Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An understanding of the molecular aspects of cell signaling is clinically relevant to uncover potential targets of novel therapeutics for the treatment of metabolic dysfunctions and diseases.
Signaling molecules can transmit information by acting as ligands , binding to receptors expressed by their target cells. Some signaling molecules can act on the cell surface after binding to cell surface receptors. Others can cross the plasma membrane and bind to intracellular receptors in the cytoplasm and nucleus.
Cell signaling and feedback action ( 3-1 and 3-2 ) Signaling molecules use different routes to reach their targets:
Endocrine cell signaling involves a signaling molecule, a hormone , secreted by an endocrine cell and transported through the circulation to act on distant target cells (see 3-1 ).
An example is the steroid hormone testosterone, produced in the testes, which stimulates the development and maintenance of the male reproductive tract using the vascular route.
Neuroendocrine cell signaling is a specific form of endocrine signaling involving a product secreted by a nerve cell into the bloodstream and acting on distant cells.
Paracrine cell signaling is mediated by a molecule acting locally to regulate the behavior of a nearby cell . A paracrine molecule diffuses over small distances to reach a target cell.
Neurotransmitter or synaptic cell signaling is a specialized form of paracrine signaling. Neurons secrete neurotransmitters that diffuse short distances and bind to receptors on target cells.
