Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter describes the tissue types present in early embryos and the interactions between these tissues.
The membrane systems and cytoskeletal elements within a typical cell are reviewed.
Early embryos contain only epithelial and mesenchymal populations.
Each tissue type produces specialised extracellular matrix molecules and proteins which permit and encourage tissue interactions.
Specific interactions between developing epithelia and mesenchyme are presented, and the common cytokines and growth factors are discussed.
This chapter provides a brief overview of concepts of cells and tissues and the terminology by which developmental processes are described. It should be noted that the genetic programme of the early conceptus is now being studied by analysis of substances secreted and metabolites produced, some of the very early cell interactions between the fertilised oocyte and the maternal endothelium have been revealed, and the ways in which cell types interact before and during organogenesis is now being explored in human embryonic stem cell and human-induced pluripotent stem cell culture.
All cell types have a plasma membrane and internal organelles, and all are supported by a range of cytoskeletal structures. The details and arrangement of proteins within cells and within the matrices they synthesise encompass a vast region of research beyond the scope of this text. Only the main proteins and structures necessary for the appreciation of developmental processes are presented. Readers are recommended to consult other texts for further details.
The plasma membrane of cells is composed of phospholipids molecules arranged in a bilayer. Within this layer are vast numbers of proteins which may reside entirely within the bilipid layer or protrude intracellularly, extracellularly, or both. Proteins which project exteriorly are covered with carbohydrates as glycoproteins or proteoglycans and contribute to the glycocalyx of the cell ( Fig. 2.1 ). The glycocalyx also contains glycoproteins and proteoglycans which have been secreted into the extracellular space around the cells and then absorbed onto the cell surface. Thus it is difficult to specify exactly where a cell plasma membrane ends and the surrounding extracellular matrix (ECM) begins. The glycocalyx is fundamental in cell–cell and cell–matrix communication. Cells place specific protein and carbohydrate groups into the glycocalyx when touching and adhering to other cells, when displaying cell markers to other cells, in blood clotting cascades and in inflammatory responses.
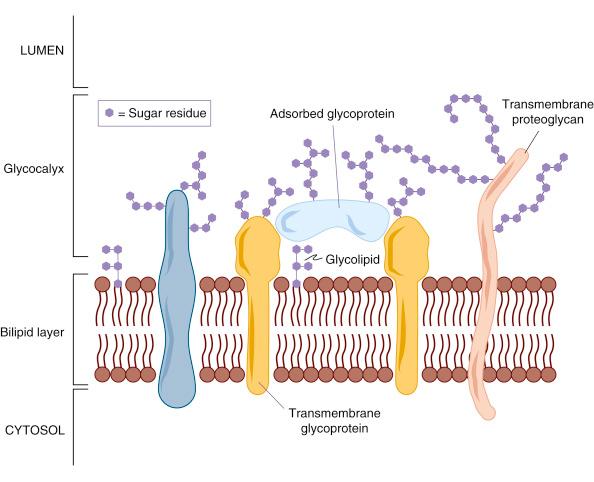
The membrane systems (organelles) within the embryonic cells (i.e., nucleus, mitochondria, endoplasmic reticulum, Golgi apparatus, lysosomes and secretory vesicles) are also made of the plasma membrane; secretory vesicles can become incorporated into the external plasma membrane to release contained contents. Intracellular membrane systems are supported within the cytoplasm by a range of cytoskeletal elements which also allow cells to maintain surface specialisations (microvilli), to change shape (as in the movements of endocytosis and exocytosis), to move in specific directions (with the glycocalyx molecules) and to adhere strongly to a substrate when movement ceases.
The cytoskeleton is a highly dynamic network of protein filaments that extends throughout the cell. As it also allows the cell to move or move portions of its plasma membrane, the cytoskeleton is less like a bony framework and more like a moveable muscular system. Three types of protein filaments produce a diverse range of cytoskeletal elements, including actin filaments , microtubules and intermediate filaments ( Fig. 2.2 ); these are synthesised from actin, tubulin and a range of fibrous proteins (e.g., vimentin, laminin, respectively).
