Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiopulmonary interactions are crucial considerations in the intensive care management of critically ill neonates. The understanding of the impact of mechanical ventilation on the heart and lungs permits the prediction of a neonate’s response to the initiation or modification of positive pressure ventilation, or the effect of cardiovascular therapeutics. These interactions are guided by the laws of hydrodynamics as applied to a distensible or compressible structure. The thoracic cavity limits the space available for the heart and lungs. With this external constraint, changes in intrathoracic pressure (ITP) throughout the respiratory cycle may have varying effects on cardiac and respiratory function. Conversely, the serial nature of the pulmonary and systemic circulations implies that cardiac dysfunction may adversely impact vascular pressures in the lung and thereby deleteriously affect gas exchange. This chapter will illustrate core principles of cardiopulmonary interactions and their importance in the clinical management of critically ill neonates.
Systemic venous return occurs due to a pressure gradient between the systemic venous reservoirs (P venous , upstream pressure) and the right atrium (P RA , downstream pressure) such that
The venous system pressure (P venous ) is influenced by both intravascular volume and venous compliance. Low-pressure venous reservoirs in the hepatic, splanchnic, and splenic circulations contain most of the circulating blood volume, and healthy neonates have sufficient capacity to receive substantial increases in intravascular volume without appreciable increases in venous pressure. Venous compliance is dependent upon both capacitance and venomotor tone. Increased skeletal muscle contraction and systemic vascular resistance augment the venous tone.
Right atrial pressure is the major resistor to systemic venous return and is, under normal conditions, typically low. The P venous – P RA pressure gradient is augmented, facilitating improvement in systemic venous return, in the setting of higher peripheral venous pressure (e.g., intravascular volume administration or increased skeletal muscle activity) and lower right atrial pressure. Catecholamines mediate acute compensatory increases in systemic venous return by facilitating vascular smooth muscle contraction (which reduces venous capacitance and increases P venous ), and this may be induced by neurosympathetic activation or through exogenous catecholamine administration ( Table 4.1 ).
| Increase Systemic Venous Return | Decrease Systemic Venous Return |
|---|---|
|
|
During normal, spontaneous respiration, ventilation occurs through the generation of negative ITP. Diaphragm descent and rib cage expansion increase the chest cavity volume, creating negative pleural pressure and reducing ITP. The lungs expand to fill the chest cavity and the negative ITP is transmitted to the vena cava and right atrium, resulting in increased transmural RA pressure, RA expansion, and reduced P RA . Simultaneously, intraabdominal pressure increases due to diaphragm descent, resulting in decreased venous reservoir capacitance and an increase in P venous . , The combined increase in P venous and reduction in P RA increase the P venous – P RA pressure gradient, increasing systemic venous return, right heart preload, and right ventricular stroke volume.
The augmentation in systemic venous return and right heart preload associated with the reduction in P RA associated with negative pressure inspiration is limited by the collapsibility of the vena cava. While negative ITP and lung expansion result in reduced P RA , there is an associated reduction in transmural pressure (P TM ) of the intrathoracic great veins. Increasingly, negative ITP and consequently reduced P RA will result in increasing systemic venous return up until vena cava P TM becomes negative at the thoracic inlet. At this point, vena cava collapse occurs at the thoracic inlet, and further systemic venous return is determined by the difference between P venous and vena cava pressures at the thoracic inlet (intraabdominal pressure for inferior vena cava; atmospheric pressure for superior vena cava).
Altogether, under normal physiological conditions of spontaneous respirations, these cardiopulmonary interactions culminate in small but detectable and cyclical variations in ventricular stroke volume and systolic blood pressure during the respiratory cycle. During inspiration, the increase in RV preload alters the interventricular septal configuration and increases LV elastance, resulting in reduced LV preload and stroke volume and reduced systolic blood pressure. During expiration, this process is reversed and is associated with an increase in systolic blood pressure.
The administration of positive pressure ventilation, through increases in ITP, reverses the cardiopulmonary interactions that occur with spontaneous ventilation. Inspiration is associated with a reduction in right ventricular preload and an increase in systolic blood pressure ( Figure 4.1 ). While these breath-to-breath hemodynamic fluctuations that occur over the respiratory cycle illustrate the dynamic cardiopulmonary interactions that exist, they have limited clinical importance in the administration and titration of intensive care.
