Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Histology is the study of normal cells and tissues, mainly using microscopes. This book describes the histology of normal human tissues, although much of the material applies to other mammals and, indeed, non-mammals. Structure and function are interdependent. Histological structure determines and is determined by the functions of different organs and tissues; the study of one has enriched the understanding and study of the other. When diseases such as cancer or inflammation affect a tissue, there are often specific changes in the microscopic structure of the tissue. The microscopic study of these changes is known as histopathology, anatomical pathology or sometimes just pathology . Obviously a sound knowledge of normal structure is essential for an understanding of pathology.
The cell is the functional unit of all living organisms. The simplest organisms, such as bacteria and algae, consist of a single cell. More complex organisms consist of many cells as well as extracellular matrix (e.g. the matrix of bone). The term eukaryote refers to organisms whose cells consist of cytoplasm and a defined nucleus bounded by a nuclear membrane ; this includes plants, fungi and animals, both unicellular and multicellular. The cytoplasm contains variable numbers of several different recognisable structures called organelles, each with a defined function. The nucleus may be considered to be the largest organelle.
Prokaryote refers to bacteria and archaea, whose cells do not have a membrane-bound nucleus; they also have other major structural differences and will not be discussed further here.
The cells of multicellular organisms, such as humans, show a great variety of functional and morphological specialisation, with amplification of one or another of the basic functions common to all living cells. The process by which cells adopt a specialised structure and function is known as differentiation . Despite an extraordinary range of morphological forms, all eukaryotic cells conform to a basic structural model, which is the subject of this chapter.
The major tool in the study of histology is the microscope. The basic techniques used in histology, including tissue preparation, slide production, light microscopy and electron microscopy, are outlined in Appendix 1 . Various staining techniques are used to prepare tissues to make them visible through the microscope and these are described in Appendix 2 . The student is strongly urged to read these appendices first; it really will make all that follows more comprehensible. Appendix 3 is a glossary of common histological terms and, like the other appendices, can be dipped into at any time.
C adjacent cell ER endoplasmic reticulum F collagen fibrils G Golgi apparatus IS intercellular space L lysosome M mitochondrion N nucleus NE nuclear envelope PM plasma membrane V transport vesicles
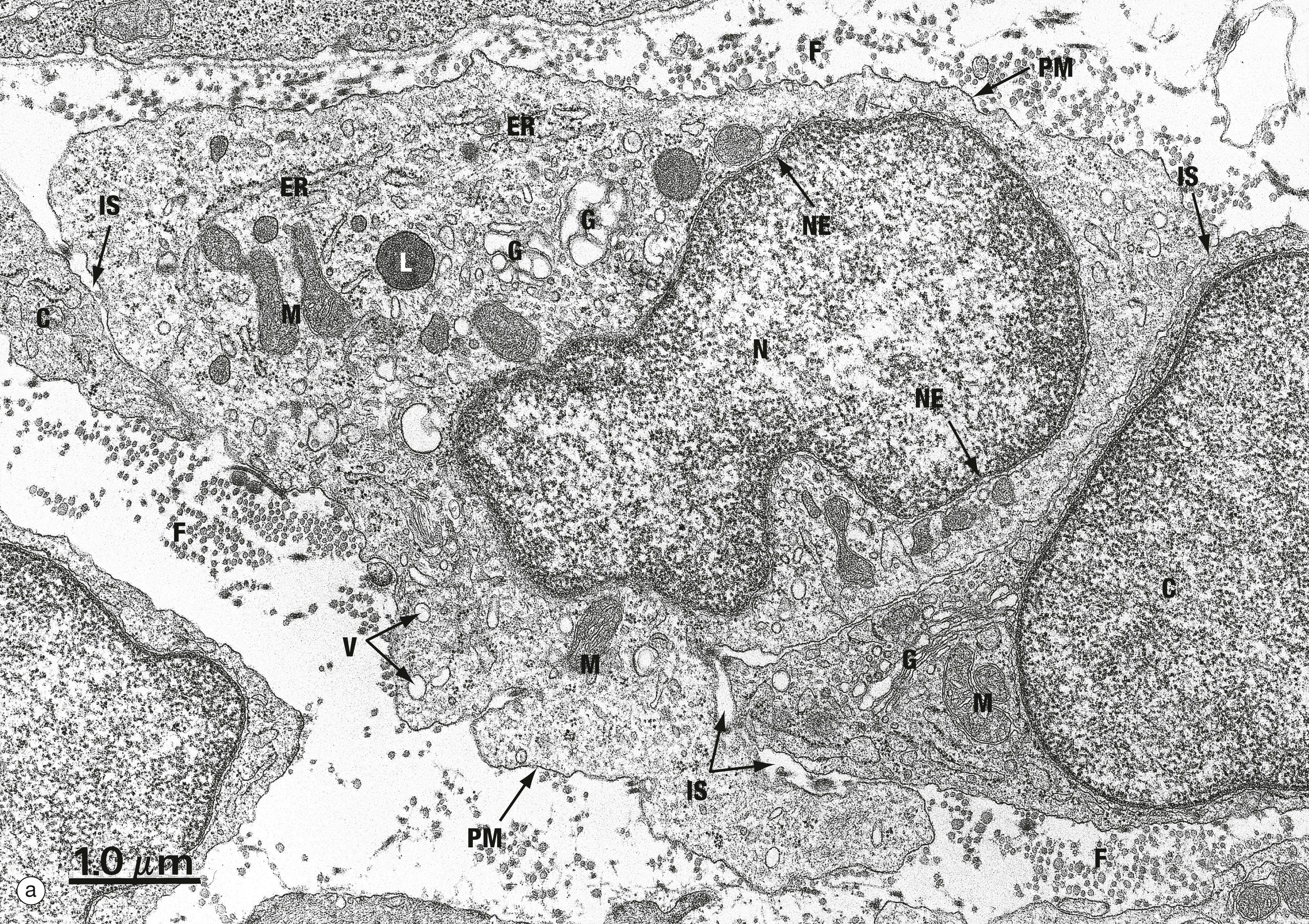
Cell membranes, including the outer plasma membrane and the internal membranes, are composed of approximately equal amounts (by weight) of lipids and proteins. The unique properties of cell membranes allow them to separate the interior of the cell from the external milieu and the internal compartments of the cell from each other. Most of the chemical reactions of cells take place in aqueous polarised solution. The immiscibility of lipids with water leads them to form lipid bilayers , which effectively prevent passage of polarised ions and molecules; thus the contents of different compartments are kept separate and ion gradients between different compartments are maintained. Proteins embedded within the lipid bilayer act as channels to allow selective passage of particular ions and molecules. Some types of cell signalling are also mediated by membrane proteins.
