Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bisphosphonates (BPs) are well established as the gold standard treatment for metabolic bone diseases associated with excessive bone destruction, including osteoporosis, bone metastases, and cancer therapy–induced bone loss. By virtue of their ability to chelate calcium ions, BPs bind strongly to the skeleton upon administration, thus selectively affecting bone-resorbing osteoclasts. However, preclinical and epidemiological cancer studies support the notion that BPs have additional beneficial effects beyond simply inhibiting bone resorption. This review describes the biochemical and molecular actions underlying the antiresorptive effect of these drugs on osteoclasts and their potential direct and indirect antitumor activities.
BPs are analogues of inorganic pyrophosphate (PPi) ( Fig. 62.1 ), containing two phosphonate groups linked by stable phosphoether bonds to a central (geminal) carbon atom [ ]. This creates a backbone P-C-P structure with two side chains, R 1 and R 2 , attached to the geminal carbon. While R 1 is usually a hydroxyl (-OH) group, R 2 is more varied and determines the type of bisphosphonate, its pharmacological activity, and potency. There are two types of bisphosphonates: simple or nitrogen-containing bisphosphonates, and they inhibit osteoclast activity by different mechanisms. Simple bisphosphonates closely resemble pyrophosphate (PPi) (e.g., clodronate, R 2 = Cl) and were the first antiresorptive compounds to be developed for clinical use. The more recent nitrogen-containing bisphosphonates (e.g., pamidronate, alendronate, ibandronate, zoledronate) contain a primary or tertiary amine in an alkyl chain or heterocyclic group in the R 2 side chain, and are several orders of magnitude more potent at inhibiting bone resorption in vivo than simple bisphosphonates [ ] ( Fig. 62.1 ).