Juxtacrine cell signaling is contact-dependent signaling . It requires the contact of proteins of adjacent plasma membranes for signaling to occur.
An example is the immunologic synapse , a combination of cell-cell adhesion and signaling that takes place when the the corresponding receptors of an antigen-presenting cells and a T cell are in contact with each other.
Autocrine cell signaling is defined by cells responding to signaling molecules that they themselves produce .
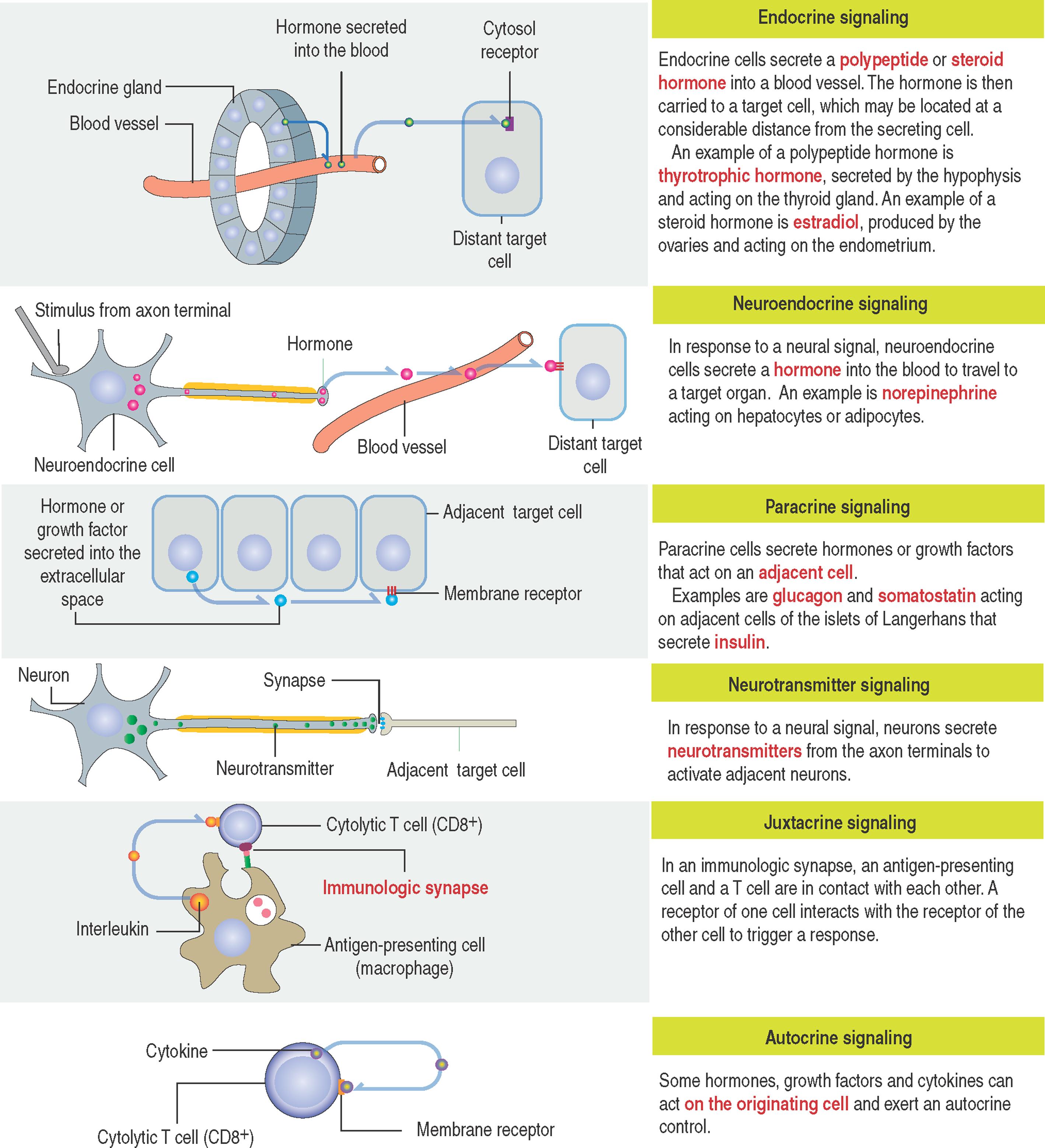
A classic example is the response of cells of the immune system to foreign antigens or growth factors that trigger their own proliferation and differentiation. Abnormal autocrine signaling leads to the unregulated growth of cancer cells.
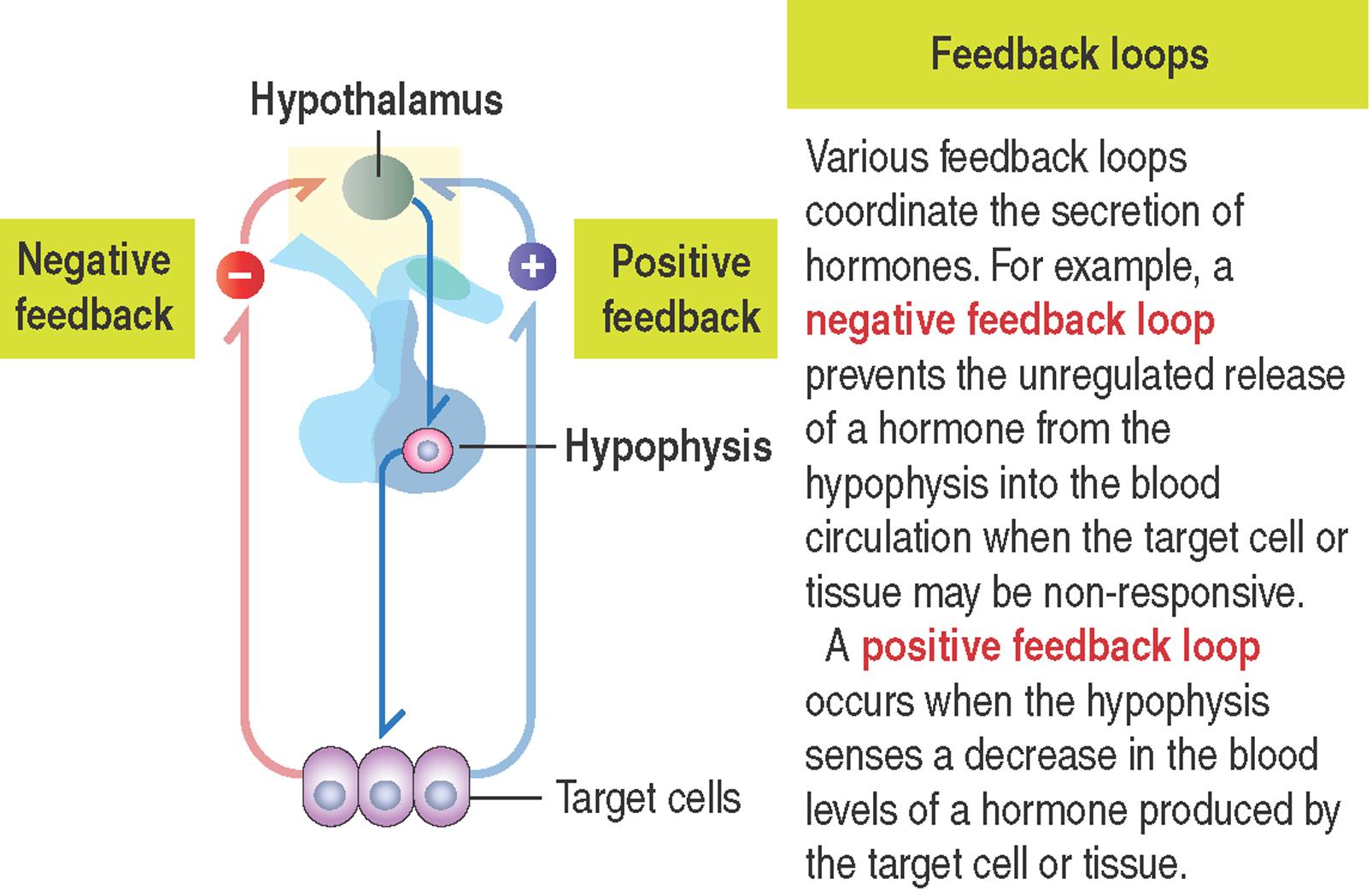
Cell signaling mechanisms require a feedback action. In general, after a signaling molecule binds to its receptor, the target cells exerts either a negative or positive feedback action to regulate the release of the targeting hormone (see 3-2 ).
Binding of a hormone or ligand to its receptor initiates a cascade of intracellular reactions (called s ignal transduction) to regulate critical functions such as embryonic and fetal development, cell proliferation and differentiation, movement, metabolism and behavior .
Hormones or ligands include:
Steroid hormones .
Peptide hormones, neuropeptides and growth factors .
Nitric oxide .
Neurotransmitters .