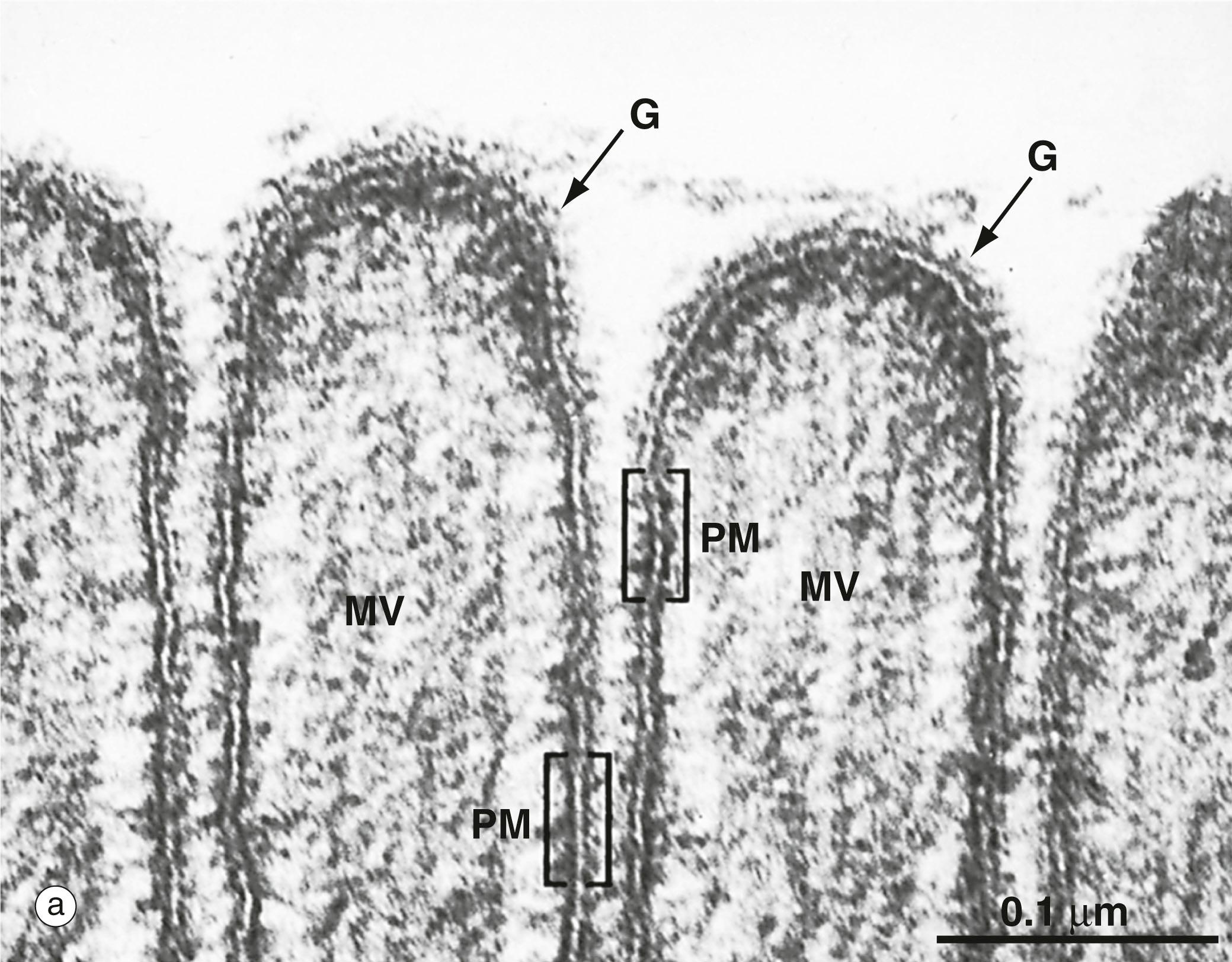
G glycocalyx MV microvilli PM plasma membrane
The nucleus is the largest organelle in the cell and is usually the most obvious feature of the cell seen by light microscopy. The nucleus contains the genetic material of the cell, deoxyribonucleic acid (DNA) , arranged in the form of chromosomes. Each chromosome contains a number of genes joined end to end, with each gene encoding the structure of a single protein according to the sequence of nucleotides along the length of the gene ( Fig. 1.6 ). Thus the genetic blueprint for all proteins, whether structural or enzymes, is contained within the nucleus of every cell in the body except for red blood cells, which have no nucleus (see Ch. 3 ). The substance of the nucleus is known as the nucleoplasm and it is surrounded by the nuclear membrane. The first step in cell division is replication of the DNA so that a copy of the genome goes to each of the daughter cells. Cell division is the subject of Ch. 2 .
BN bilobed eosinophil nucleus C colonic crypt Cap capillary E euchromatin ER endoplasmic reticulum FN flattened endothelial cell nucleus H heterochromatin LP lamina propria M mitochondrion MM muscularis mucosae N crypt epithelial cell nucleus Nu nucleolus SM submucosa SN smooth muscle cell nucleus
DFC dense fibrillar component E euchromatin ECS extracellular space ER endoplasmic reticulum FC fibrillar centre GC ganglion cell G granular component H heterochromatin M mitochondrion N nucleus NE nuclear envelope NP nuclear pore PM plasma membrane R ribosome ST sustentacular cell nucleus
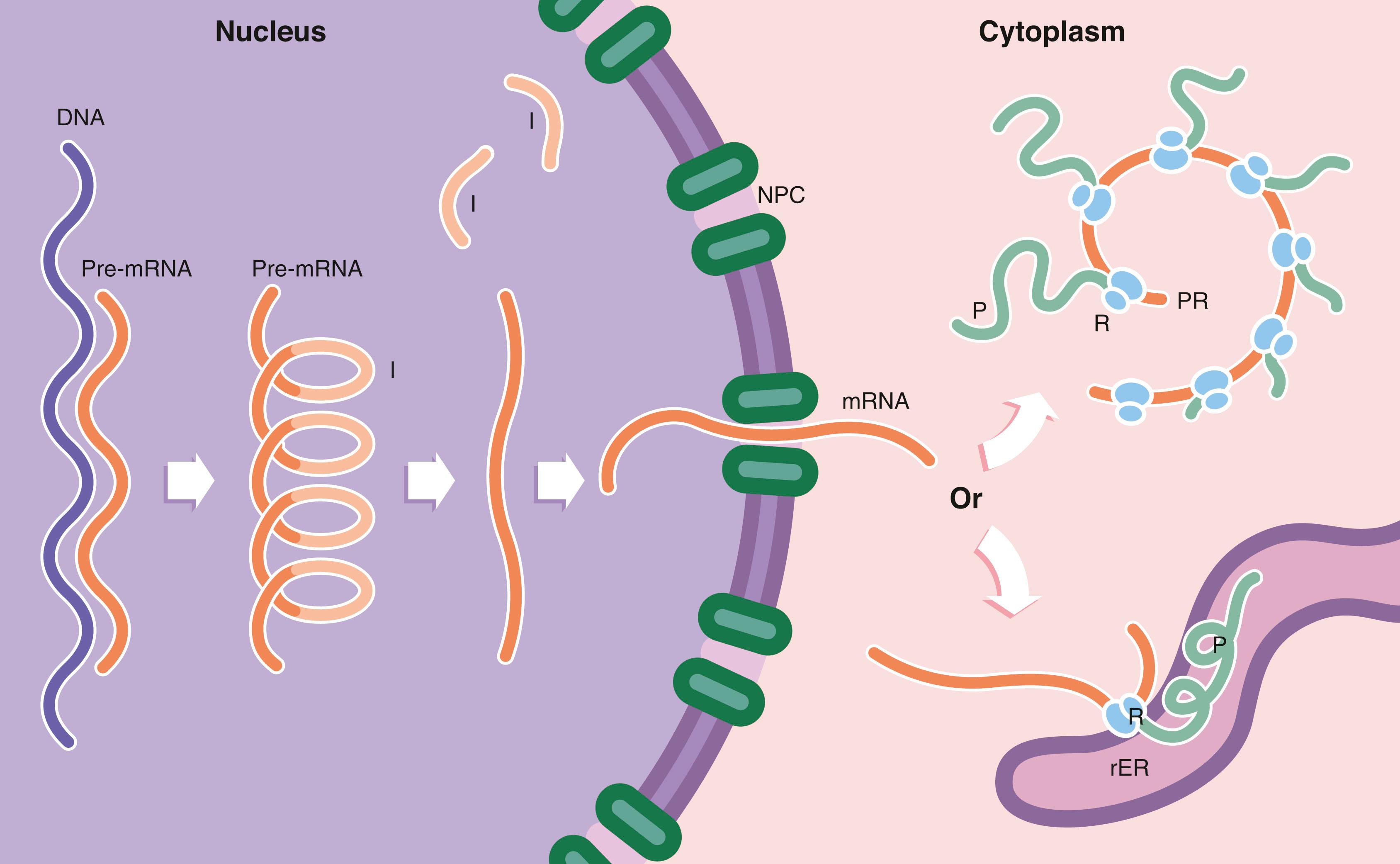
Proteins are not only a major structural component of cells, but, as enzymes, transport and regulatory proteins, they mediate many metabolic processes. The nature and quantity of proteins within a cell determine its activity and thus the study of proteomics can be very informative about the functions of a particular cell. All cellular proteins are replaced continuously. Many cells also synthesise proteins for export, including glandular secretions and extracellular structural proteins like collagen. Protein synthesis is therefore an essential and continuous activity of all cells and the major function of some cells. The ribosome is the protein factory of the cell. Every cell contains within its DNA the code for every protein that individual could produce. Production or expression of selected proteins only is characteristic of differentiated cells.
