Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Jay A. Berzofsky, MD, PhD
Branch Chief
Vaccine Branch
Center for Cancer Research, National Cancer Institute, Bethesda
Maryland
United States
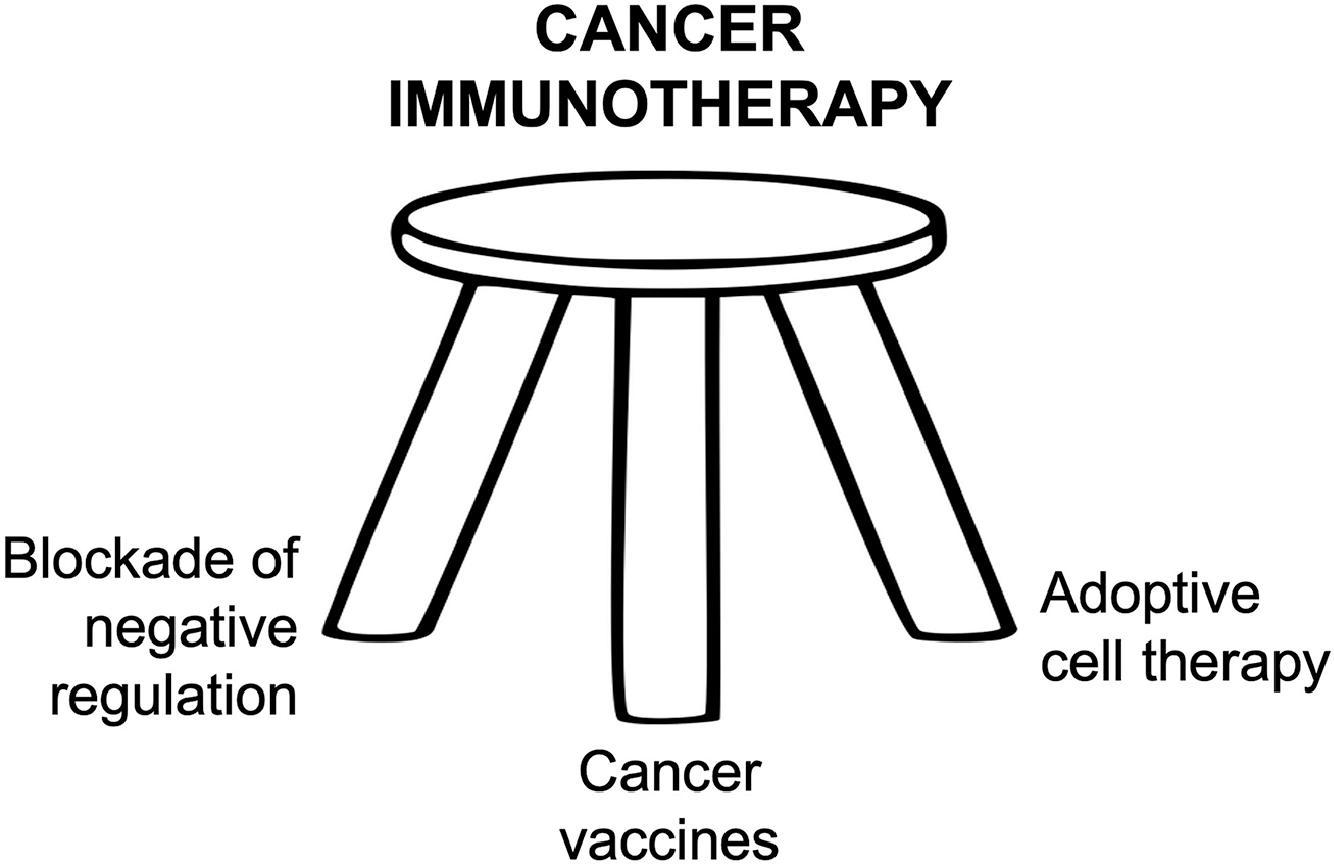
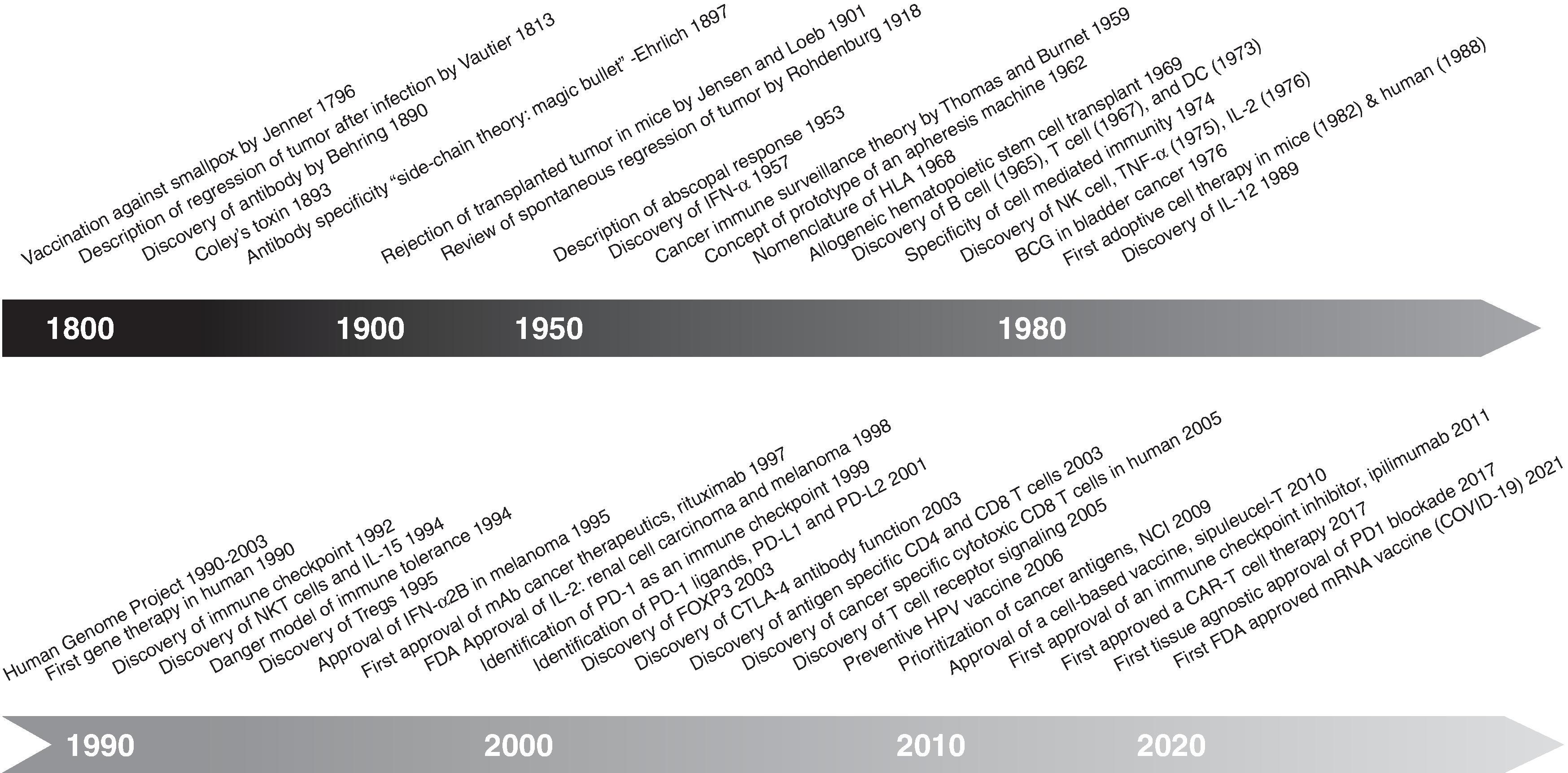
Immunologists have long hoped to exploit the exquisite specificity of the immune system to eradicate cancers. Almost nothing is as specific as the immune system to distinguish cancer cells from normal cells, as it can detect single amino acid changes in a protein antigen, corresponding to a single codon mutation. Unlike surgery or radiotherapy, which cannot reach distant metastases, or chemotherapy, which is almost never uniquely specific for cancer cells, the immune system can reach all parts of the body and target cancers with exquisite specificity. Early cancer vaccines induced T cell and antibody responses but sustained tumor regression was not seen. We now know a major reason for this difficulty, namely the myriad of immunoregulatory mechanisms that cancers exploit to suppress the immune system. Early setbacks discouraged many cancer researchers and clinicians from further investigating immunotherapy. The breakthrough success of immune checkpoint inhibitors, initially with anti-CTLA-4 and anti-PD-1 antibody, revolutionized the field. Currently, cancer vaccines are one of the three legs of the immunotherapy stool, of which the other two are immune checkpoint inhibitors (ICIs) (and other approaches to block immunosuppression) and adoptive cell therapy ( Fig. 14.1 ). The last of these came from the pioneering work of Rosenberg and others using tumor-infiltrating lymphocytes (TIL) , and more recently genetically engineered T cells with defined T-cell receptors (TCRs) or chimeric antigen receptors (CAR) consisting of an antibody binding region fused to a TCR-like and/or costimulatory transmembrane signaling base. The CAR T cells have recently had their greatest success in treating several B cell malignancies. , Of the three legs, cancer vaccine therapy has been the slowest to move into licensed therapies for human cancer ( Fig. 14.2 ) .