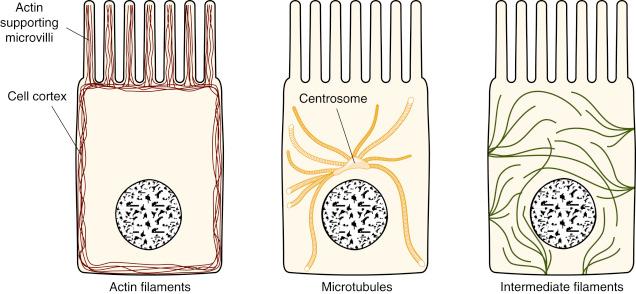
Actin filaments are polar structures composed of globular molecules of actin arranged as a helix. They work in networks and bundles, often found just beneath the plasma membrane, where they crosslink to form the cell cortex. Actin filaments are used to change the shape of the plasma membrane, moving it outwards in projections or inwards in invaginations. Whereas discrete bundles of actin, anchored into the cortex, can produce thin spiky protrusions of the plasma membrane, microvilli, sheet-like extensions of the membrane (lamellipodia) are supported by continuous flattened bundles of actin similarly anchored. Conversely, actin filaments can pull portions of the membrane inwards in the formation of endocytotic vesicles or in cell division. Here, contractile bundles of actin associated with the motor protein myosin form. Although myosin is most familiar in muscle fibres, nonmuscle cells contain various myosin proteins. Contractile bundles of actin filaments and myosin filaments are synthesised for specific functions and then disassembled (e.g., during cell division after chromosomal separation, the plasma membrane constricts to the middle of the cell, allowing two daughter cells to separate). Such assemblies of actin and myosin are also found in development near the apical surface of epithelial cells where they play a role in folding of epithelial sheets and in mesenchyme where they can form stress fibres which allow the cells to exert tension on the ECM.
Microtubules are long, hollow cylinders composed of the globular protein tubulin. They are much more rigid than actin filaments. Microtubules emanate from the centre of the cell in a region termed the centrosome . They lengthen by adding tubulin to the proximal end of each microtubule while subunits are lost from the distal end. The centrosome offers a focus and region of stabilisation for the proximal ends of the hundreds of microtubules in a cell; it also contains the two centrioles which are used by the cell when dividing. Vast numbers of microtubules extend in all directions to the plasma membrane and seem to ensure that the centrosome is at the centre of the cell. From this position, the microtubule array sites the other cellular organelles and holds them in place using a range of contact proteins. If a cell touches another cell, there may be internal movements of the organelles driven by the microtubules, resulting in repositioning of the centrosome. Microtubules display a dynamic instability, with new subgroups being added or subtracted very rapidly. The turnover of distal units can be slowed by contact with proteins close to the plasma membrane; this allows cells to maintain a particular shape and polarity. Microtubules are also used in cell-surface specialisations, where they form the basis of cilia in the familiar 9+2 arrangement of nine microtubule doublets around a pair of single microtubules.
Intermediate filaments are made of a variety of proteins all formed from highly elongated fibrous molecules. They are arranged as rope-like fibres which span each cell often from one cell junction to another. They are termed intermediate filaments because their apparent diameter on electron microscopy is between that of actin filaments and thick myosin filaments. Specific varieties of intermediate filaments are present in epithelial and mesenchymal cells. Whereas epithelial cells contain keratin filaments, mesenchymal cells have vimentin and vimentin-related filaments, and in the cells which will develop a myogenic lineage, desmin filaments are seen. Neuroepithelial cells develop neurofilaments and glial fibrillary acidic protein filaments are seen in astrocytes.
Two early tissue arrangements can be seen in embryos – epithelia and mesenchyme. Individual cells within each arrangement secrete extracellular proteins which form the ECM. This structures the space around and within cell populations and provides the appropriate conditions for development.
Cells composing epithelia are polarised with apical and basal surfaces. Whereas the apical surface commonly displays specialised features such as microvilli, the basal surface is the site of extracellular protein deposition in the form of a basal lamina. Laterally, the cells contact their neighbours via varieties of juxtaluminal junctional complexes which bridge the narrow intercellular clefts. Epithelial cell polarity factors regulate the relative size of the surfaces or domains and the internal organisation of the cytoskeleton. The transmembrane protein Crumbs specifies the apical domain, Baz/PAR-3 controls the position and extent of the junctions, Scribble restricts the size of the junctional domains, an internal contractile actomyosin network which produces the planar polarity of epithelia is contiguous across the lateral borders of the cells through E-cadherin adhesion, and the basal region displays integrins which link to the basal lamina.