The ability of BPs to selectively affect osteoclasts, and thereby inhibit bone resorption, is based on the three-dimensional structure of BPs that allows the chelation of divalent metal ions, especially Ca 2+ [ ]. Hence, upon administration, BPs are rapidly cleared from the circulation [ ] by binding with high affinity to exposed hydroxyapatite bone mineral surfaces ( Fig. 62.2 ).
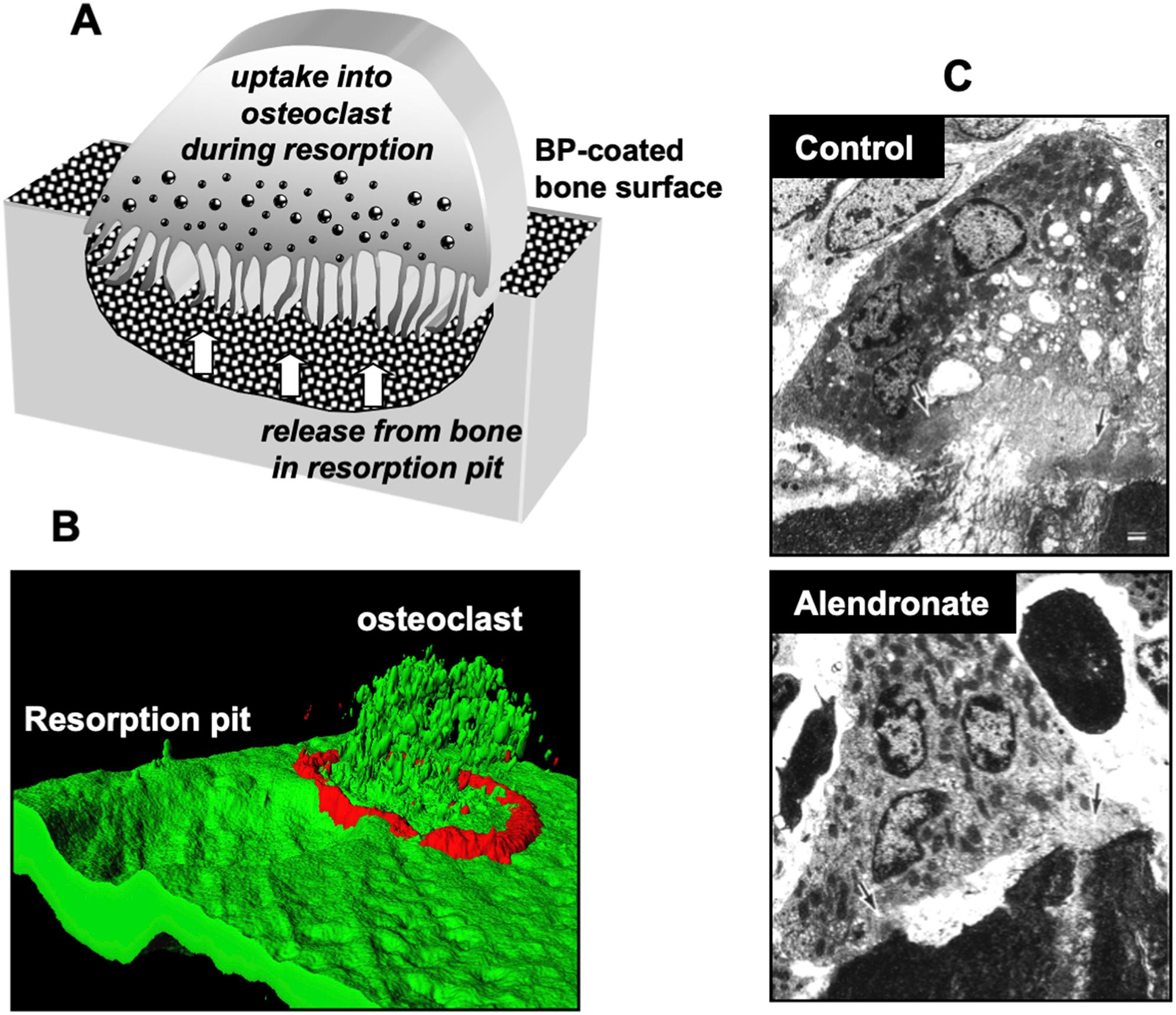
During bone resorption by osteoclasts, the acidic pH in the resorption lacuna causes the dissociation of BP from the mineral surface, followed by cellular uptake. The newly released drug is most likely incorporated into the cytoplasm as a complex with calcium ions and bone matrix proteins by fluid-phase endocytosis [ ]. Fluorescently labeled BPs have been extremely useful tools to visualize the skeletal distribution and cellular incorporation of BPs in vivo , as well as their intracellular localization in endocytic vesicles within osteoclasts [ ] ( Fig. 62.2 ). In this regard, vesicle acidification appears to be necessary for the translocation of BPs from the vesicles into the cytosol [ ] where these drugs exert their biochemical effect. Passive diffusion from the endosomal compartment into the cytosol is highly unlikely given the negative charge of BP molecules. SLC37A3, a transporter in the vesicle membrane, was identified as a likely route by which BPs may transfer from the acidic endosome into the cytosol [ ] ( Fig. 62.2B ). ATRAID also appears to be required for cellular uptake, and cells lacking ATRAID are resistant to the antiproliferative effects and pharmacologic actions of N-BPs [ ].
Given their ability to acidify and dissolve the bone surface, osteoclasts are generally considered to be the only cell type within the bone microenvironment capable of releasing and internalizing sufficient amounts of BPs to exert pharmacologic effect on cell function in vivo . However, it is becoming increasingly clear that other highly endocytic cells such as monocytes and macrophages may also be capable of incorporating the drug transiently present in the circulation, bone marrow, and soft tissues [ ].