Eicosanoids .
Steroid hormones are lipid-soluble molecules that diffuse across the phospholipid bilayer of the plasma membrane of target cells, bind to intracellular receptors in the cytoplasm, enter the nucleus as steroid hormone-receptor complexes and bind to specific receptor sites on chromatin (specifically hormone-response elements at the DNA) to activate or repress gene expression. Steroid receptors are members of the steroid receptor superfamily .
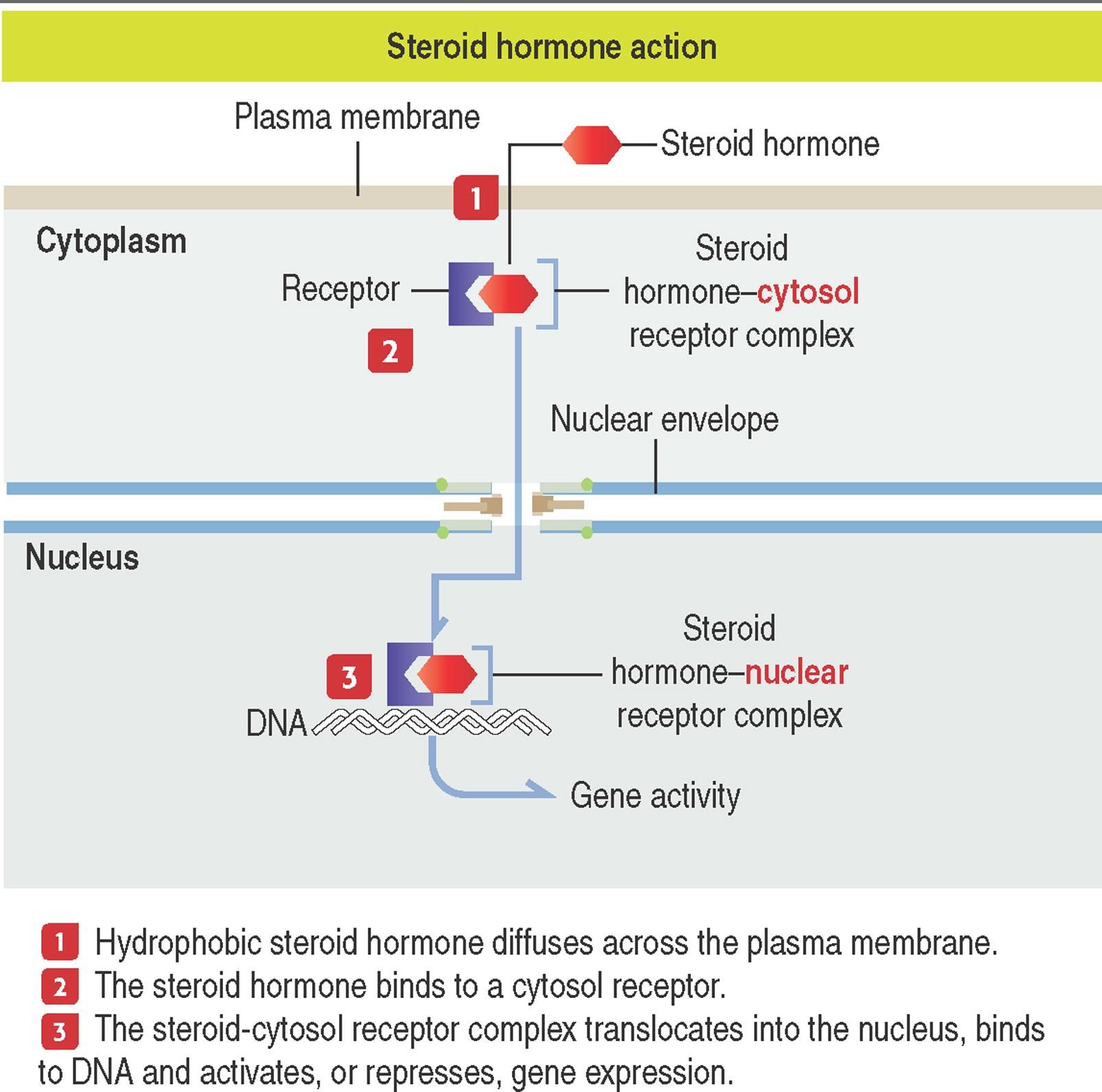
Steroid hormones are synthesized from cholesterol and include testosterone, estrogen, progesterone and corticosteroids (see Box 3-A ). Steroid hormones are usually secreted as they are synthesized and are transported in the bloodstream bound to protein carriers.
Steroid hormones derive from cholesterol and bind mainly to intracellular receptors in the cytosol and nucleus.
They circulate in blood bound to a protein , are non-polar molecules and are not stored in the producing endocrine cell .
Steroid hormones can be administered orally and are readily absorbed in the gastrointestinal tract.
Testosterone, estrogen and progesterone are sex steroids and are produced predominantly by the gonads. In the androgen insensitivity syndrome (also known as the testicular feminization syndrome, Tfm) , there is a mutation in the gene expressing the testosterone receptor such that the receptor cannot bind the hormone and hence the cells do not respond to the hormone. Although genetically male, the individual develops the secondary sexual characteristics of a female.