Basal laminae are thin, flexible sheets of ECM which are made by and underlie epithelial cells ( Fig. 2.3 ). Basal laminae are also found surrounding individual skeletal muscle fibres, fat cells and Schwann cells. The presence or absence of a basal lamina beneath an epithelium during development is of consequence. Basal laminae organise the proteins in adjacent cell membranes, induce cell differentiation and cell metabolism, serve as routes for cell migration and can influence cell polarity in those cells that touch them; they can change with time during development and thus can maintain a developmental impetus.
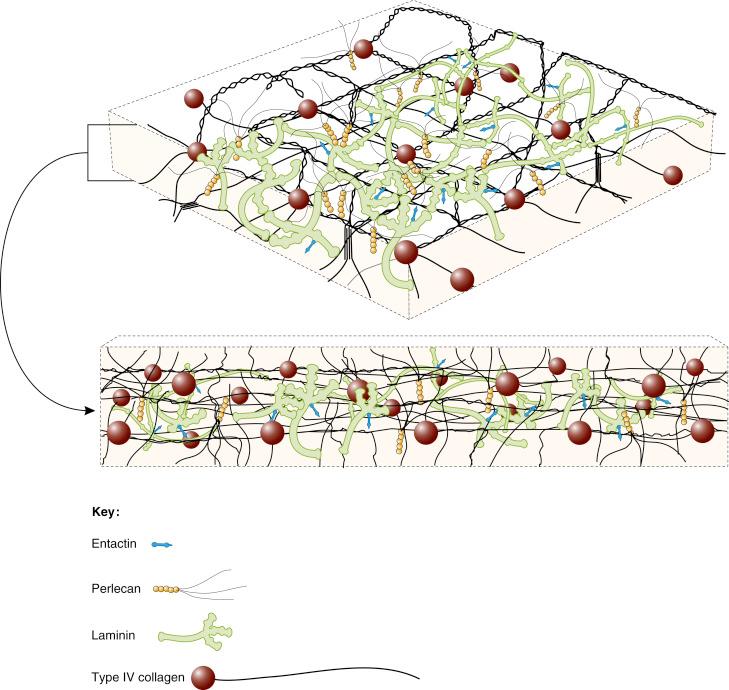
The basal lamina is described in electron microscopic studies as having an electron lucent layer, the lamina lucida or rara , closest to the basal surface of the cell and an electron-dense layer, the lamina densa below. If the epithelial layer rests on underlying mesenchyme, a layer of collagen fibrils connects the basal lamina to the underlying tissue, sometimes with specialised anchoring collagen fibrils. The strength of this connection is important for development and growth. Many textbooks do not distinguish between the basal lamina as described and the basement membrane , a thicker layer which includes the basal lamina and extracellular components of the underlying connective-tissue matrix.
Embryonic basal laminae are composed chiefly of laminin . Later, other extracellular molecules such as type IV collagen , perlecan and entactin (see below) contribute to the feltwork of the layer (see Fig. 2.3 ). In some regions of the body, basal laminae form specialised structures or units which have a specific function during development or in adult life. An example of this arrangement is seen in tooth development, where initially ameloblasts and odontoblasts are separated by a basal lamina. The ameloblasts deposit enamel directly onto one side of this basal lamina, and the odontoblasts deposit dentine onto the other (see Fig. 2.12 ); in this way, the tooth is formed. In both the kidneys and the lungs, the basal lamina from the specialised cells of the organ abuts directly onto the endothelial basal lamina, producing a selectively permeable barrier. In the kidneys, this is the glomerular basement membrane.
In development, the basal lamina acts as a selective barrier to the movement of cells, and migrating cells will move along basal laminae but not through them. Cells beneath an epithelial layer see only the basal lamina which the overlying cells produce. Changes in the local basal laminal composition is one way by which the epithelial cells can communicate with the cells migrating beneath them. In adult tissues, the basal lamina permits the movement of macrophages, lymphocytes and nerve processes and plays an important part in tissue regeneration after injury.
