Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
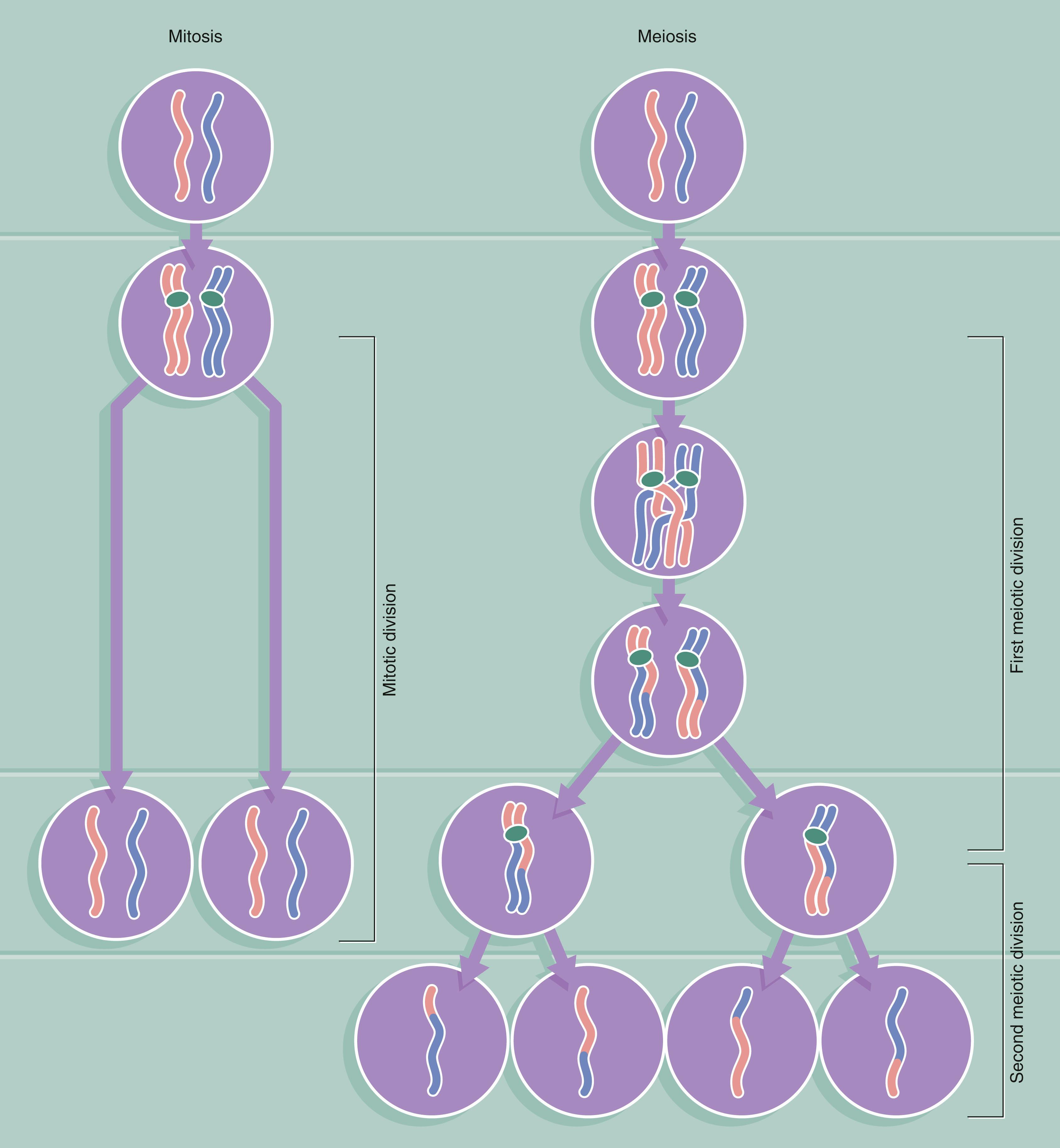
The development of a single fertilised egg cell to form a complex multicellular organism involves cellular replication, growth and progressive specialisation ( differentiation ) for a variety of functions. The fertilised egg ( zygote ) divides by a process known as mitosis to produce two genetically identical daughter cells, each of which divides to produce two more daughter cells and so on. Some of these daughter cells progressively specialise and eventually produce the terminally differentiated cells of mature tissues, such as muscle or skin cells. Most tissues, however, retain a population of relatively undifferentiated cells ( stem cells ) that are able to divide and replace the differentiated cell population as required. The interval between mitotic divisions is known as the cell cycle . All body cells divide by mitosis except for male and female germ cells, which divide by meiosis to produce gametes ( Fig. 2.6 ).