DNA deoxyribonucleic acid I intron M mitochondrion mRNA messenger ribonucleic acid N nucleus NE nuclear envelope NPC nuclear pore complex Nu nucleolus P polypeptide chain PR polyribosome pre-mRNA pre–messenger RNA R ribosome rER rough endoplasmic reticulum
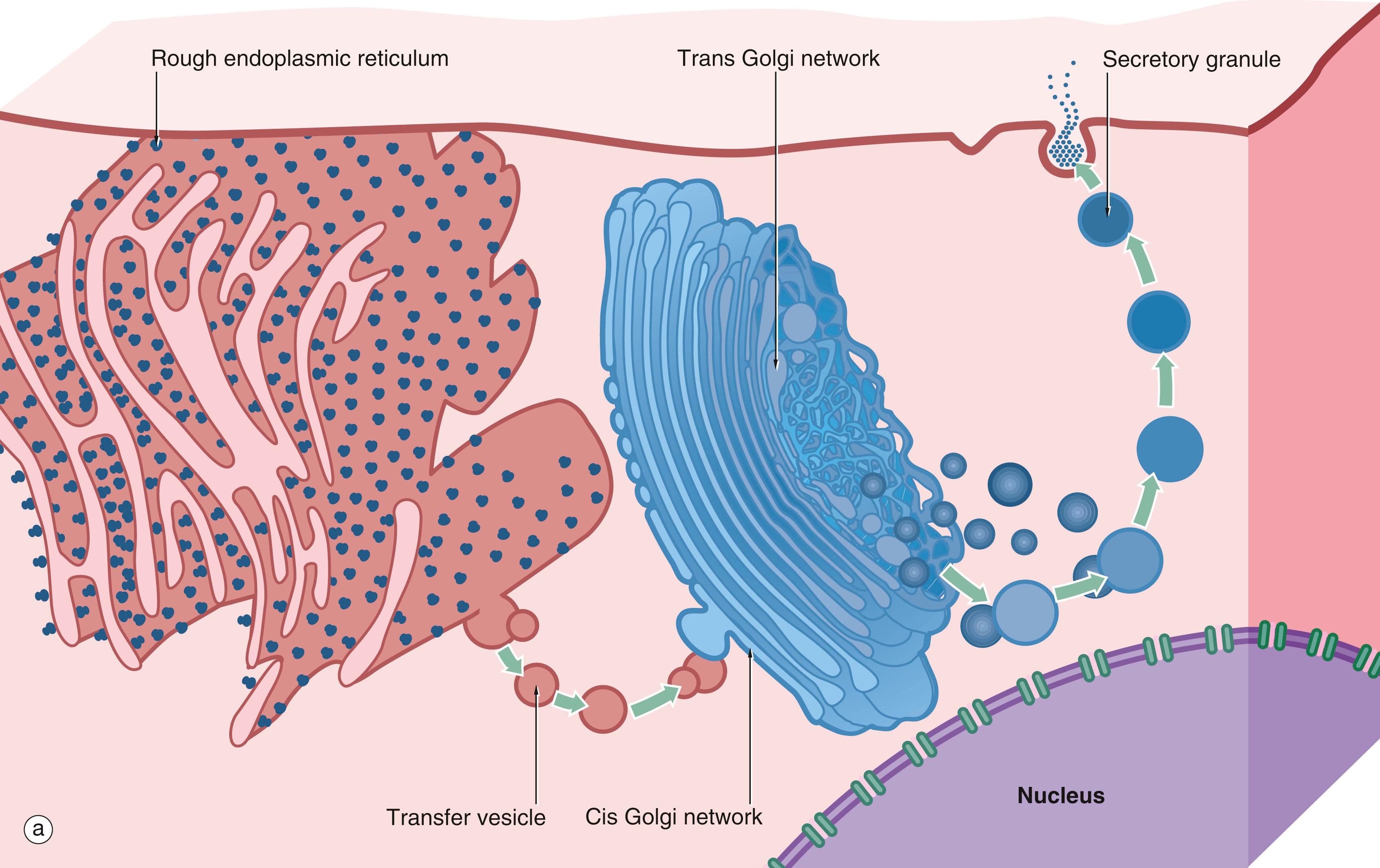
Movement of materials into and out of cells and between separate compartments of a cell involves crossing lipid membranes. The plasma membrane thus controls the interaction of the cell with the external environment, mediating the exchange of nutrients and waste products, secretions and signalling mechanisms. Lipid membranes also separate different compartments within the cell, many of which contain mutually incompatible biochemical reactions. For instance, the process of protein synthesis and export which takes place in the rough endoplasmic reticulum and Golgi apparatus must be kept separate from the garbage disposal and recycling plant, the lysosome . Likewise, microorganisms phagocytosed by cells must be killed and disposed of without damage to normal structures and mechanisms.
Information must also cross membranes, telling the cell when to divide, release secretions, contract or perform many other functions. Many of the mechanisms used for transport of cargo also serve to transmit messages to the interior of the cell. There are also dedicated mechanisms for the transfer of information, such as the transient depolarisation of the plasma membrane along the length of a nerve axon in the conduction of a nerve impulse. Histologically, these transport processes can only be observed indirectly: for example, cells suspended in hypotonic solutions swell due to passive uptake of water, whereas cells placed in hypertonic solutions tend to shrink due to outflow of water. Radioisotope labelling techniques can be used to follow active transport processes. Bulk transport, however, is readily observable by microscopy ( Fig. 1.13 ). Both active and passive transport processes are enhanced if the area of the plasma membrane is increased by folds or projections of the cell surface, as exemplified by the absorptive cells lining the small intestine ( Fig. 1.2 ).
M mitochondrion P peroxisome R ribosomes RB residual body rER rough endoplasmic reticulum sER smooth endoplasmic reticulum
The main mechanisms by which materials and information are transported across cell membranes are:
Passive diffusion . This type of transport is dependent on the presence of a concentration gradient across the membrane and also on the size and polarity of the molecule. Lipids and lipid-soluble molecules such as the hormones oestrogen and testosterone pass freely through lipid membranes, as do gases such as oxygen, nitrogen and carbon dioxide. Uncharged but polar small molecules such as water and urea diffuse through lipid membranes slowly, but charged molecules such as sodium (Na + ) and potassium (K + ) ions diffuse through very slowly indeed.