Juxtaposed cells usually do not touch. For contact to be established, the cells produce specific molecules which promote the development of a cell–cell junction between them ( Fig. 2.4 ). Junctional complexes allow sheets of epithelial cells to act in concert in maintaining a barrier or in producing alterations in the overall epithelial morphology; they also permit cell–cell communication and are in this respect especially important in development. Cell junctions are classified into three main groups: (i) tight junctions , which prevent leakage of molecules between cells from one side of a sheet of cells to the other ; (ii) anchoring junctions , where the neighbouring cell membranes attach and are supported by cytoskeletal elements within the cells, either actin or intermediate filaments (this type of junction also anchors epithelial cells to the ECM); and (iii) communicating junctions , which mediate the passage of electrical or chemical signals from one cell to another.
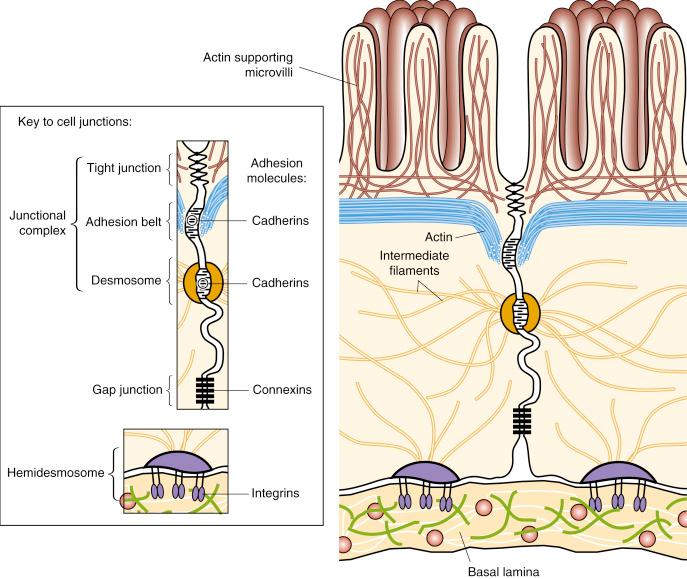
The formation of these junctional complexes is dependent on a range of cell adhesion molecules (CAMs) (see Fig. 2.4 ). In cell–cell anchoring junctions (adhesion belts and desmosomes), the CAMs involved are termed cadherins ; they are attached intracellularly to intermediate or actin filaments in the cell cortex. The latter run parallel to the plasma membrane; thus the actin bundles of adjacent cells are linked. Concomitant contraction of the actin bundles results in narrowing of the apices of the epithelial cells and rolling of the epithelial layer into a deep groove or a tube.
Epithelial cells contact the underlying basal lamina they synthesise by different types of anchoring junctions (hemidesmosomes and focal contacts). In these cases, the transmembrane linker proteins belong to the integrin family of ECM receptors. Cytoskeletal filaments support the connection of the integrin within the cell membrane to the ECM.
Communication between adjacent epithelial cells is mediated by gap junctions . In forming a gap junction, each cell contributes six identical protein subunits (called connexins ) which form a structure, similar to an old-fashioned cotton reel, termed a connexon . This is situated across the bilaminar membrane with the thicker rims extending into the extracellular and intracellular spaces. Each connexon is capable of opening and closing, thus controlling the gap. When two connexons from adjacent cells are aligned, a tubular connection is made between the cells. Each gap junction is really a cluster of apposed connexons which each permit molecules smaller than 1000 daltons to pass through them. In early embryos, most cells are electrically coupled to one another by gap junctions. Later in development, epithelial cells synthesise gap junctions at particular stages when it is inferred that information is passing from cell to cell. When gap junctions are removed, there is often a difference in differentiation in the cellular progeny. Gap junctions are seen in adult tissues (e.g., connecting cardiac myocytes to permit transmission of the electrical signals of the cardiac cycle). (For further information on CAMs, see Alberts et al. )
Mesenchymal cells, in contrast to epithelial cells, have no polarity and thus no directional surface specialisations. They have junctional complexes which are not juxtaluminal, and they produce extensive ECM molecules and fibres from the whole cell surface ( Fig. 2.5 ). As development proceeds, proliferating mesenchymal populations begin to differentiate. This is often first seen by the upregulation of specific mRNA in the cell or in the production of different ECM molecules by selected progeny.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here