As previously mentioned, simple BPs share the backbone P-C-P structure, and bone-binding R 1 side chain with N-BP. The R 2 , however, is much smaller and very similar to pyrophosphate (PPi), an abundant metabolic by-product. In contrast to N-BPs, simple BPs such as clodronate are only active when converted to nonhydrolyzable AppCp-type metabolites upon cellular uptake. Intracellular accumulation of these toxic metabolites causes osteoclast apoptosis, thus inhibiting bone resorption [ ]. The mechanisms of action of simple BPs was elucidated through observations that the simplest compound of this class, methylene bisphosphonate (medronate), could be metabolically incorporated into methylene-containing analogues of adenosine triphosphate (ATP) and diadenosine tetraphosphate (Ap 4 A) in Dictyostelium slime mold amebae [ ] ( Fig. 62.3 ). Further studies demonstrated that clodronate and other BPs of simple chemical structure could also be metabolized by Dictyostelium amebae and by cultured mammalian cells to methylene-containing (AppCp-type) analogues of ATP that inhibited cell growth [ ].
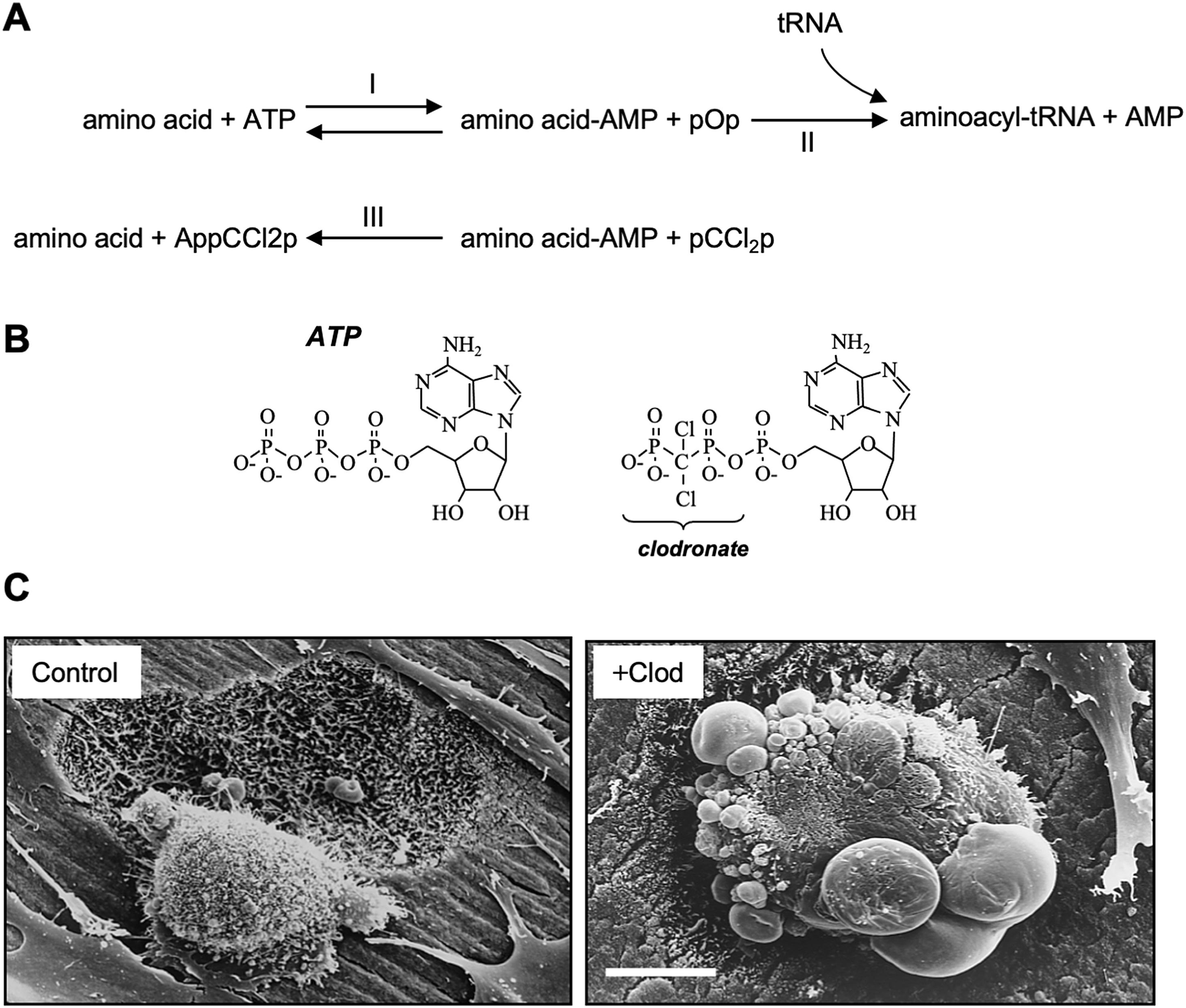
The incorporation of simple BPs into nucleotide analogues appears to be brought about by type II class of aminoacyl-tRNA synthetases [ , ] ( Fig. 62.3A ), although other intracellular enzymes such as T4 RNA ligase may also be capable of metabolizing simple BPs [ ]. Simple BPs resemble PPi and substitute it in the active site of aminoacyl-tRNA synthetases. This allows a back-reaction involving the condensation of a BP with adenosine monophosphate (AMP) derived from an aminoacyladenylate to form an AppCp-type nucleotide ( Fig. 62.3B ). However, the sterically bulkier N-BPs, such as alendronate, pamidronate, and ibandronate, cannot be metabolized by these enzymes [ ].