Facilitated diffusion . This type of transport involves the movement of hydrophilic molecules such as water, ions, glucose and amino acids. This process is strictly passive, moving polar or charged substances along an electrochemical gradient, but requires the presence of protein carrier molecules. There are two types of protein carrier molecule: the first type (known as pores or channels ) form a water-filled channel across the membrane through which selected molecules or ions can pass depending on concentration, size and electrical charge, while the second type binds a particular molecule or ion and then undergoes a change in conformation, moving the substrate to the other side of the membrane (a transporter or carrier ). Aquaporins, which allow water to cross membranes at a much faster rate than by diffusion alone, are an important and common example of a transmembrane channel. There are many different aquaporin molecules, some of which are highly specific for water molecules, while others allow the passage of other small molecules such as urea or glycerol. Some facilitated diffusion pores are gated , which means that the pore is open or closed depending on different physiological conditions (e.g. open only at a particular pH).
Active transport . This mode of transport is not only independent of electrochemical gradients, but also often operates against extreme electrochemical gradients. The classic example of active transport is the continuous movement of Na + out of the cell and K + into the cell by the Na + -K + pump, which moves Na + ions out of the cell and K + ions into the cell across the plasma membrane. Adenosine triphosphate (ATP) is converted to adenosine diphosphate (ADP) in the process to generate the energy required, hence the name Na + -K + ATPase .
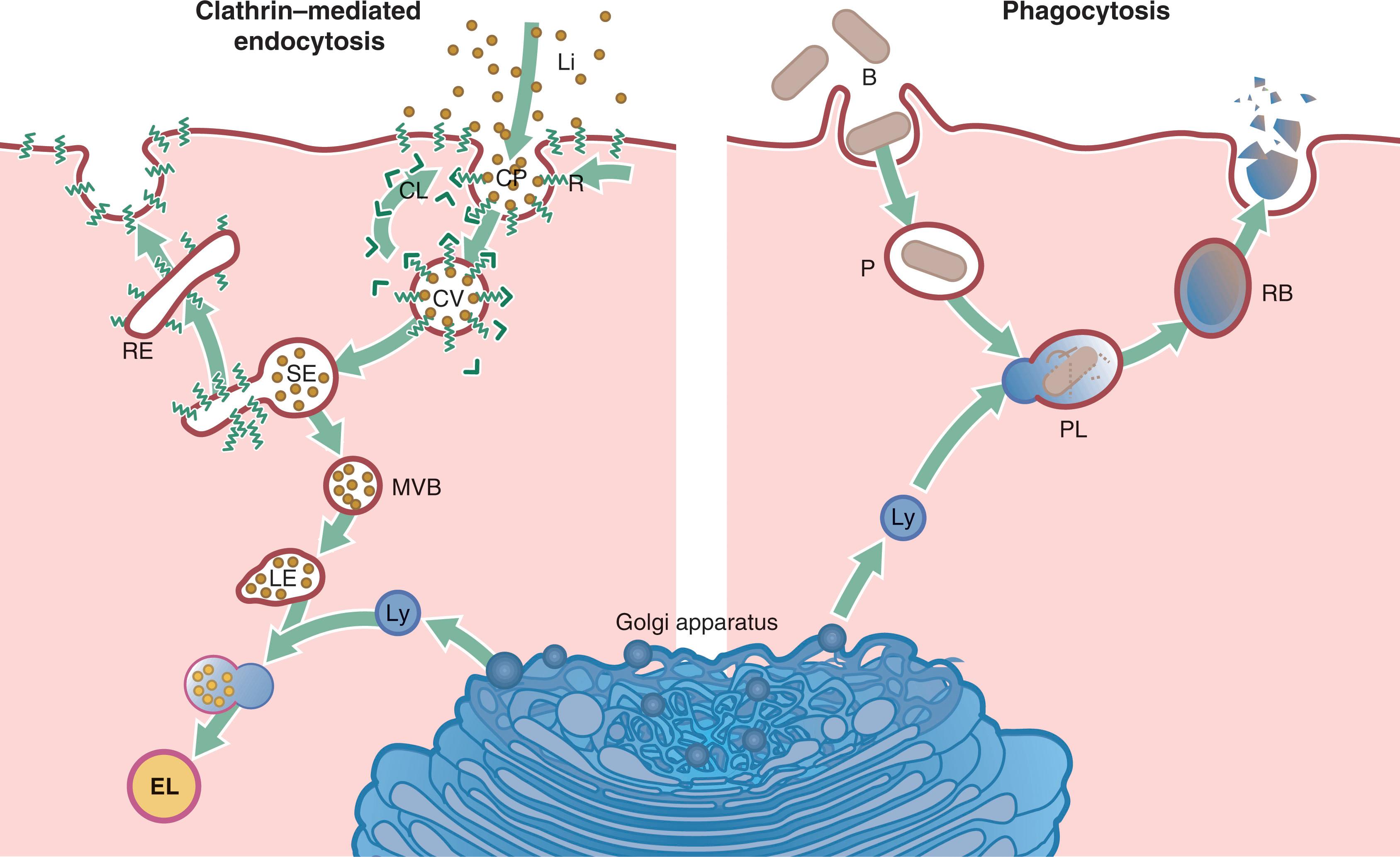
Bulk transport . Transport of large molecules or small particles into, out of or between compartments within the cell is mediated by subcellular, transient membrane-bound vesicles. These vesicles transport proteins embedded in the membrane of the vesicle (e.g. proteins destined for the plasma membrane) and soluble cargo within the lumen. Transport vesicles are formed by the assembly of a protein ‘coat,’ leading to budding of a section of membrane which is pinched off to form a vesicle. At their destination, the reverse process takes place when the transport vesicle fuses with the target membrane, incorporating into it and releasing its contents. These mechanisms are dependent on the fluidity and deformability of lipid membranes and the mobility of intrinsic membrane proteins within the plane of the membrane. Specific examples such as endocytosis, exocytosis and intracellular transport vesicles are given in Figs 1.10–1.13 .
Transmembrane signalling . There are various ways in which signals may cross a plasma membrane to deliver information to a cell. One example is lipid-soluble molecules, such as the hormone oestrogen, which diffuse through the plasma membrane to bind to an intracellular receptor. Non–lipid soluble molecules, such as the hormone insulin, bind to a protein receptor embedded in the plasma membrane, which is thus activated, and pass the signal on to an intracellular signalling pathway. Other signalling molecules, such as neurotransmitters at nerve synapses (see Ch. 7 ), bind to an ion channel in the postsynaptic membrane, allowing ions to enter the cell and initiating depolarisation of the membrane.