We discuss the androgen insensitivity syndrome in Chapter 21 , Sperm Transport and Maturation. We discuss their functional roles in Chapter 20 , Spermatogenesis and Chapter 22 , Folliculogenesis and Menstrual Cycle.
Corticosteroids are steroid hormones synthesized in the cortex of the adrenal gland and include two major classes: glucocorticoids , which stimulate the production of glucose and mineralocorticoids , which act on the kidneys to regulate water and salt balance.
We address structural and functional aspects of corticosteroids in Chapter 19 , Endocrine System.
There are types of cell signaling molecules structurally and functionally distinct from steroids that act on target cells by binding to intracellular receptors after entering the cell by diffusion across the plasma membrane. They include thyroid hormones (produced in the thyroid gland to regulate development and metabolism), vitamin D 3 (regulates calcium metabolism; see Chapter 19 , Endocrine System) and retinoids (synthesized from vitamin A to regulate wound healing and epidermal differentiation; see Chapter 11 , Integumentary System). The synthetic pathway of thyroid hormones and some of their actions are presented in Chapter 19 , Endocrine System.
Peptide hormones and growth factors bind to cell surface receptors. Unlike intracellular steroid receptors, membrane-bound receptors of peptide/protein ligands affect cellular function by transduction signaling (see Box 3-B ).
