia described above predisposes to ischemic injury when both nitric oxide and vasodilator prostaglandin synthesis are blocked. Iodinated contrast reduces NO synthesis in primary cultures of renal artery smooth muscle cells. Injection of radiocontrast results in an immediate decrease in renal blood flow that is counteracted by an increase in renal prostaglandin formation. When prostaglandin synthesis is inhibited by cyclooxygenase inhibitors, prolonged endothelin-mediated renal vasoconstriction is observed. Endothelin antagonists have not been successful in preventing contrast nephropathy. However, the only clinical trial using endothelin antagonists utilized a nonselective endothelin antagonist that promoted both an increase in circulating endothelin of longer duration than the duration of antagonism and antagonism of ET-B receptors, which would have suppressed NO production. Subsequent experimental studies have demonstrated protection by ET-A selective antagonists, but it is uncertain whether this protection was hemodynamically mediated.
Modulation of NO also contributes to calcineurin-inhibitor and cisplatin toxicity. Acute dose-dependent vasoconstriction of the renal microcirculation by cyclosporine and tacrolimus is NOS dependent and reversed by supplementation with l-arginine. Chronic cyclosporine involves downregulation of eNOS, which can be reversed by administration of l-arginine or pravastatin. Inhibition of NOS (by 2-amino-4-methylpyridine aggravates experimental cisplatin-induced nephrotoxicity with exaggeration of both histological and metabolic features of cisplatin toxicity including reduction in glutathione -peroxidase activity and elevation of platinum accumulation, but prevents the typical reduction in GSH and increase in malondialdehyde.
Both vasodilator and vasoconstrictor prostaglandins generated by COX from arachidonic acid are critical in the regulation of vascular tone and sodium and water homeostasis in the kidney. Endogenous prostaglandins have been found to modulate the regulatory status of the perfused kidney and there is growing evidence to indicate that COX-2 is involved in the modulation of afferent arteriolar autoregulatory responses. The interplay between endogenous vasodilators and vasoconstrictors—for instance, prostaglandins and endothelin—and the increase in vasoconstrictor production when vasodilators are stimulated or vice versa, highlights the important homeostatic effects of local autoregulation. This is critical in determining regional blood flow and toxicity, but can make interpretation difficult.
Significant exposure of the kidney to toxicants typically results in ARF. The primary target for most nephrotoxin and ischemic injury are epithelial cells of the proximal tubule. Other renal target cells include endothelial cells—for example, in response to Shiga toxin-2 or ischemia. Endothelial cells can also be modified by cytotoxins to sensitize them to other forms of injury. For example, incubation of endothelial cells with calcineurin inhibitors has been shown to increase subsequent adhesion by dendritic cells. Vascular smooth muscle cells can be indirect or direct targets. For instance, impaired vasorelaxation through impaired NO formation characterizes cyclosporine, contrast, and myoglobin toxicity as described. Severe ischemia may injure smooth muscle cells directly. Podocyte injury is critical in the development of several forms of glomerular disease, and is induced experimentally by the nephrotoxin puromycin. Tubular interstitial cells may be targeted by drugs with resultant tubulointerstitial nephritis, glomerular cells to cause proteinuria, and distal tubular cells to cause functional injury manifested as inhibition of transport (e.g., lithium and demeclocycline cause diabetes insipidus, and cotrimoxazole causes hyperkalemia) or structural damage (e.g., with fibrosis induced by lithium or other drugs). Secondary parenchymal injury may result from activation of inflammatory chemokines resulting in an inflammatory response with leukocyte activation and infiltration. This is a critical component of ischemia-reperfusion injury, but has been inadequately explored in nephrotoxic injury.
Most nephrotoxic and ischemic injury involves proximal tubular cells. Proximal tubule dysfunction is primarily responsible for activation of several of the main pathophysiological mechanisms, which leads to loss of GFR in acute renal failure as discussed earlier. The S3 segment of the proximal tubule and the medullary thick ascending limb (MTAL) of the loop of Henle traverse the outer stripe of the outer medulla and medullary rays in close proximity. These are regions of the kidney that are marginally oxygenated because of diffusional shunting of oxygen, and where blood flow is reduced in the setting of ischemic and some toxic injuries. Although both nephron segments have high transport activity and concomitant high metabolic activity, only proximal tubular cells are susceptible to injury causing cell death in most animal models. This greater susceptibility probably arises because proximal tubular cells rely principally on fatty acid oxidation for energy, and have low glycolytic capacity compared with MTAL cells under conditions of oxygen deprivation. Thus, the higher glycolytic capacity of MTAL cells allows continued generation of ATP in the setting of rapid ATP depletion resulting from impaired oxidative phosphorylation. There may also be greater cytoprotective reserve in MTAL cells in contrast to proximal tubule cells against early apoptotic injury through early and differential upregulation of anti-apoptotic Bcl-2 family members, and of the protective mitogen-activated kinases (MAP kinase) pathway regulated by extracellular signal kinase (ERK), as will be discussed further below. However, while ATP availability may make distal nephron cells less vulnerable to immediate cell death by necrosis, MTAL cells do undergo sublethal changes and produce various chemokines, cytokines, and growth factors that may have autocrine and/or paracrine effects on the injury and regeneration process of the kidney post-ischemia; MTAL cells may also undergo apoptotic cell death pathways under severe stress conditions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here