The metabolism of the simple BP clodronate by osteoclasts results in the accumulation of AppCCl 2 p [ ] at cytosolic concentrations reaching up to 1 mM [ ]. Owing to the nonhydrolyzable nature of these metabolites, their intracellular accumulation is likely to inhibit numerous enzymatic processes, thus severely impacting cell function and inducing apoptosis [ ] ( Fig. 62.3C ). In support of this, treatment of cultured osteoclasts with AppCCl 2 p (encapsulated in liposomes) causes osteoclast apoptosis and inhibits bone resorption to the same extent as clodronate [ ]. Induction of apoptosis appears to involve the adenine nucleotide translocase (ANT), a component of the mitochondrial permeability transition pore. ANT inhibition by the clodronate metabolite AppCCl 2 p in osteoclasts prevents ATP translocation, which in turn trigger inner membrane hyperpolarization [ ], breakdown of the mitochondrial membrane potential, activation of caspase-3 [ ], and cleavage of the apoptosis-promoting kinase Mst-1 [ ]. Thus, osteoclast apoptosis appears to be the primary mechanism of action of simple BPs, and this is consistent with historical observations that treatment with these drugs causes morphological changes in osteoclasts in vitro and in vivo that are now recognized as features of apoptosis ( Fig. 62.3C ). Furthermore, the effects of clodronate and etidronate on blocking bone resorption can be overcome when osteoclast apoptosis is prevented using a caspase inhibitor [ ]. The ability of clodronate to promote apoptotic cell death is also the basis for using clodronate-encapsulated liposomes to deplete macrophages in vivo . Macrophages selectively internalize liposomes loaded with clodronate, resulting in the accumulation of the toxic AppCCl 2 p metabolite at high intracellular concentrations triggering cell death [ ].
The apoptosis-inducing effects of BPs can be rescued, at least in part, by the actions of receptor-activator of nuclear factor kappa-β ligand (RANKL) and tumor necrosis factor alpha (TNF-α) on enhancing expression of the antiapoptotic Bcl2-family members Mcl-1 and Bcl-xL. This may explain why BPs have not been as effective in inflammatory models of bone loss in which there are high levels of RANKL and TNF-α [ , ].
Nitrogen-containing BPs (N-BPs) are not metabolized in the same manner as simple BPs and have a different molecular mechanism of action on osteoclasts. This was elucidated from initial observations that the N-BPs ibandronate and incadronate inhibited the mevalonate-cholesterol biosynthesis pathway ( Fig. 62.4A ) in J774 macrophages [ , ]. This cell line constituted an appropriate and convenient model in which to study the actions of N-BPs, since even small changes to the R 2 side chain known to affect antiresorptive potency similarly affected the ability to cause apoptosis [ ]. The close similarity between induction of J774 macrophage apoptosis by cholesterol-lowering statins and N-BPs was consistent with the involvement of the mevalonate pathway, and explained the effects of N-BPs on the inhibition of bone resorption [ ].