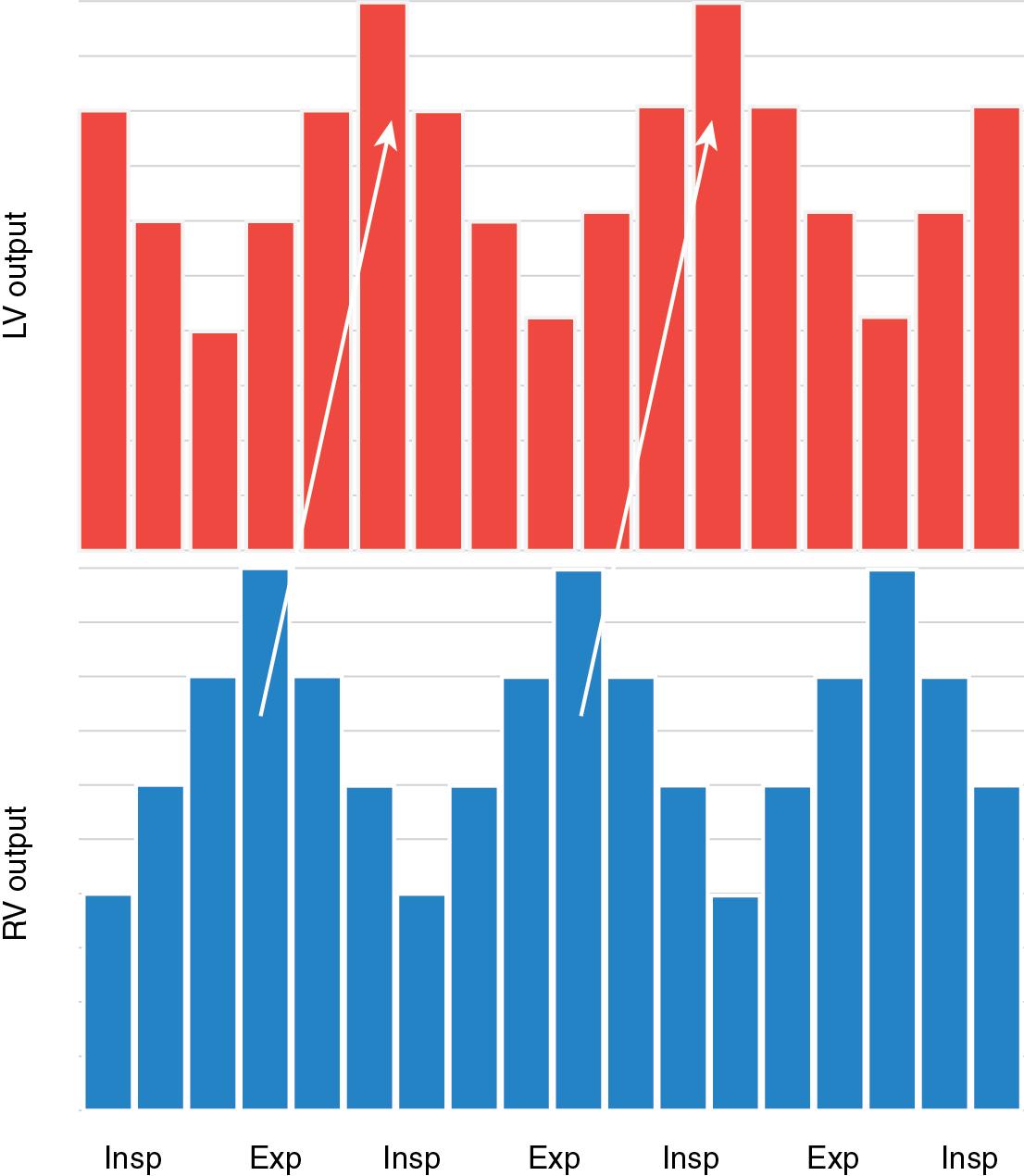
In contrast, sustained influences of positive pressure ventilation on the circulation have the potential to have a significant impact on cardiorespiratory stability, which necessitates consideration in clinical decision making. In contemporary practice, mechanical ventilation is universally administered with positive end-expiratory pressure (PEEP), resulting in an ITP that is higher than atmospheric pressure throughout the respiratory cycle. For mechanically ventilated neonates, the effect of ITP on systemic and pulmonary hemodynamics is mediated by lung volumes. During the administration of positive pressure ventilation, lung compliance is the predominant determinant of the lung volume attained for the given pressure administered. In the setting of excessive lung volumes (“overdistention”), the lungs exert positive pressure on adjacent structures, including the pleura and pericardium, resulting in an increase in intrapleural, pericardial, and ultimately intracardiac pressure. The transmission of positive pressure from the lungs to the heart results in increased pressure within the cardiac chambers, including P RA . Increased P RA decreases the P venous – P RA pressure gradient, thereby reducing systemic venous return. The reduction in systemic venous return reduces both right ventricular preload and stroke volume and, ultimately, left ventricular (LV) output. If P RA rises to the level of P venous , then systemic venous return ceases and both RV stroke volume and pulmonary blood flow fall precipitously, representing tamponade physiology. Mean airway pressure–induced reductions in cardiac output have been confirmed in preterm neonates.