In the fully developed organism, the terminally differentiated cells of some tissues, such as the neurones of the nervous system, lose the ability to undergo mitosis. In contrast, the cells of certain other tissues, such as the stem cells of gut and skin, undergo continuous cycles of mitotic division throughout the lifespan of the organism, replacing cells lost during normal wear and tear. Between these extremes are cells such as liver cells that do not normally divide but retain the capacity to undergo mitosis should the need arise ( facultative dividers ). Cell division is tightly controlled to meet the needs of the organism; uncontrolled cell division is one of the features of cancer.
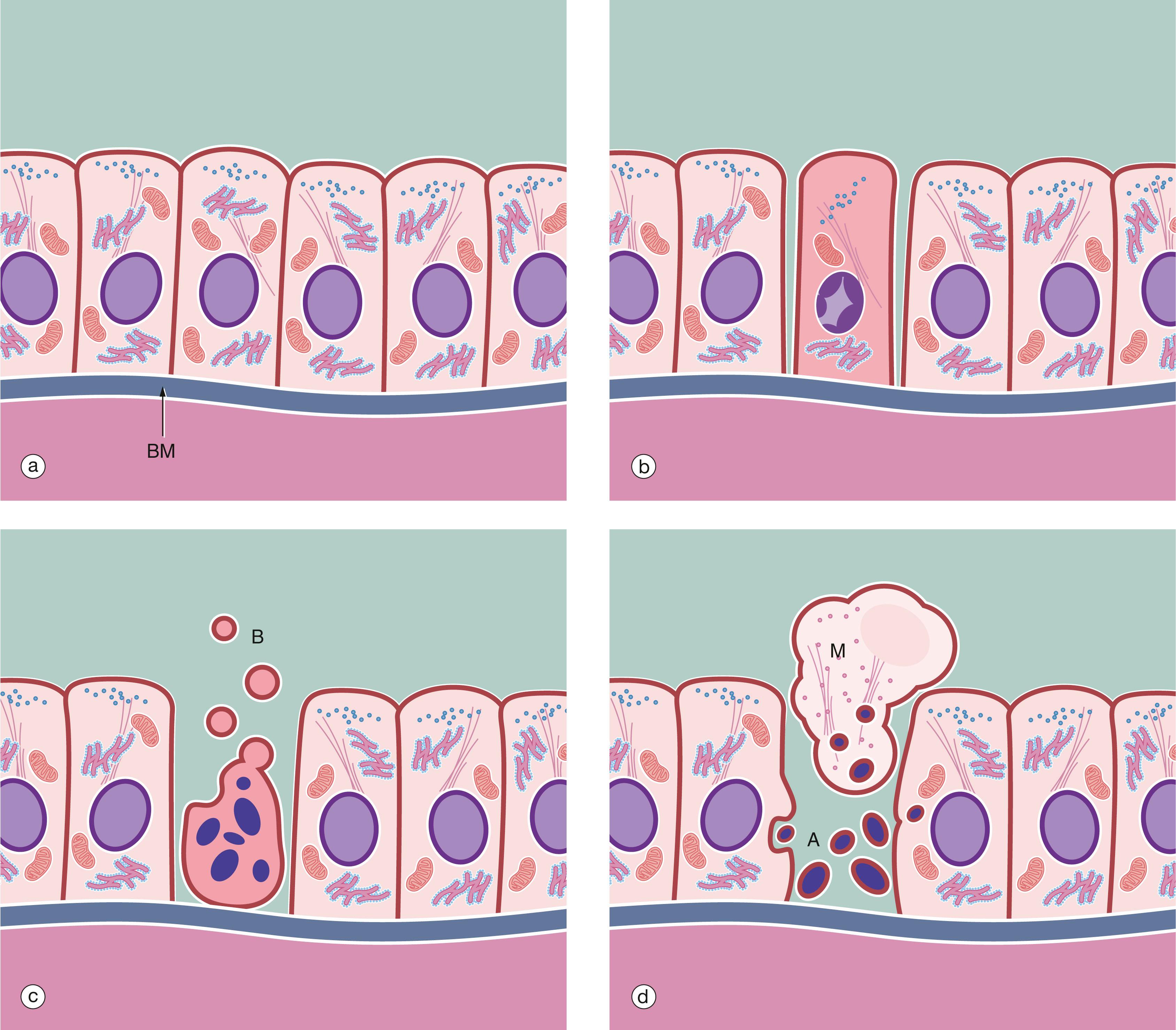
Cell division and differentiation are balanced by cell death, both during the development and growth of the immature organism and in the mature adult. In these circumstances, cell death occurs by a mechanism known as apoptosis ( Fig. 2.7 ).