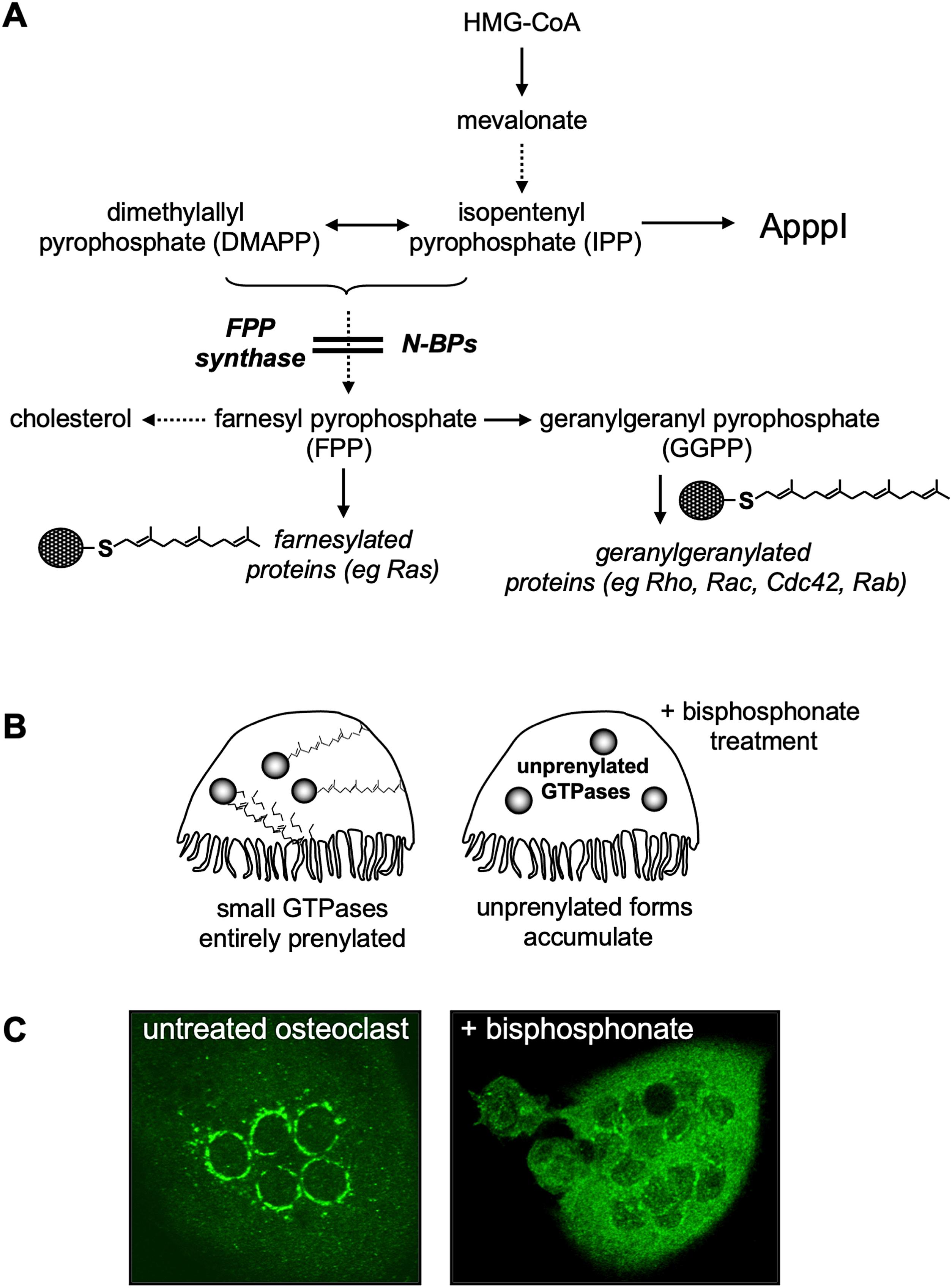
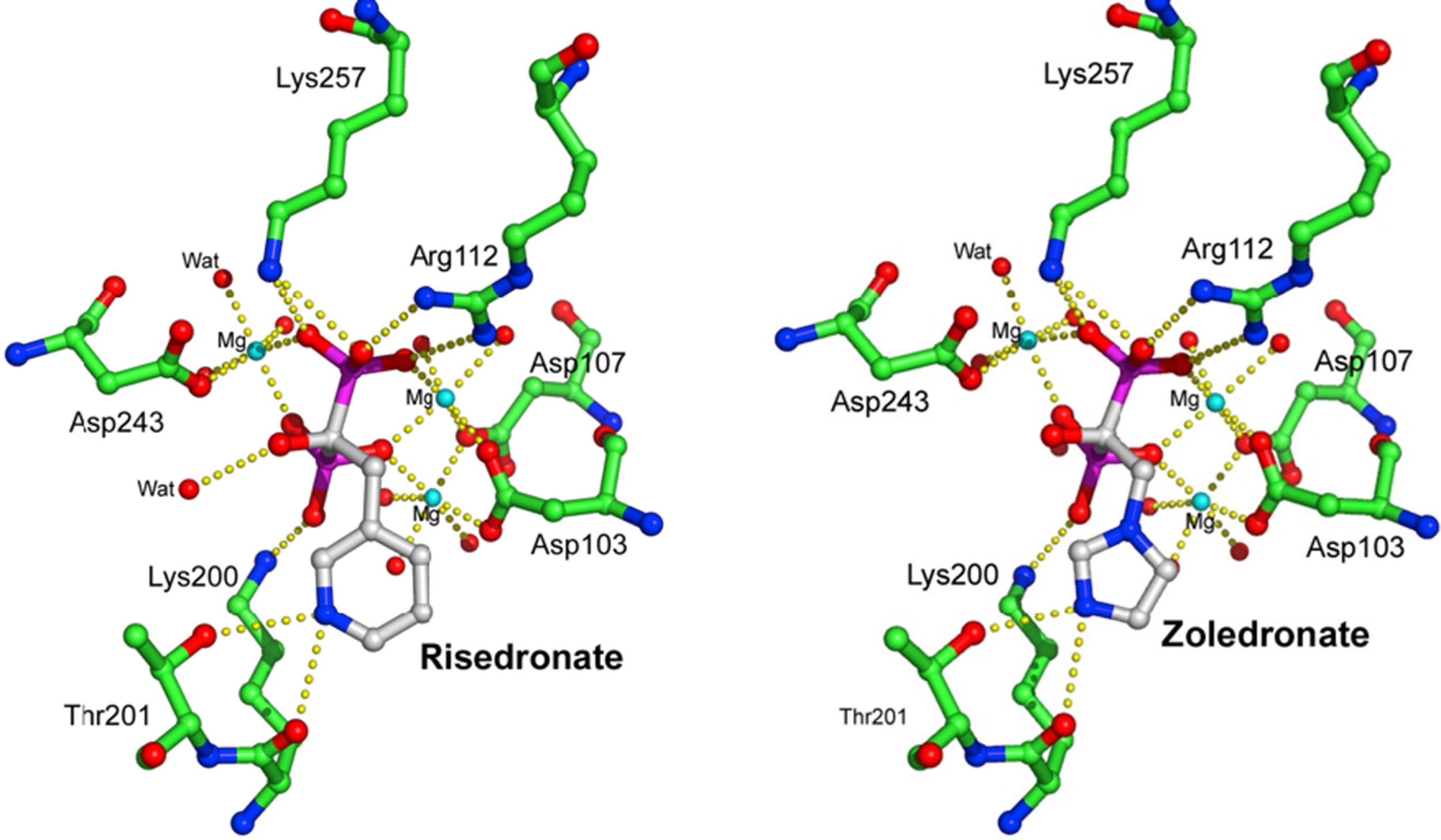
Around the late 1990s a flurry of studies in J774 macrophages and osteoclasts identified farnesyl pyrophosphate (FPP) synthase as the major target of N-BPs ( Fig. 62.4A ) [ ], although some compounds also showed weaker inhibitory effects on other enzymes in the mevalonate pathway, including isopentenyl pyrophosphate (IPP) isomerase, squalene synthase, and geranylgeranyl pyrophosphate (GGPP) synthase [ , , ] ( Fig. 62.4A ). N-BPs mimic the structure of the natural isoprenoid pyrophosphate substrates of FPP synthase (GPP/DMAPP) and compete for binding to the enzyme [ ] in a "slow-tight" binding mechanism that leads to potent and virtually irreversible enzyme inhibition [ , ]. The exact manner in which N-BPs inhibit FPP synthase was revealed by X-ray crystallography. These studies confirmed the binding of N-BPs in one of two substrate docking sites and involving (1) the positioning of the R 2 side chain of the N-BP in the hydrophobic cleft normally reserved for the isoprenoid lipid substrate, and (2) the interaction of the phosphonate groups in the N-BP molecule with a cluster of magnesium ions that would normally accommodate the pyrophosphate moiety of DMAPP/GPP [ , ]. The binding of a molecule of N-BP in the first substrate docking site, and of a molecule of IPP in the second one, causes conformational changes