However, compensatory mechanisms exist that mitigate the reduction in systemic venous return associated with increased ITP and P RA . Although increased ITP may increase P RA and reduce systemic venous return, the associated reduction in cardiac output elicits arterial baroreceptor–mediated neurosympathetic activation, which results in decreased venous capacitance and increased P venous through increased venomotor tone. The potential reduction in systemic venous return associated with an increase in ITP is thus partially mitigated ( Figure 4.2 ).
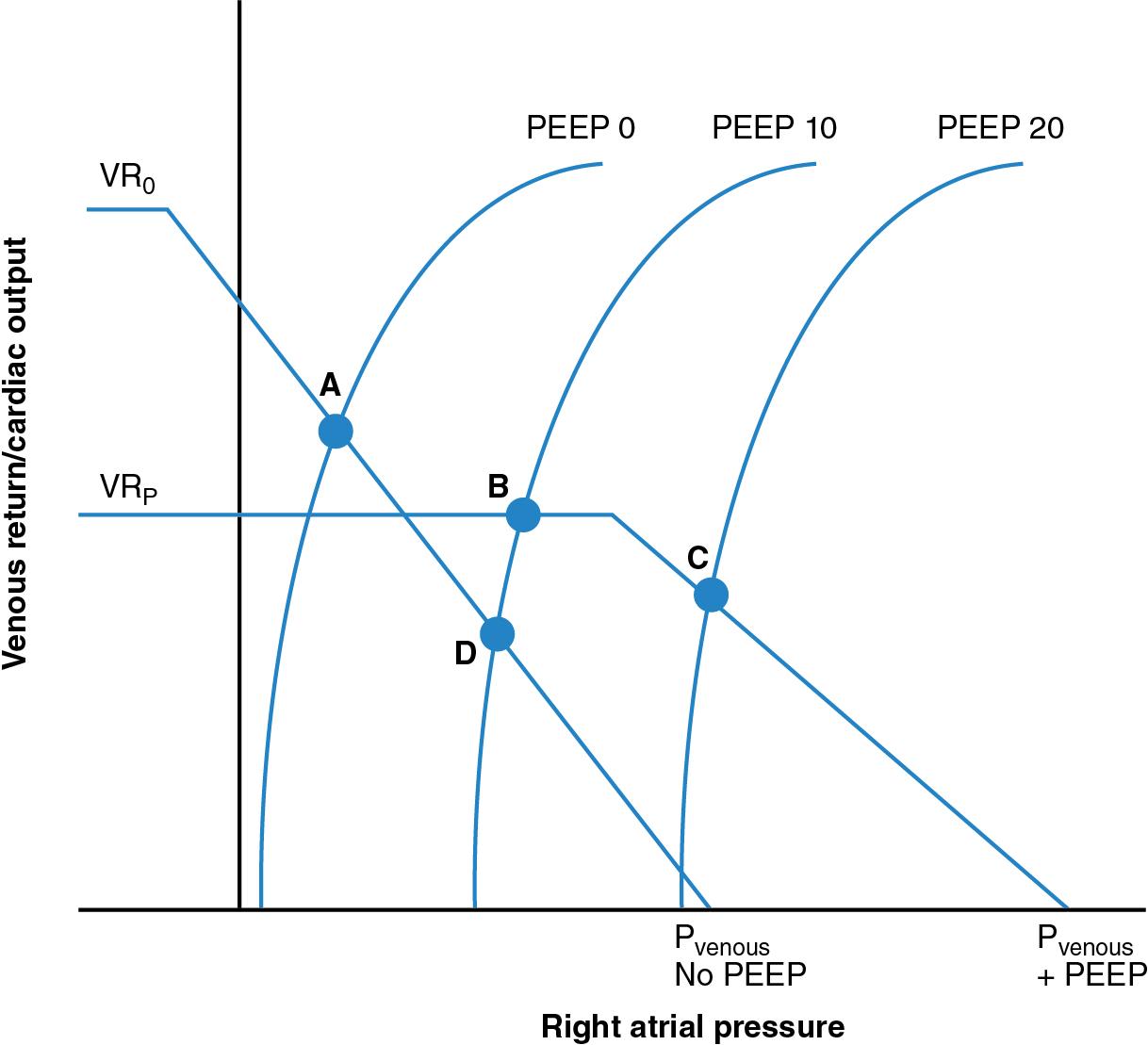
Among ventilated patients where higher ITP and increased lung volumes are deemed to be necessary, or for whom there is uncertainty regarding the magnitude of the P Venous – P RA pressure gradient, the administration of intravenous fluid may transiently augment systemic venous return. Among mechanically ventilated patients who have sustained reductions in ventricular preload associated with progressive increases in mean airway and ITP, a bolus of 0.9% saline restores end-diastolic volume to pre-ventilation levels.
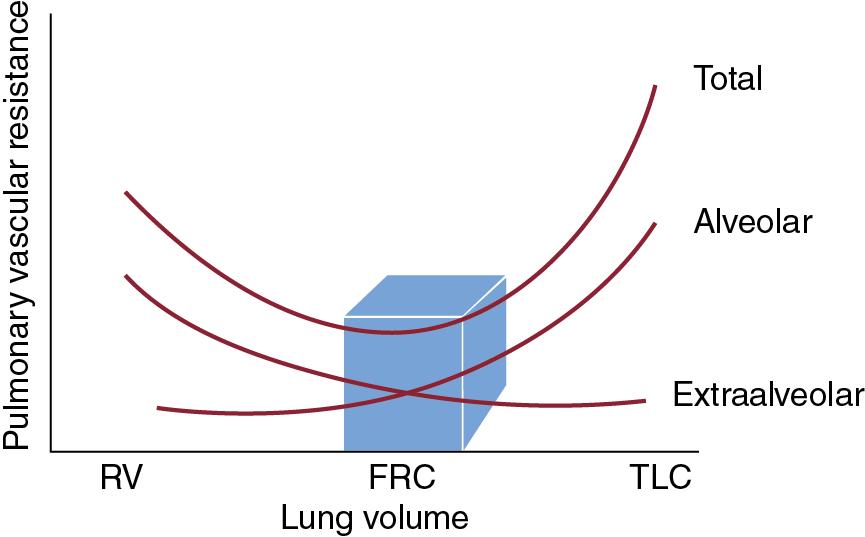
The impact of mechanical ventilation on right ventricular afterload is mediated through the relationship of PVR and lung volumes. PVR is the net resistance across alveolar vessels (contributing to gas exchange and exposed to alveolar pressure) and extra-alveolar vessels (larger vessels, located in the interstitium and exposed to intrapleural pressures). Alveolar and extra-alveolar vessels lie in series, and thus the net PVR is the sum of their total resistances.
At low lung volumes, alveoli are atelectatic, resulting in more tortuous extra-alveolar vessels (high resistance), while alveolar vessels have low resistance owing to low intra-alveolar pressure. Atelectasis also promotes V/Q mismatch and intra-pulmonary shunting, leading to hypoxia, which increases PVR. At high lung volumes, alveolar overdistention compresses alveolar vessels (resulting in high resistance), while extra-alveolar vessels assume a straighter configuration and develop lower resistance. Overdistention also results in V/Q mismatch by increasing ventilatory dead space, leading to hypercarbia, which increases PVR. As net PVR is the sum of the alveolar and extra-alveolar vascular resistance, PVR is minimized and pulmonary blood flow maximized, when lung volumes are at functional residual capacity (FRC) ( Figure 4.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here