CP checkpoint G 0 continuous differentiated function G 1 gap phase 1 G 2 gap phase 2 M mitotic phase S synthesis phase
Cell cycle checkpoints are critical in regulating the process of cell division and involve a series of processes which aim to avoid incomplete or damaged DNA being passed on to daughter cells. This involves various regulatory proteins, mainly cyclin-dependent kinases, which control progression through the cell cycle. There are 3 main checkpoints, occurring at G 1 /S , G 2 /M and in metaphase . The G 1 /S checkpoint is known as the restriction point and indicates the point at which cells are committed to entering cell cycle, after ensuring the raw materials are available to allow DNA replication. Disruption of this signalling pathway may cause cells to enter cycle inappropriately and this dysregulation can give rise to uncontrolled cell division and cancer . The G 2 /M checkpoint involves checking for DNA damage and effecting repair if necessary. If DNA damage is not repaired before entering M phase, apoptosis can be triggered. The metaphase checkpoint is known as the spindle checkpoint and aims to ensure equal distribution of chromosomes between the two daughter cells. Failures in these checkpoints can lead to accumulation of damaged DNA and uncontrolled cellular proliferation, as occurs in cancer. Some evolving cancer therapies aim to target cyclin-dependent kinase pathways in order to disrupt cell division and inhibit tumour growth.
Division of somatic cells (all body cells except for the germ cells ) occurs in two phases. Firstly, the chromosomes duplicated in S phase are distributed equally between the two potential daughter cells; this process is known as mitosis . Secondly, the dividing cell is cleaved into genetically identical daughter cells by cytoplasmic division or cytokinesis .
Although mitosis is always equal and symmetrical, cytokinesis may, in some situations, result in the formation of two daughter cells with grossly unequal amounts of cytoplasm or cytoplasmic organelles. In other circumstances, mitosis may occur in the absence of cytokinesis, as in the formation of binucleate and multinucleate cells.
In tissues with a regular turnover of cells, the dividing cells are relatively undifferentiated cells and are known as stem cells . Some of the progeny of these cells undergo further cell division and finally differentiate to become the various types of mature cells, while others remain undifferentiated to maintain the pool of stem cells.
Stem cell research is currently a very controversial area. Embryonic stem cells are theoretically totipotent , i.e. able to differentiate into any other cell type, while many stem cells found in adults are either multipotent , able to produce cells of several lineages, or unipotent , producing only a single cell type. Thus multipotent haematopoietic stem cells can produce all the formed elements of the blood, whereas unipotent epidermal stem cells of the skin can produce only epithelial cells. Recent research has hinted that, under certain circumstances, haematopoietic stem cells can produce other cell types. The advantages of such cells in the treatment of degenerative diseases are obvious – imagine being able to grow a new kidney or even a new limb to order. However, the ethical minefield created by the possible applications of such technology, especially the use of embryonic cells, is equally apparent.
A adenine C cytosine G guanine P phosphate S deoxyribose T thymine
The complex processes of meiotic cell division sometimes malfunction. It is unknown how many fetuses are conceived each year with genetic abnormalities so serious that the condition is lethal in utero and results in an early, perhaps even unrecognised, miscarriage. Most cytogenetic defects are thought to be lethal in this way and only a very few such as trisomy 21 (i.e. three copies of chromosome 21, also known as Down syndrome), trisomy 18 (Edward syndrome) and trisomy 13 (Patau syndrome) give rise to live births. In the two latter conditions, the infant usually dies within the first year, but individuals with Down syndrome may live to middle age or longer.
Trisomies arise usually due to failure of the homologous chromosome pairs to separate during the first meiotic division ( nondisjunction ) or failure of the two sister chromatids to separate during the second meiotic division ( anaphase lag ). Thus the resulting gamete has two copies of the relevant chromosome and, when fertilisation occurs, a third copy is added. In most cases, the extra chromosome is from the mother and there is a greatly increased incidence of Down syndrome with increasing maternal age, although the exact mechanism is as yet unclear.