in the enzyme that almost irreversibly “lock” it in an inactive conformation, hence inhibiting enzyme activity. From these studies it is clear that the length and orientation of the bisphosphonate R 2 side chain can affect the interaction of the nitrogen in the side chain of the N-BPs with lysine and threonine residues in the GPP/DMAPP binding site, or with the second enzyme substrate in the adjacent binding pocket of FPP synthase ( Fig. 62.5 ). They also explain why minor changes to the structure or conformation of the N-BP side chain affect its ability to inhibit FPP synthase [ , ] and markedly influence antiresorptive potency [ ]. Since the two phosphonate groups of N-BPs are also necessary for binding to magnesium ions in FPP synthase [ , ], chemical modifications (such as methylation) to either or both of the phosphonate groups of N-BPs compromises their ability to inhibit enzyme activity and reduces their antiresorptive strength [ , , ]. The phosphonate groups of N-BPs therefore have a dual role in exerting the pharmacological action of these drugs: (1) they enable their targeting to bone mineral by virtue of their calcium-chelating properties, and (2) they inhibit enzyme activity by directly binding to the cluster of magnesium ions in the catalytic site in FPP synthase [ , ]. Together, these observations shed light on the relationship between BP structure and antiresorptive efficacy, and further confirmed that FPP synthase is the major pharmacological target of N-BPs in osteoclasts in vivo .
The high degree of evolutionary conservation of FPP synthase explains why N-BPs also inhibit this enzyme in Dictyostelium slime mold amebae [ ] and in other eukaryotes such as Leishmania and Trypanosoma parasites [ , ]. Furthermore, the development of resistance to the growth-inhibitory or cytotoxic effect of N-BPs in spontaneous mutant strains of Dictyostelium [ ] , as well as tumor cells [ , ], is accompanied by increased FPP synthase activity. Similarly, upregulation of endogenous levels of FPP synthase can also confer partial resistance to N-BPs [ ], and a variant in FPP synthase gene has been associated with lower bone mineral density [ ]. Whether mutations or polymorphisms in FPP synthase may underpin interindividual differences in the response to N-BP therapy, or the development of resistance to N-BPs in some patients, remains largely unexplored. Differences in cellular uptake of BPs could also confer differences in sensitivity to their pharmacologic actions. For example, whole-genome sequencing analysis revealed two rare variants in the ATRAID gene in patients that developed osteonecrosis of the jaw (ONJ) and/or atypical femoral fractures (AFFs) after BP therapy, suggesting a personalized approach to identify patients most at risk of developing these side effects [ ].