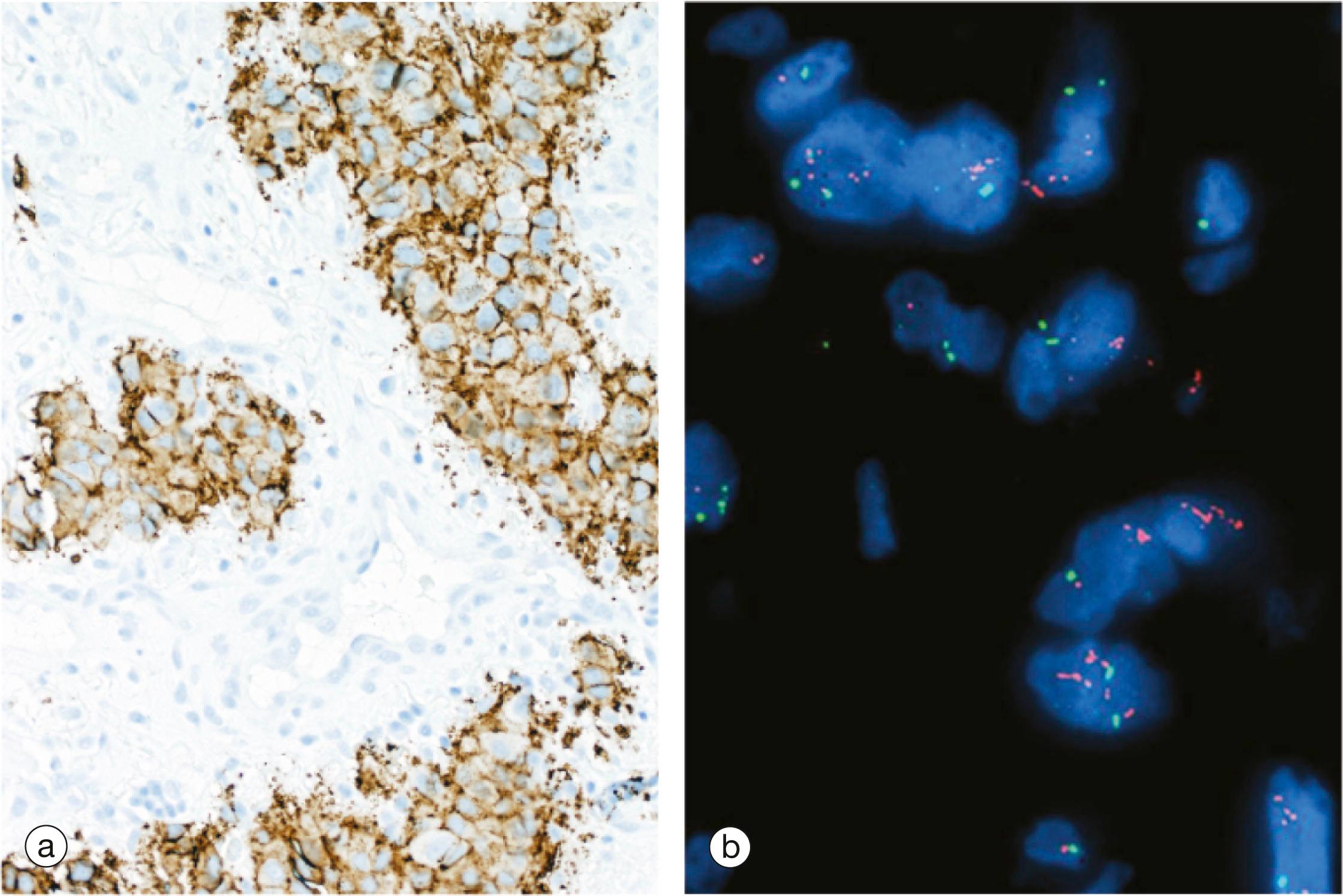
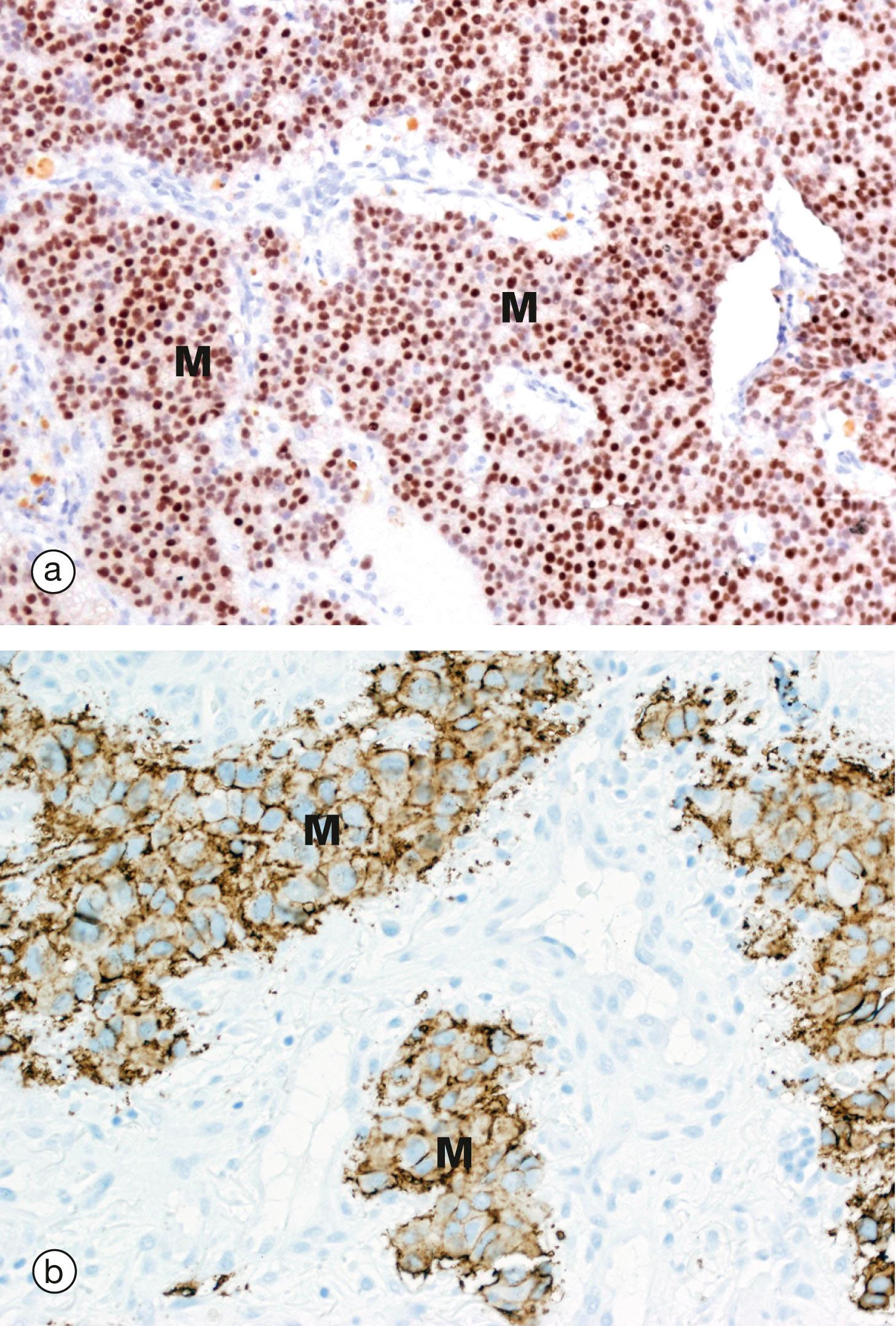
Drugs that modify membrane receptors can be used in the treatment of disease. One example of this is the use of drugs such as trastuzumab in the treatment of some breast cancers. Normal breast epithelium expresses a signalling molecule called human epidermal growth factor type 2 ( Her2 , also known as Her2/neu or ErbB-2 ) on the plasma membrane. Her2 (along with Her1, Her3 and Her4) regulate growth and survival of normal breast epithelium cells. Her2 is a transmembrane protein with three functional domains: an extracellular receptor component, a hydrophobic transmembrane component and an intracellular enzyme, a tyrosine kinase, that passes on the received signal within the cell. When a ligand binds to Her2 it passes a signal to the cell to divide and also promotes longer survival of the cell.
In approximately 20% to 30% of breast cancers there is gene amplification of the HER2 gene, i.e. there are more than the normal number of copies of the gene in the nucleus. This results in more than the normal number of molecules of Her2 at the cell surface, i.e. overexpression , which seems to be one of the mechanisms whereby the cells undergo uncontrolled growth and survival to form a cancer. The excess Her2 expression can be detected by various techniques ( Fig. 1.9 ). Patients with cancers that overexpress Her2 are treated with drugs such as trastuzumab, a monoclonal antibody that binds to the extracellular domain of Her2 and blocks its activation. Thus the effects of Her2 overexpression by the tumour cells are blocked and the patient survives for longer.