Peptide hormones . This group includes insulin, glucagon and hormones secreted by the hypophysis and peptides secreted by neurons (enkephalins and endorphins) , that decrease pain responses in the central nervous system. See Chapter 18 , Neuroendocrine System, and Chapter 19 , Endocrine System, for a detailed discussion of peptide hormones and neuropeptides.
Growth factors . This group of peptides controls cell growth and differentiation. It includes nerve growth factor (NGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and others that we discuss in several chapters.
Peptide hormones are synthesized as precursor molecules (prohormones), are stored in membrane-bound secretory vesicles and are generally water soluble (polar).
Peptide hormones circulate in blood as unbound molecules and cannot be administered orally .
Peptide hormones usually bind to cell surface receptors .
NGF is a member of a family of peptides called neurotrophins , which regulate the development and viability of neurons. EGF stimulates cell proliferation and is essential during embryonic development and in the adult. PDGF is stored in blood platelets and released during clotting.
Nitric oxide is a simple gas synthesized from the amino acid arginine by the enzyme nitric oxide synthase . It acts as a paracrine signaling molecule in the nervous, immune and circulatory systems.
Like steroid hormones, nitric oxide can diffuse across the plasma membrane of its target cells. Unlike steroids, nitric oxide does not bind to an intracellular receptor to regulate transcription. Instead, it regulates the activity of intracellular target enzymes .
The following are relevant characteristics of nitric oxide:
It is an unstable molecule with a limited half-life (seconds).
It has local effects.
A well-defined function of nitric oxide signaling is the dilation of blood vessels . For example, the release of the neurotransmitter acetylcholine from nerve cell endings in the blood vessel muscle cell wall stimulates the release of nitric oxide from endothelial cells.
Nitric oxide increases the activity of the second messenger cyclic guanosine monophosphate (cGMP) in smooth muscle cells, which then causes cell muscle relaxation and blood vessel dilation (see Chapter 21 , Sperm Transport and Maturation).
Nitroglycerin , a pharmacologic agent used in the treatment of heart disease, is converted to nitric oxide, which increases heart blood flow by dilation of the coronary blood vessels.
This interaction activates G protein, which then dissociates from the receptor and triggers an intracellular signal to an enzyme or ion channel. We return to G protein when we discuss the cyclic adenosine monophosphate (cAMP) pathway.
Tyrosine kinases are enzymes capable of transferring a phosphate group from adenosine triphosphate (ATP) to a protein in a cell.

There are two main classes of tyrosine kinases:
Receptor tyrosine kinases are transmembrane proteins with a ligand-binding extracellular domain and an intracellular kinase domain.
N on-receptor tyrosine kinases lack a transmembrane domain and are located in the cytosol, nucleus and inner side of the plasma membrane.
Receptor tyrosine kinases , in contrast with G protein–coupled receptors, are enzymes that phosphorylate substrate proteins on tyrosine residues. EGF, NGF, PDGF, insulin and several growth factors are receptor tyrosine kinases .
Most of the receptor tyrosine kinases consist of single polypeptides, although the insulin receptor and other growth factors consist of a pair of polypeptide chains.
Binding of a ligand (such as a growth factor) to the extracellular domain of a receptor tyrosine kinase induces receptor dimerization which results in receptor autophosphorylation (the two polypeptide chains phosphorylate one another).
The autophosphorylation of the receptors determines the binding of downstream signaling molecules to the tyrosine kinase domain.
Downstream signaling molecules bind to phosphotyrosine residues through SH2 domains (for Src homology 2). Src (for sarcoma) is a gene present in the tumor-producing Rous sarcoma virus and encodes a protein that functions as a tyrosine kinase.
The subfamily of non-receptor tyrosine kinases includes the Src family , the Fujinami poultry sarcoma/feline sarcoma (Fps/Fes) and Fes-related (Fer) subfamily. They are found inside the cell (cytosol and nucleus).
How do receptor and non-receptor tyrosine kinases differ functionally from each other?
In the absence of a ligand, a receptor tyrosine kinase is unphosphorylated and remains monomeric, whereas a non-receptor tyrosine kinase is maintained in an inactive state by cellular inhibitor proteins. Activation occurs when the inhibitors are dissociated or are recruited to transmembrane receptors to trigger autophosphorylation. Tyrosine kinase activity terminates when tyrosine phosphatases hydrolyze tyrosyl phosphates or by induction of inhibitory molecules.