The primary function of the mevalonate pathway is the production of cholesterol as well as the synthesis of isoprenoid lipids such as farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP). These isoprenoid lipids are the building blocks for the production of a variety of metabolites, such as dolichol and ubiquinone [ ], but are also required for the posttranslational modification of proteins known as prenylation ( Fig. 62.4A ). The process of prenylation involves the transfer of the 15-carbon or 20-carbon chain isoprenoid groups (FPP or GGPP, respectively) onto a cysteine residue in characteristic carboxy-terminal motifs of specific target proteins [ , ]. The farnesylated and geranylgeranylated prenylome comprise up to 2% of mammalian proteins, predominantly small GTPases, but also γ-subunits of heterotrimeric G proteins, phosphodiesterase subunits and nuclear lamins. Prenylation serves to anchor proteins in cell membranes ( Fig. 62.4B and C ) and to modulate protein–protein interactions (as in the case of small GTPases with regulatory GAPs and GDIs) [ , ], thus is required for appropriate localization, regulation, and signaling. Prenylation is particularly important for the function of small GTPases. These proteins act as molecular switches that control crucial signaling pathways; therefore their activity must be tightly regulated. Inhibition of protein prenylation by treatment with N-BP results in the cytosolic accumulation of unprenylated small GTPases ( Fig. 62.4C ) in their active (GTP-bound) state, causing inappropriate activation of downstream signaling kinases such as p38 [ ]. Importantly, small GTPases such as those of the Ras, Rho, Rac, Cdc42, and Rab families govern cellular processes essential for osteoclast function, including cytoskeletal arrangement, membrane ruffling, trafficking of intracellular vesicles, and apoptosis [ ]. Loss of prenylation and the accumulation of proteins in their unprenylated dysregulated form account for most, if not all, of the known effects of N-BPs on osteoclasts [ , , ]. For example, in osteoclasts, Rho, Rac, or Cdc42 are required for cytoskeletal organization and the formation of F-actin adhesion rings. In addition, Rab GTPases are crucial regulators of vesicular trafficking [ , , ]. Together, these small GTPases also govern the formation of the ruffled border and other cellular processes necessary for bone resorption. In vitro treatment of osteoclasts with N-BP inhibits F-actin ring [ ] and ruffled border formation [ , ], and interferes with the trafficking of lysosomal enzymes and transcytosis of degraded bone matrix [ , ] ( Fig. 62.2C ). Loss of prenylation of small GTPases such as Rac also results in disruption of downstream signaling pathways promoting cell survival, a likely route by which osteoclasts undergo apoptosis when exposed to sufficiently high concentrations of N-BP [ ]. However, unlike for simple BP, induction of osteoclast cell death does not account for the antiresorptive actions of N-BP, since blocking osteoclast apoptosis in vitro using a caspase inhibitor did not restore function [ ]. Furthermore, pharmacological activity of N-BPs is not always accompanied by signs of cell toxicity or a decrease in osteoclast number, unless at high concentrations or doses [ , , ]. Indeed, N-BP therapy can lead to the formation of giant osteoclasts, identified histologically as hypernucleated cells that are usually detached from the bone surface or associated with superficial resorption lacunae [ , ]. These presumably occur as a result of accumulation of functionally inactive osteoclasts that continue to undergo cell fusion.
The importance of prenylated proteins for osteoclast function has been confirmed using specific inhibitors that prevent either protein farnesylation (FTI-277, an inhibitor of farnesyl transferase) or protein geranylgeranylation (GGTI-298, an inhibitor of geranylgeranyl transferase I). Whereas loss of farnesylated proteins in osteoclasts has little effect, loss of geranylgeranylated proteins causes disruption of actin rings, inhibits bone resorption, and stimulates osteoclast apoptosis [ ]. This is supported by the finding that replenishing cells with an isoprenoid lipid substrate that restores geranylgeranylation can overcome the effects of N-BPs on osteoclast formation, apoptosis, and bone resorption [ , , ]. Together, these observations demonstrate that inhibition of FPP synthase by N-BPs affects bone resorption by altering the geranylgeranylation of proteins that are fundamentally important for osteoclast function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here