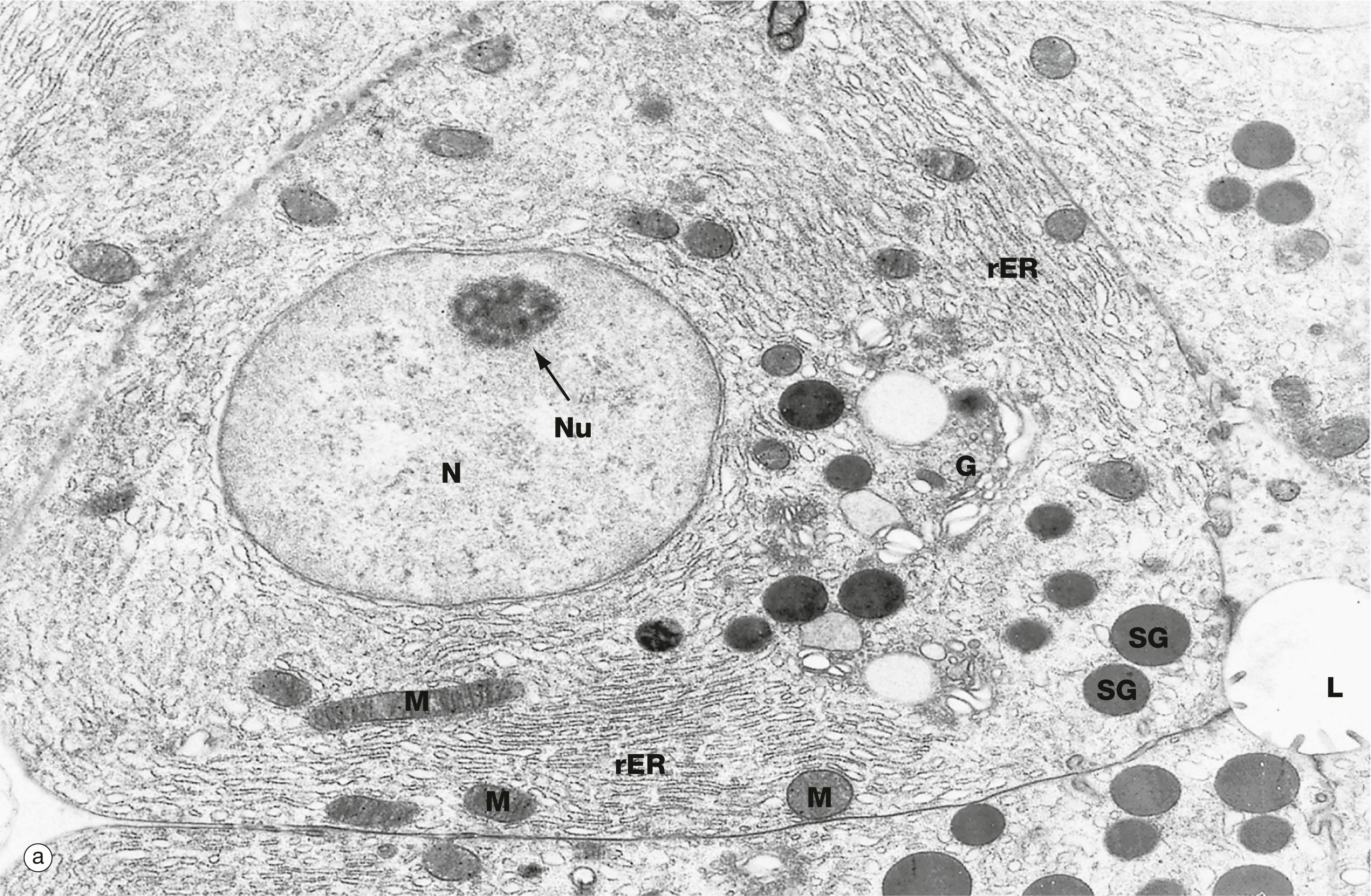
C Golgi cisternae D central duct G Golgi apparatus N nucleus NM nuclear membrane rER rough endoplasmic reticulum SG secretory granules T transfer vesicles V vesicles
B bacterium CL clathrin CP coated pit CV coated vesicle EL endolysosome G Golgi apparatus JC junctional complex L gland lumen LE late endosome Li ligand Ly lysosome M mitochondrion MV microvilli MVB multivesicular body N nucleus Nu nucleolus P phagosome PL phagolysosome R receptor RB residual body RE recycling endosome rER rough endoplasmic reticulum SE sorting endosome SG secretory granules
Phagocytosis is a vital component of the innate immune system (see Ch. 11 ). Phagocytosis of bacteria in most cases results in bacterial cell death with lysis of the dead organisms within a phagolysosome. However, some pathogenic organisms have learned to use the phagocytic mechanism to their own advantage to gain entry to the cell and grow there in a protected environment, safe from other elements of the immune system. For instance, Mycobacterium tuberculosis , the agent responsible for the important worldwide infection tuberculosis, is able to grow and divide within macrophages.
M. tuberculosis does this by preventing the phagosome from fusing with a lysosome and thus lives and divides safely within the phagosome. Listeria monocytogenes , a rare cause of food poisoning, is able to disrupt the phagosomal membrane and escape into the cell cytoplasm. Legionella pneumophila modifies the membrane of the phagosome so that it resembles ER and thus remains untouched. Some viruses, on the other hand, gain entry to the cell by receptor-mediated endocytosis. Both Poliovirus and Adenovirus use this mechanism, casting their protein coats inside the endosome and allowing their genome to escape into the cytoplasm.
B bacterium ER endoplasmic reticulum GC multinucleate giant cell Ly1 lysosomes Ly2 secondary or phagolysosome M mitochondrion MB multivesicular body N nucleus NE nuclear envelope Pp pseudopodia Ps phagosome rER rough endoplasmic reticulum S suture material
Every cell has a supporting framework of minute filaments and tubules, the cytoskeleton , which maintains the shape and polarity of the cell. Nevertheless, the cell membrane and intracellular organelles are not rigid or static structures but are in a constant state of movement to accommodate processes such as endocytosis, phagocytosis and secretion. Some cells (e.g. white blood cells) propel themselves about by amoeboid movement ; other cells have actively motile membrane specialisations such as cilia and flagella (see Ch. 5 ); while other cells (e.g. muscle cells) are highly specialised for contractility. In addition, cell division is a process that involves extensive reorganisation of cellular constituents. The cytoskeleton incorporates features that accommodate all these dynamic functions.
The cytoskeleton of each cell contains structural elements of three main types: microfilaments , microtubules and intermediate filaments . The cytoskeleton structures are made up of protein subunits ( monomers ) that are non-covalently bound together into filaments ( polymers ). Many accessory proteins link these structures to one another, to the plasma membrane and to the membranes of intracellular organelles. Other associated proteins are the motor proteins responsible for movement, the best known of which are the myosin , dynein and kinesin protein families.
Microfilaments . Microfilaments are extremely fine strands (5 to 9 nm in diameter) of the protein actin . Each actin filament (F-actin) consists of two protofilaments twisted together to form a helix. The protofilaments are made up of multiple globular actin monomers (G-actin) joined together head to tail and associated with ATP molecules to provide energy for contraction. The actin filament is then assembled into larger filaments, networks and 3-dimensional structures. Actin filaments are best demonstrated histologically in skeletal muscle cells where they form a stable arrangement of bundles with the motor protein myosin. Contraction occurs when the actin and myosin filaments slide relative to each other due to the rearrangement of intermolecular bonds, fuelled by the release of energy from associated ATP molecules (see Ch. 6 ). However, all eukaryotic cells contain a dynamic actin network. Beneath the plasma membrane, actin, in association with various transmembrane and linking proteins, forms a robust supporting meshwork called the cell cortex , which protects against deformation and yet can be rearranged to accommodate changes in cell morphology. Membrane specialisations such as microvilli (see Fig. 5.14 ) also contain a skeleton of actin filaments. Actin plays a central role in cell movement, pinocytosis and phagocytosis. Actin may also bind to intrinsic plasma membrane proteins to anchor them in position.