The activity of tyrosine kinases in cancer cells can be affected by unregulated autophosphorylation in the absence of a ligand, by disrupting autoregulation of the tyrosine kinase or by overexpression of receptor tyrosine kinase and/or its ligand.
Abnormal activation of tyrosine kinases can stimulate the proliferation and anticancer drug resistance of malignant cells.
Tyrosine kinase activity can be inhibited by imatinib mesylate , a molecule that binds to the ATP–binding domain of the tyrosine kinase catalytic domain.
Imatinib can induce hematologic remission in patients with chronic myeloid leukemia and tumors caused by activated receptor tyrosine kinase PDGF receptor (chronic myelomonocytic leukemia) and c-kit (systemic mastocytosis and mast cell leukemias) . Imatinib has been successfully used in the treatment of gastrointestinal solid tumors.
Neurotransmitters are released by neurons and act on cell surface receptors present in neurons or other type of target cells (such as muscle cells).
This group includes acetylcholine, dopamine, epinephrine (adrenaline), serotonin, histamine, glutamate and γ -aminobutyric acid (GABA).
The release of neurotransmitters from neurons is triggered by an action potential . Released neurotransmitters diffuse across the synaptic cleft and bind to surface receptors on the target cells.
There are differences that distinguish the mechanism of action of neurotransmitters . For example, acetylcholine is a ligand-gated ion channel . It induces a change in conformation of ion channels to control ion flow across the plasma membrane in target cells.
Neurotransmitter receptors can be associated with G proteins (see section on G protein–coupled receptors), a class of signaling molecules linking cell surface receptors to intracellular responses.
Some neurotransmitters have a dual function . For example, epinephrine (noradrenaline; produced in the medulla of the adrenal gland) can act as a neurotransmitter and as a hormone to induce the breakdown of glycogen in muscle cells.
Eicosanoids (Greek eikos , twenty; compounds containing 20 carbon atoms) are lipid-containing inflammatory mediators produced in leukocytes and other cells of the immune system that, in contrast to steroids, bind to cell surface receptors (see Box 3-C ).
Eicosanoids derive from polyunsaturated fatty acids with 18, 20 and 22 carbons. Arachidonic acid is the main precursor.
This group includes prostaglandins, leukotrienes, thromboxanes and prostacyclin .
Eicosanoids have primary autocrine and paracrine actions and their synthesis is regulated by hormones .
Eicosanoids usually bind to cell surface receptors .
Prostaglandins, prostacyclin, thromboxanes and leukotrienes are members of this group of molecules. They stimulate blood platelet aggregation, inflammatory responses and smooth muscle contraction.
Leukotrienes (Greek leukos , white; Chemistry triene , a compound containing three double bonds) are synthesized by the oxidation of arachidonic acid by the enzyme arachidonate lipoxygenase.
During the synthesis of prostaglandins, arachidonic acid is converted to prostaglandin H 2 by the enzyme prostaglandin synthase . This enzyme is inhibited by aspirin (acetylsalicylate) and anti-inflammatory drugs. Inhibition of prostaglandin synthase by aspirin reduces pain, inflammation, platelet aggregation and blood clotting (prevention of strokes).
Most peptide hormones and growth factors bind to receptors on the surface of target cells.
Ligand binding to hormone and growth factor receptors activates a series of intracellular targets located downstream of the receptor , in particular the activity of intracellular proteins, or, like neurotransmitter receptors, the control of the flow of water (aquaporins) and electrolytes across ligand-gated ion channels located on the plasma membrane.
Let us now consider several functional aspects of specific cell surface receptors:
G protein–coupled receptors.
Receptor and non-receptor tyrosine kinases.
Cytokine receptors.
Tyrosine phosphatases and serine-threonine kinases.
Members of a large family of G proteins (more than 1000 guanine nucleotide–binding proteins) are present at the inner leaflet of the plasma membrane.

When a signaling molecule or receptor ligand binds to the extracellular portion of a cell surface receptor, its cytosolic domain undergoes a conformational change that enables binding of the receptor to the G protein complex .