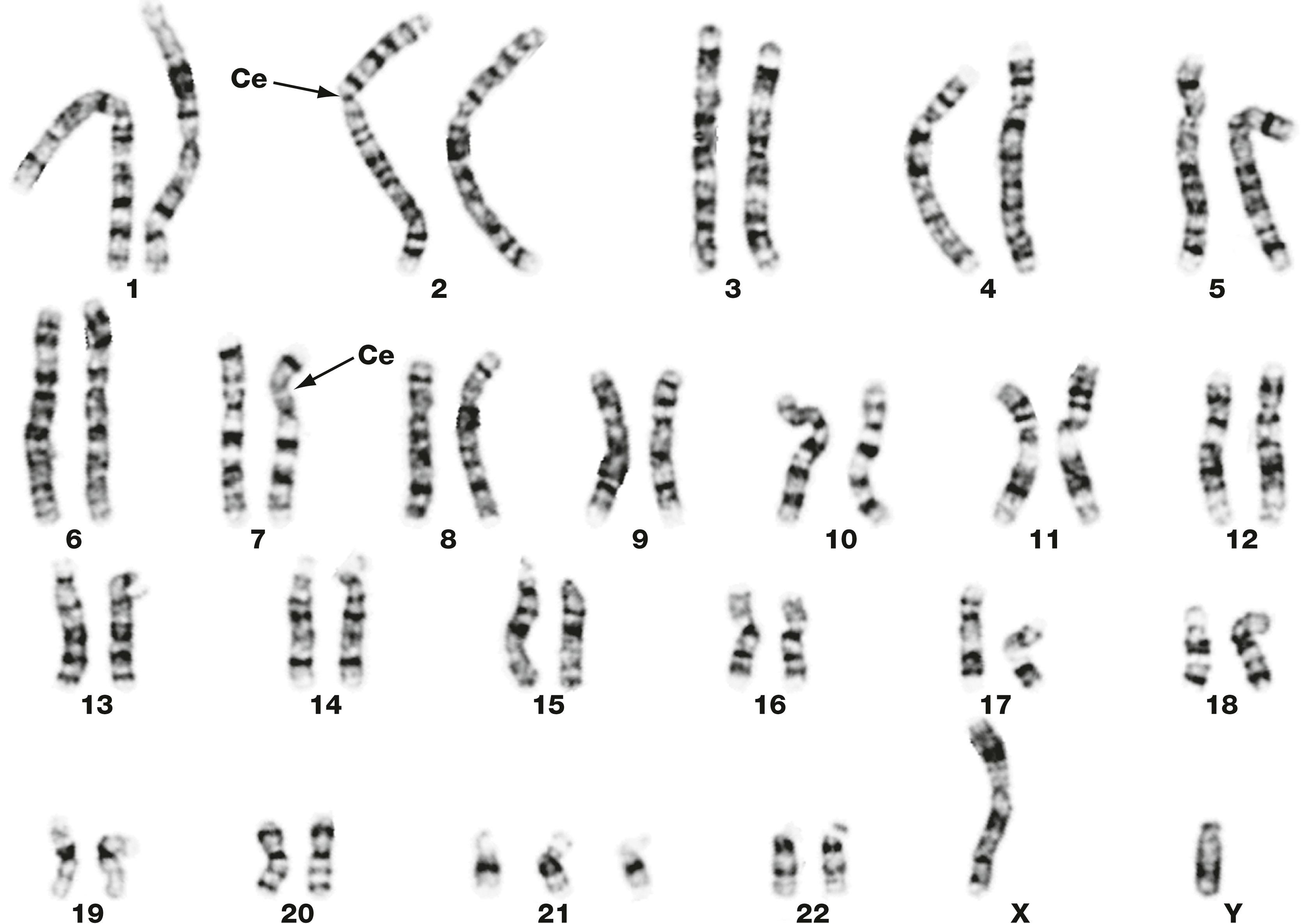
Other types of chromosomal abnormalities that may be detected by karyotyping include translocation , both balanced and unbalanced, abnormal chromosome structures, such as ring chromosomes , and amplification or deletion of parts of a chromosome.
Karyotyping of fetal cells is widely available to detect cytogenetic abnormalities by the techniques of amniocentesis or chorionic villous sampling . These are invasive diagnostic tests which present some fetal and maternal risks.
More recently, novel techniques have been developed to allow non invasive prenatal testing for certain genetic conditions, including fetal trisomies. Cell free fetal DNA is detectable in maternal plasma during pregnancy and these DNA fragments, obtained from a simple blood test in the mother, can be amplified, sequenced and then mapped to the human genome to detect fetal genetic abnormalities. In Down syndrome, genetic material from chromosome 21 is over-represented.