Intermediate filaments . Intermediate filaments (approximately 10 nm in diameter) are, as their name implies, intermediate in size between microfilaments and microtubules. These proteins have a purely structural function and consist of filaments that self-assemble into larger filaments and bind intracellular structures to each other and to plasma membrane proteins. In humans, there are more than 50 different types of intermediate filament, but these can be divided into different classes, with some classes characteristic of particular cell types. This feature is used in diagnostic pathology to identify different varieties of tumour. For example, the keratin (or cytokeratin ) intermediate filament family is characteristic of epithelial cells, where they form a supporting network within the cytoplasm and are anchored to the plasma membrane at intercellular junctions. Specific keratin types form hair and nails. Likewise, vimentin is found in cells of mesodermal origin, desmin in muscle cells, neurofilament proteins in nerve cells and glial fibrillary acidic protein in glial cells. Lamin intermediate filaments form a structural layer on the inner side of the nuclear membrane.
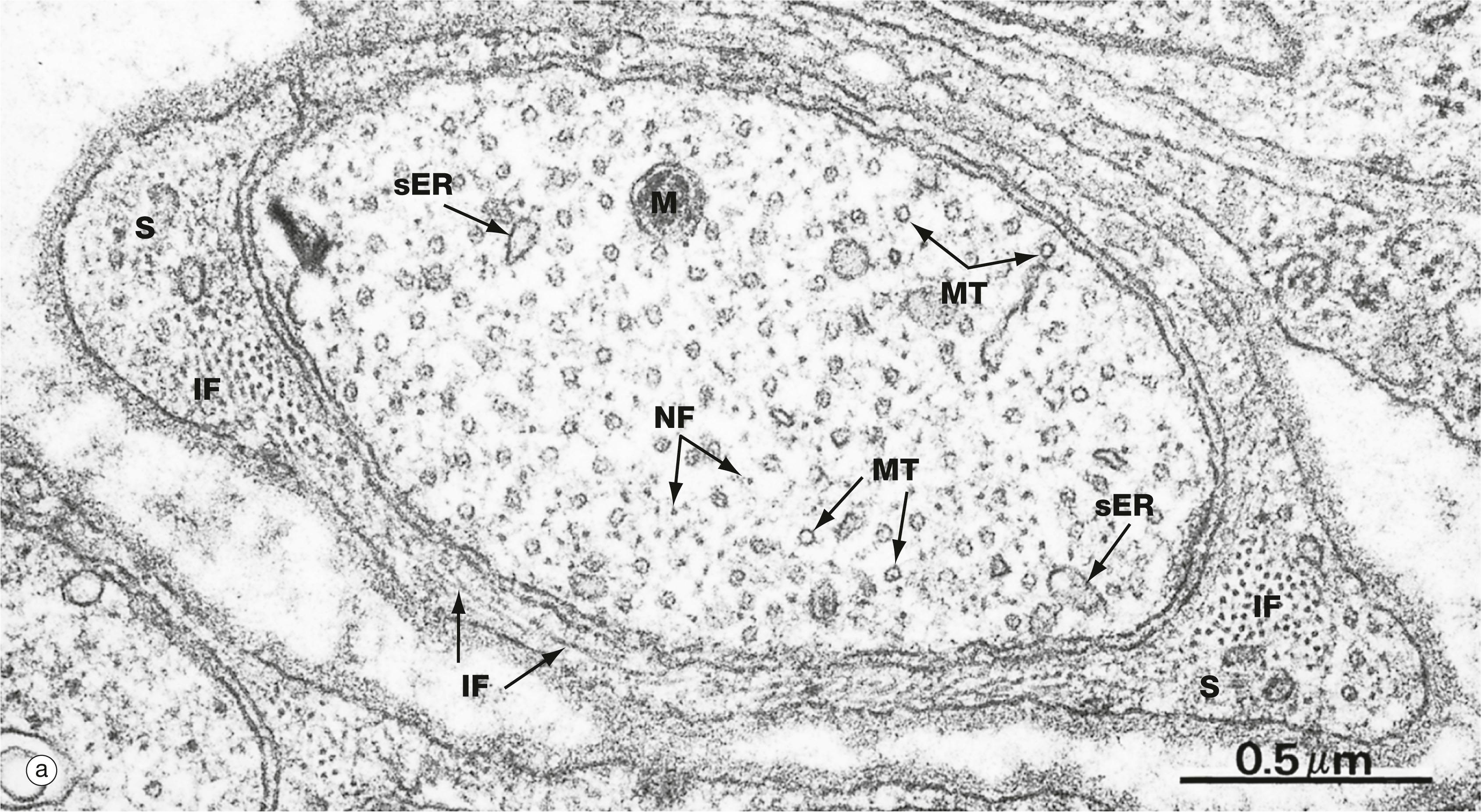
Microtubules . Microtubules (25 nm in diameter) are much larger than microfilaments but, like them, are made up of globular protein subunits which can readily be assembled and disassembled to provide for alterations in cell shape and position of organelles. The microtubule subunits are of two types, α- and β-tubulin, which polymerise to form a hollow tubule; when seen in cross-section, thirteen tubulin molecules make up a circle. Microtubules originate from a specialised microtubule organising centre , the centriole , found in the centrosome (see below), and movement may be effected by the addition or subtraction of tubulin subunits from the microtubules, making them longer or shorter. Microtubule-associated proteins (MAPs) stabilise the tubular structure and include capping proteins , which stabilise the growing ends of the tubules. The motor proteins dynein and kinesin move along the tubules towards and away from the cell centre, respectively. These motors attach to membranous organelles (e.g. mitochondria, secretory vesicles) and move them about within the cytoplasm, rather like an engine pulling cargo along a railway track. The function of the spindle during cell division is a classic example of this process on a large scale ( Fig. 2.3 ). The centrosome, consisting of a pair of centrioles, each of nine triplets of microtubules, organises the microtubules of the cell spindle during cell division ( Figs 1.17 and 1.18 ). In cilia, nine pairs of microtubules form a cylindrical structure and movement occurs by rearrangement of chemical bonds between adjacent microtubule pairs (see Fig. 5.13 ).
A number of blistering diseases of the skin are caused by abnormalities of the cytoskeleton. In the rare congenital disorder epidermolysis bullosa simplex, mutations have been found in the genes coding for cytokeratins 5 and 15. These intermediate filaments normally provide basal epidermal cells of the skin with resistance to friction and, in this condition, clumps of abnormal intermediate filaments can be seen within the cells. The result is a loss of cohesion between the basal epithelial cells and the underlying basement membrane, causing blister formation and fluid loss. Mutations in the gene for plectin, a cross-linking protein for intermediate filaments, give rise to various syndromes of epidermolysis bullosa and muscular dystrophy or epidermolysis bullosa and pyloric atresia.