Cytokine receptors are cell-surface glycoproteins that lack tyrosine kinase activity but depend on associated cytoplasmic tyrosine kinases known as Janus kinases (JAKs) to mediate changes in gene expression following binding to specific cytokine ligands. All cytokine receptors are associated with one or more members of the JAK-STAT pathway .
The functional principles are the following: Upon ligand binding, the cytokine receptors oligomerize and allow the associated JAKs to cross-phosphorylate, become activated and phosphorylate the cytokine receptors. This event creates a docking site for the SH2 domain present in members of the signal transducers and activators of transcription (STAT ) family of transcription factors.
Cytokines and cytokine receptors regulate hematopoiesis, immune responses, inflammation and tissue healing through the JAK-STAT pathway which represents a potential therapeutic target. We discuss below additional details of the JAK-STAT pathway.
The family of cytokine receptors consists of several subfamilies classified on their differing structure and activities. They include:
Type I cytokine receptors (to which interleukin ligands bind) and type II cytokine receptors (mainly for interferon ligands).
What happens when a cytokine receptor does not work as expected? Hyperactivation mutations of the type I cytokine receptor signaling pathway are associated with myeloproliferative diseases and other hematologic defects. Abnormal activation of type I cytokine receptor correlates with leukemias and lymphomas . Defective type II cytokine receptor signaling can be associated with immune deficiencies and inflammatory conditions.
Chemokine receptors and chemokine ligands (designated CC, CXC, CX3C and CXCR1; where the spacing between cysteins [C] determines the bound type of chemokine ligand).
What is the difference between a cytokine and a chemokine? A cytokine elicits a direct biological function. A chemokine is a type of cytokine that has a chemoattractant function (for example, it recruits cells to sites of infection or inflammation).
Chemokine ligands are 8 kd to 14 kd in size. Binding of chemokine ligands to chemokine receptors induce chemotaxis (cell migration during homing) of target inflammatory cells. Migrating cells are attracted to sites with higher concentrations of chemokines (concentration gradient). We discuss homing and inflammation in Chapter 6 , Blood and Hematopoiesis.
The tumor necrosis factor receptor (TNRF) superfamily (known as death receptors) belongs to the cytokine receptor group.
The receptors and ligands (TNFL) of this family participate in signaling pathways for cell proliferation, survival and differentiation. TNFR/TNFL participate in chronic inflammatory conditions such as rheumatoid arthritis (see Chapter 5 , Osteogenesis) and inflammatory bowel disease (see Chapter 16 , Lower Digestive Segment).
TNFR are active as self-assembling non-covalent trimers. The cytoplasmic domain of TNFR is the docking site of signaling molecules, such as the cytoplasmic adaptor protein TRAF (TNF receptor–associated factors) and Dead Domain (DD).
From a functional perspective, adaptor proteins allow the regulatory flexibility of the dead receptors. As we discuss in the Apoptosis section of this chapter, the Fas receptor has a DD that binds to the Fas-associated DD (FADD) protein adapter which ultimately recruits and activates caspase 8 to cause cell death.
RANKL (for transmembrane receptor for activation of nuclear factor kappa B ligand) is a member of the TNF superfamily with binding affinity to RANK receptor.
It has a significant tole in the development of osteoclasts from monocyte precursors (see Chapter 4 , Connective Tissue). RANK/RANKL signaling regulates the differentiation of mammary gland alveolar buds into tubulo-alveolar structures in preparation for lactation.
Transforming growth factor-β (TGF-β) type I and type II serine/threonine kinase receptors . The transforming growth factor-β (TGF-β) superfamily of cytokines consists of TGF-βs, activins, inhibins, bone morphogenetic proteins (BMPs) and anti-müllerian hormone.
TGF-β is secreted by several cell types that participates in cell growth, cell differentiation and apoptosis after binding to its receptors.
Three TGF-β ligand isoforms (TGF-β1, TGF-β2 and TGF-β3) with similar biological activities have been identified in mammals.
The ligands share a receptor complex and signal in similar ways, but their expression levels vary depending on the target tissue.
Binding of the ligand to the TGF-β receptor induces first receptor dimerization and then formation of a heteromeric complex and a change in the conformation of the intracellular serine/threonine kinase domains of the receptors.
This event facilitates the phosphorylation and activation of multiple signaling pathways downstream from the receptor, including the SMAD transcription factors and cell cycle proteins.