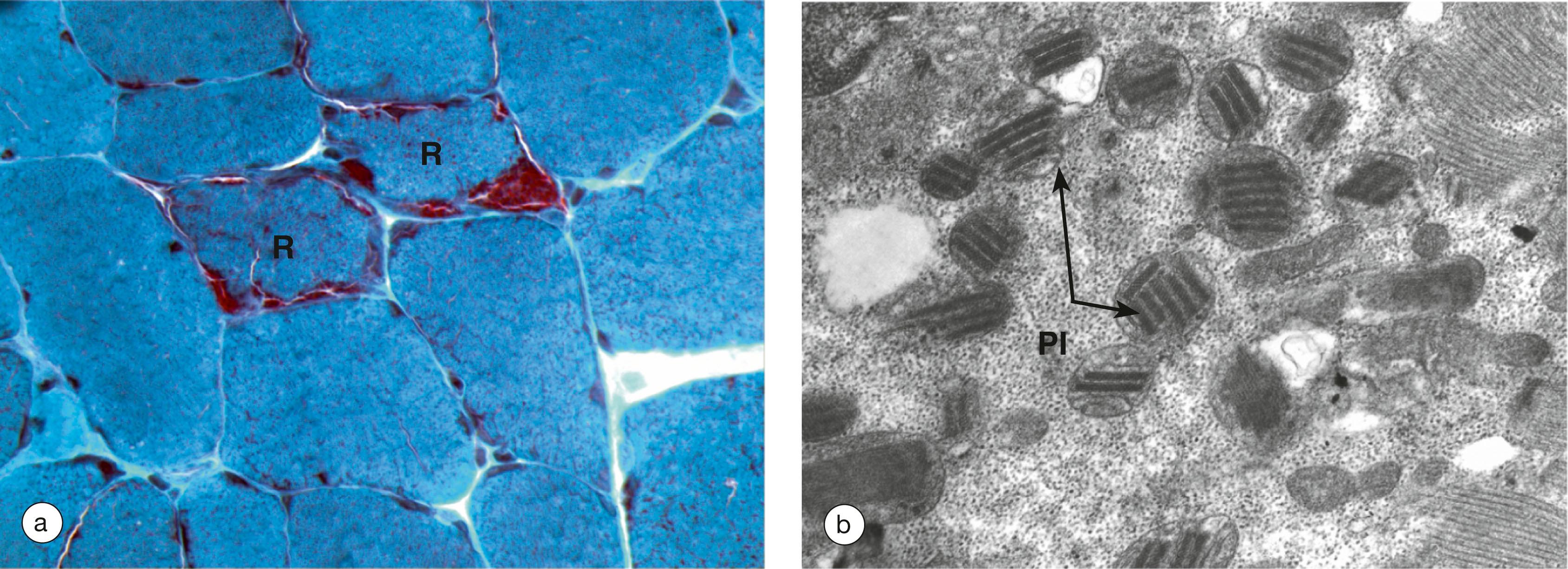
A late anaphase C chromatin Ce centromere G gland M mitochondrion MP metaphase plate MT microtubules P prometaphase PM plasma membrane R ribosomes S stroma sER smooth endoplasmic reticulum
In dividing somatic cells, cell division (mitosis) results in the formation of two daughter cells, each one genetically identical to the mother cell. Somatic cells contain a full complement of chromosomes (the diploid number ) which function as homologous pairs, one member of each pair deriving from the father and one from the mother. The process of sexual reproduction involves the production by meiosis of specialised male and female cells called gametes ; meiotic cell division is thus also called gametogenesis . Each gamete contains the haploid number of chromosomes (23 in humans), i.e. one from each homologous pair. When the male and female gametes fuse at fertilisation to form a zygote , the diploid number of chromosomes (46 in humans) is restored. The member of each chromosome pair assigned to a particular gamete is entirely random, so that a particular gamete contains a mix of chromosomes from the mother and father of the individual forming the gamete. This mixing of chromosomes contributes to the genetic diversity of the next generation. Further genetic diversity, and therefore possible evolutionary advantage, is added by the mechanism of crossing over (see below). The process of meiosis involves many of the mechanisms and control systems that regulate mitosis. The process of meiosis is outlined below and compared to mitosis in Fig. 2.6 opposite.
Before meiosis can begin, the chromosomes are duplicated as for mitosis ( meiotic S phase ).
This is immediately followed by crossing over of the chromatids, so that genetic information is exchanged between the two chromosomes of the homologous pair. As one of each pair of chromosomes is derived from the father and one from the mother, crossing over mixes up these paternally and maternally derived alleles (alternative forms of the same gene) so that the haploid gamete ends up with only one of each chromosome pair, but each individual chromosome includes alleles from each parent ( Fig. 2.5 ). The mechanism of crossing over is called chiasma formation . This phase is known as prophase I . As well as generating great genetic diversity, this mixing up of genes explains the conviction of many teenagers that they must be adopted!
The first meiotic division then proceeds, involving separation of the homologous pairs of chromosomes, each one consisting of a pair of chromatids still joined together at the centromere ( metaphase I and anaphase I ). This separation of chromosomes is carried out by a microtubules spindle, as in mitosis. Thus at the end of the first meiotic division, each daughter cell contains a half complement of duplicated chromosomes, one from each homologous pair of chromosomes.
The second meiotic division involves splitting of the chromatids by pulling apart the centromeres ( metaphase II ). The chromatids then migrate to opposite poles of the spindle ( anaphase II, telophase II ).
Thus, meiotic cell division of a single diploid germ cell gives rise to four haploid gametes. In the male, each of the four gametes undergoes morphological development into a mature spermatozoon . In the female, unequal distribution of the cytoplasm results in one gamete gaining almost all the cytoplasm from the mother cell, while the other three acquire almost none; the large gamete matures to form an ovum and the other three, called polar bodies , degenerate.