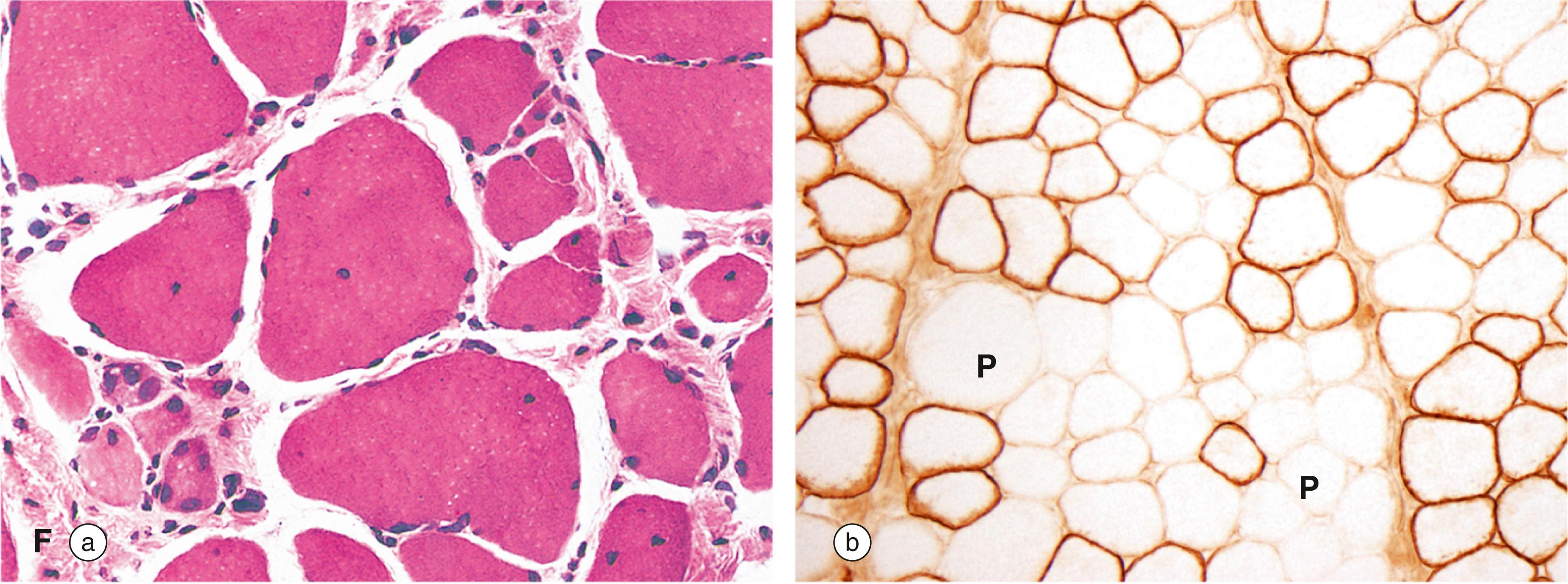
Cytokeratins are the main intermediate filaments present in epithelial tissues. There are many different keratin molecules, often identified by numbers, e.g. CK7, CK20, and these are typically expressed in specific tissues and organs. In diagnostic pathology, a broad stain covering many different cytokeratin molecules can be used to confirm that a poorly differentiated tumour is of epithelial origin and is a carcinoma . More specific cytokeratin stains can help in identifying the likely site of origin of a carcinoma. For example, cytokeratin 20 (CK20) is mainly found in glandular epithelium of the intestine whilst CK5 is associated with squamous epithelial cells.
Different intermediate filaments are typical of other tissue types and immunohistochemical staining for these molecules can be used to confirm tissue type. For example, desmin is found in muscle cells as part of the sarcomere (see Ch. 6 ) whilst neurofilaments are associated with neurones (see Ch. 7 ). Staining for these structural proteins is often helpful in diagnosing tumours, and in other inherited diseases such as muscular dystrophy, as described above (see textbox ‘Abnormalities of the cytoskeleton can produce life-threatening diseases’).
C crypt E epithelial cells G goblet cells LP lamina propria M mitochondrion MF microfilaments MM muscularis mucosae R ribosomes V villus
C centriole F filament G Golgi apparatus IF intermediate filament M mitochondrion MT microtubule N nucleus NF neurofilament S Schwann cell sER smooth endoplasmic reticulum T triplet
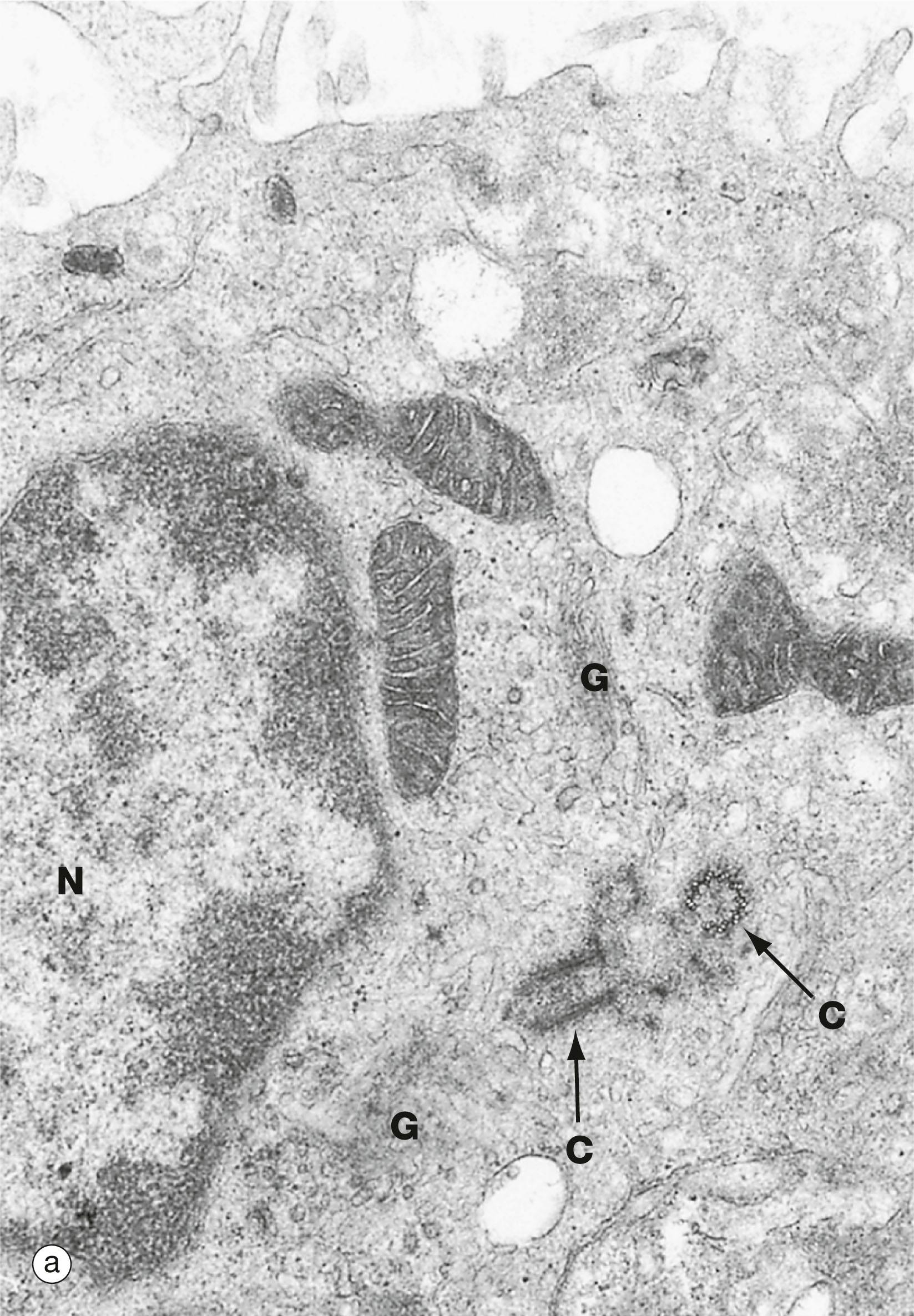
C centriole G Golgi complex M mitochondrion MT microtubules N nucleus rER rough endoplasmic reticulum
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here