SMADs translocate to the nucleus, where they interact with other transcription factors (cofactors) to regulate transcriptional responses.
So far, we have seen that some receptors have enzymatic activity to stimulate protein phosphorylation at tyrosine residues. However, receptors can associate with proteins that have phosphatase enzymatic activities to remove phosphate groups from phosphotyrosine residues. Therefore, they regulate the effect of tyrosine kinases by arresting signals initiated by tyrosine phosphorylation .
Upon ligand binding, most cell surface receptors stimulate intracellular target enzymes t o transmit and amplify a signal . An amplified signal can be propagated to the nucleus to regulate gene expression in response to an external cell stimulus.
The major intracellular signaling pathways include:
The cAMP pathway .
The cGMP pathway .
The phospholipids–Ca 2+ pathway .
The Ca 2+ -calmodulin pathway .
The Ras (for rat sarcoma virus), Raf (for rapidly accelerated fibrosarcoma) and MAP (for mitogen-activated protein) kinase pathway .
The JAK-STAT (for Janus kinase–signal transducers and activators of transcription) pathway .
The NF-κB (for nuclear factor involved in the transcription of the κlight chain gene in B lymphocytes) transcription factor pathway .
The integrin-actin pathway .
The intracellular signaling pathway mediated by cAMP was discovered in 1958 by Earl Sutherland while studying the action of epinephrine , a hormone that breaks down glycogen into glucose before muscle contraction.

When epinephrine binds to its receptor, there is an increase in the intracellular concentration of cAMP. cAMP is formed from adenosine triphosphate (ATP) by the action of the enzyme adenylyl cyclase and degraded to adenosine monophosphate (AMP) by the enzyme cAMP phosphodiesterase .
This mechanism led to the concept of a first messenger (epinephrine) mediating a cell-signaling effect by a second messenger , cAMP. The epinephrine receptor is linked to adenylyl cyclase by G protein, which stimulates cyclase activity upon epinephrine binding.
The intracellular signaling effects of cAMP are mediated by the enzyme cAMP-dependent protein kinase (or protein kinase A). In its inactive form, protein kinase A is a tetramer composed of two regulatory subunits (to which cAMP binds) and two catalytic subunits . Binding of cAMP results in the dissociation of the catalytic subunits . Free catalytic subunits can phosphorylate serine residues on target proteins.
In the epinephrine-dependent regulation of glycogen metabolism, protein kinase A phosphorylates two enzymes:
Phosphorylase kinase , which in turn phosphorylates glycogen phosphorylase to break down glycogen into glucose-1-phosphate.
Glycogen synthase , which is involved in the synthesis of glycogen. Phosphorylation of glycogen synthase prevents the synthesis of glycogen.
Note that an elevation of cAMP results in two distinct events: the breakdown of glycogen and, at the same time, a blockage of further glycogen synthesis .
Also note that the binding of epinephrine to a single receptor leads to a signal amplification mechanism during intracellular signaling mediated by many molecules of cAMP.
cAMP signal amplification is further enhanced by the phosphorylation of many molecules of phosphorylase kinase and glycogen synthase by the catalytic subunits dissociated from protein kinase A. It is important to note that protein phosphorylation can be rapidly reversed by protein phosphatases present in the cytosol and as transmembrane proteins. These protein phosphatases can terminate responses initiated by the activation of kinases by removing phosphorylated residues.
cAMP also has an effect on the transcription of specific target genes that contain a regulatory sequence called the cAMP response element (CRE) . Catalytic subunits of protein kinase A enter the nucleus after dissociation from the regulatory subunits. Within the nucleus, catalytic subunits phosphorylate a transcription factor called CRE-binding protein (CREB) , which activates cAMP-inducible genes.
Finally, cAMP effects can be direct, independent of protein phosphorylation. An example is the direct regulation of ion channels in the olfactory epithelium .
Odorant receptors in sensory neurons of the olfactory epithelium are linked to G protein, which stimulates adenylyl cyclase to increase intracellular cAMP (see Chapter 13 , Respiratory System).
cAMP does not stimulate protein kinase A in sensory neurons but acts directly to open Na + channels in the plasma membrane to initiate membrane depolarization and nerve impulses.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here