During both the first and second meiotic divisions, the cell passes through stages that have many similar features to prophase, metaphase, anaphase and telophase of mitosis. Unlike mitosis, however, the process of meiotic cell division can be suspended for a considerable length of time. For example, in the development of the human female gamete, the germ cells enter prophase of the first meiotic division during the fifth month of fetal life and then remain suspended until some time after sexual maturity; the first meiotic division is thus suspended for between 12 and 50 years!
The primitive germ cells of the male, the spermatogonia , are present only in small numbers in the male gonads before sexual maturity. After this, spermatogonia multiply continuously by mitosis to provide a supply of cells which then undergo meiosis to form male gametes. In contrast, the germ cells of the female, called oogonia , multiply by mitosis only during early fetal development, thereby producing a fixed complement of cells with the potential to undergo gametogenesis.
Chromosomes are not the only source of genes in the germ cells. Mitochondria also contain DNA that codes for some intrinsic mitochondrial proteins required for energy production. Because the spermatozoa shed their mitochondria at the time of fertilisation, only maternal mitochondrial genes are passed on to the offspring. A number of inherited diseases are known to be transmitted through mitochondrial DNA (see textbox).
Mitochondria include circular stands of DNA which encode some of the mitochondrial proteins involved in cellular energy production. Mutations in these genes can cause serious illnesses. Mitochondrial DNA is maternally inherited because all of the cytoplasm of the fertilised cell is derived from the oocyte. As a result, these diseases do not follow typical Mendelian inheritance patterns and their clinical features vary widely depending upon the proportion of abnormal mitochondria in particular tissues, as well as the energy requirements of those tissues.
Most commonly, mitochondrial diseases present with features such as muscle weakness, loss of co-ordination, poor growth or a range of neurological symptoms, including autonomic dysfunction, visual and hearing problems. Examples of some mitochondrial diseases include mitochondrial myopathies , Leber’s hereditary optic neuropathy and MERRF syndrome (myoclonic epilepsy with ragged red fibres) . The age of onset of symptoms varies greatly and these conditions may be difficult to diagnose.
Muscle biopsy is often used in diagnosis and typically reveals the presence of so-called ‘ragged red fibres’, muscle cells with clumps of diseased mitochondria beneath the sarcolemma . It is worth noting that some forms of mitochondrial myopathy occur due to mutations in mitochondrial proteins which are encoded by nuclear DNA and these disorders show more conventional patterns of inheritance.
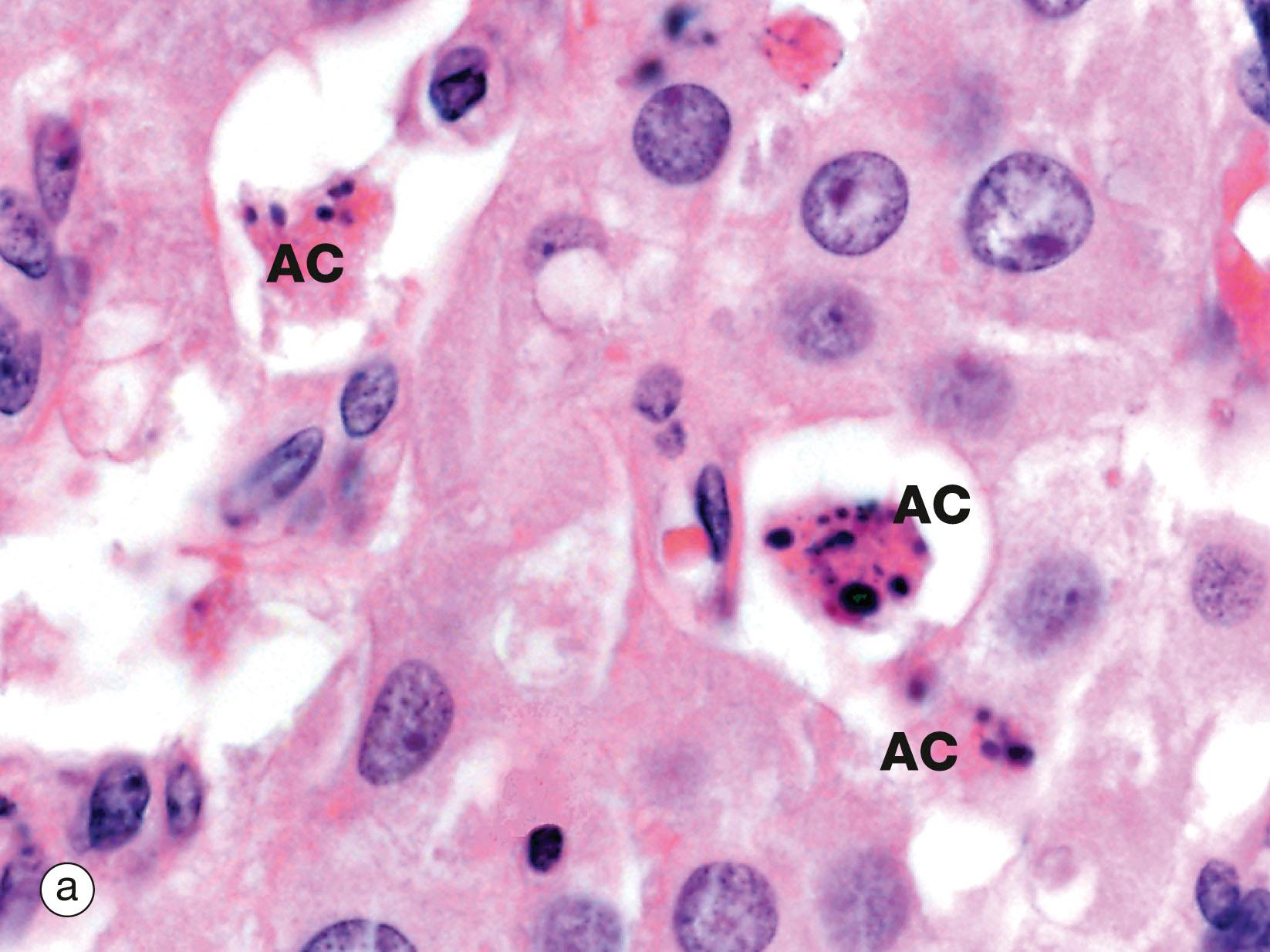
Programmed cell death is an essential part of normal fetal development, growth of juveniles and control of cell numbers in adults, where it exactly balances cell division. The most common and best understood method of programmed cell death is apoptosis , which also occurs in certain pathological conditions. Apoptosis has characteristic microscopic features and is a highly controlled and ordered mechanism that removes cells in a way that causes minimal disruption to the surrounding tissue. Apoptosis is brought about by different mechanisms than those causing necrosis , a mode of cell and tissue death that occurs only in pathological conditions. A well-known example of necrosis is myocardial infarction, where heart muscle dies from lack of oxygen due to blockage of a coronary artery. Apoptosis is an active process requiring the expenditure of energy, while necrosis is characterised by the inability of cells to produce the energy (ATP) required to maintain homeostasis.
Control of apoptosis is very finely balanced and a wide variety of triggers may initiate the process. The signal for apoptosis may be the binding of an external signal molecule to a membrane receptor (the ‘death receptor’ known as Fas) or may arise from intracellular signals such as DNA damage, leading to the release of the enzyme cytochrome c from the mitochondria into the cytoplasm. Within the cell, many regulatory proteins control apoptosis, including members of the bcl-2 and inhibitors of apoptosis (IAP) families. The end result of all these various signals is a common mechanism known as the caspase cascade . The activated enzymes of the caspase cascade cleave cellular proteins such as the lamins of the nucleus and activate additional enzymes such as DNAase to cleave DNA.
Examples of apoptosis include:
Some cell types have a preset limited lifetime and inevitably undergo apoptosis as part of their life cycle (e.g. epithelial cells in the skin or the lining of the gastrointestinal tract).
Other cells are triggered to destroy themselves if they behave in ways which are potentially harmful; for example, developing T lymphocytes that are capable of reacting to normal body components are triggered to self-destruct in the thymus, a process known as clonal deletion (see Ch. 11 ). Failure of clonal deletion may lead to autoimmune disorders such as autoimmune thyroiditis or pernicious anaemia.
During development certain cells are programmed to die by apoptosis; for example, in humans the webs between the fingers and toes disappear and the tadpole loses its tail as it matures into a frog.
Certain tissues in adults grow and regress in a cyclical fashion, such as the growth of ovarian follicles before ovulation in females, followed by regression of the corpus luteum by apoptosis to form a corpus albicans (see Ch. 19 ).
Apoptosis is a common mode of cell death of abnormal cells, such as those infected by viruses or with genetic mutations. Failure of apoptosis may be as important in cancer as unrestricted cell division.
A apoptotic bodies AC apoptotic cell B cytoplasmic blebs BM basement membrane M macrophage
Cancers or malignant tumours are common causes of illness and death in most societies and the diagnosis of cancer is an important part of the workload of diagnostic histopathology. Cancers cause disease by growing in an uncontrolled fashion within the body and replacing or destroying normal tissues. Importantly, as well as growing at their site of origin, cancers are able to metastasise or spread to other areas of the body such as the lungs or liver. Thus a primary cancer of the breast can spread to the lungs, causing death from respiratory failure, or a cancer of the prostate may replace the bone marrow, with consequent failure of production of white blood cells, causing death from overwhelming infection.
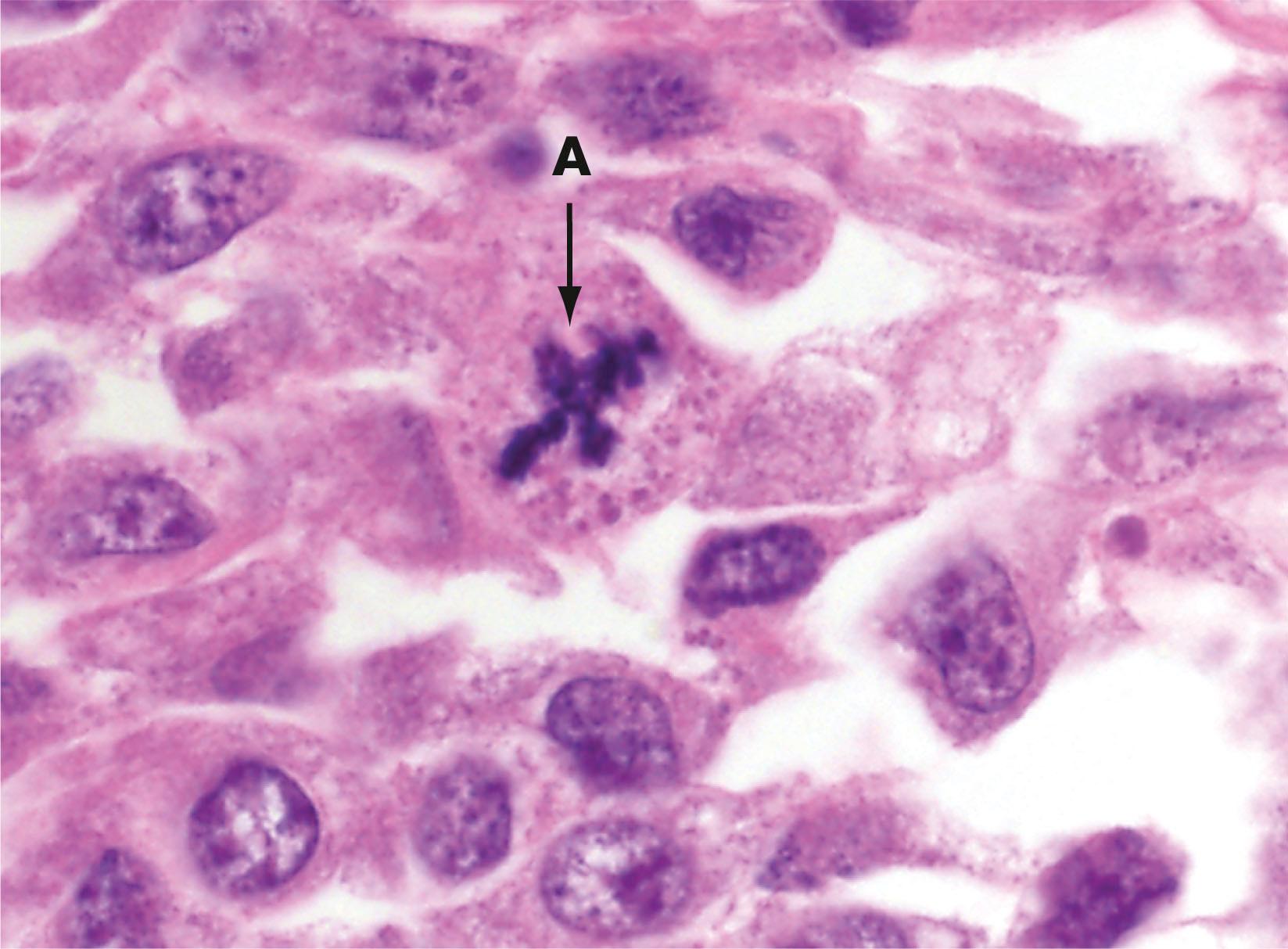
The many features of malignant tumours are well described in standard pathological texts, but one common feature is the abnormal mitotic figure ( Fig. 2.9 ), which is not seen in normal tissue but is frequently a feature of malignant tumours (and some premalignant conditions). Abnormal mitotic figures are a visually obvious example of the many genetic abnormalities that are found in cancer cells, where there is loss of control of cell division and of cell death (see Figs. 2.7 and 2.8 ). These genetic abnormalities or mutations may be caused by infections (e.g. Epstein–Barr virus), toxins (e.g. cigarette smoke), radiation (e.g. sunlight) or may be inherited. Intensive research in this area continues and more causes and mechanisms of cancer are uncovered almost daily.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here