A major reason for initial failures with cancer vaccines, as mentioned earlier, is immunosuppressive mechanisms that must be overcome for vaccines to work. Testing them in combination with other therapies like immune checkpoint inhibition has proved productive. ICIs and other counter-suppressive approaches can enable cancer vaccines and conversely, cancer vaccines can enable ICIs in patients who have not been responsive to a single agent ICI. For example, ICIs work best when a tumor is infiltrated with T cells, a so-called “hot” or “inflamed” tumor. That means the tumor has already elicited a T-cell response, but the T cells could not do their job in the face of the immunosuppression. In those cases, ICIs and the like can work as single agents. However, in other types of cancers not already infiltrated with T cells, so-called “cold” or “noninflamed,” the vaccines can present tumor antigens in a more immunogenic context and can induce tumor-specific T cells. Once those T cells are elicited, assuming they can get into the tumor (which is another barrier to be discussed), the checkpoint inhibition can work. Thus, the combination is synergistic, not unidirectional. For these reasons, there is now a critical niche and need for cancer vaccines in the three-legged stool of cancer immunotherapy. They can, in theory, turn a cold tumor into a hot one and allow ICIs and other counter-suppressive measures, such as inhibition of regulatory T cells (Tregs), , myeloid-derived suppressor cells (MDSCs), M2 macrophages, regulatory type II NKT cells, , or cancer-associated fibroblasts (CAFs) to function, and can seek out and destroy metastases wherever they are. Such a conversion of a cold pancreatic tumor to a hot one has even been seen in human cancer patients treated with a GVAX vaccine. Vaccines can also boost the efficacy of adoptively transferred T cells by stimulating them to expand in vivo, or by activating them. Likewise, blockade of negative regulation can facilitate the efficacy of adoptively transferred T cells as well as vaccines. Thus, all three legs can mutually interact pairwise and function in unison to support the three-legged stool of immunotherapy.
In this chapter, we will examine these interactions in more detail. We will also review the types of tumor antigens, such as those shared among tumors or unique for a particular patient's tumor and the pros and cons of each, and the diversity of biochemical structures of antigens used in vaccines, such as protein, carbohydrate, or even lipid. We will examine the many vaccine platforms that have been adapted from infectious disease vaccines to cancer vaccines, such as recombinant proteins, peptides, viral vectors, DNA or RNA encoding antigens, or whole cells. In this context, it should be noted that unlike infectious disease vaccines, in which antigens are almost always foreign to the host, cancers are derived from host cells and most of their antigens are derived from self (except for some virus-induced cancers), making it necessary often to overcome self-tolerance to induce immunity. This aspect may require formulations with strong adjuvants or potent vectors. We will also review the many types of cancers in which vaccines have been attempted in human trials, ranging from cold to hot tumors and across the whole panoply of organ systems that develop cancer. Finally, we will examine the promise of novel concepts for prophylactic vaccines to prevent cancer, in contrast to most conventional cancer vaccines that are therapeutic vaccines to treat existing cancers. As viral vaccines are largely prophylactic, if cancer vaccines can be developed that are prophylactic for preventing cancers, then the vaccine approach to cancer will have come full circle. We can only hope that cancer vaccines can save as many lives as infectious disease vaccines have saved over the last 200 years.
Despite the constant surveillance of the immune system, many genetic and environmental factors can cause tumors to form. Many tumors are not immunogenic on their own, or escape immune surveillance, but delivery of tumor antigens in a more immunogenic form may circumvent this barrier. Cancer vaccines typically consist of specific antigens combined with a stimulatory molecule (adjuvant). They function to activate immune cells, including antigen-presenting cells; to stimulate the host adaptive immune response to control tumor growth; induce regression of established tumors; eliminate residual disease; and establish durable immunological memory against cancer cells. It is important to note that innate immune cells, such as natural killer (NK) cells and NKT cells, can also mediate helper and killer functions, but they do not acquire specific immune memory. Effective cancer vaccine strategies should elicit efficient T-cell responses against the targeted antigenic peptides on cancer cells but not on healthy cells. Potential autoimmune side effects caused by impaired tolerance or by the vaccine's ability to break tolerance should be avoided.
The success of antigen-specific cancer vaccines largely depends on the properties of antigens in the vaccine in the context of a peptide-MHC complex for T-cell recognition or surface expression on the cancer cells for B-cell recognition. To be safe and effective, optimal choice of antigen should be (1) expressed at a high level only by cancer cells (preferably on the surface) but not expressed by normal cells, (2) highly immunogenic, and (3) essential for the survival of the cancer cells so the tumor cells cannot escape by suppressing antigen expression. However, not all tumor antigens can meet these criteria. Based on their specificity, tumor antigens can be classified into two general classes: (1) antigens with high tumor specificity or tumor-specific antigens (TSAs) and (2) antigens with low tumor specificity or tumor-associated antigens (TAAs) ( Table 14.1 and Fig. 14.3 ).
| Type of Antigen | Tumor Antigen Examples and Associated Cancer |
|---|---|
| Antigens With High Tumor Specificity (Tsas) | |
| Viral | E6 and E7 of HPV (anogenital cancer, and head and neck cancer), LMP1 and LMP2 of EBV (B cell lymphoma, Hodgkin’s disease) |
| Mutated | p53 , K-RAS , N-RAS (multiple cancers) |
| Cancer-testis | MAGE family, NY-ESO1 (melanoma) |
| Antigens With Low Tumor Specificity (TAAs) | |
| Differentiation | Melan-A (MART-1), gp100, TRP-1, and TRP-2 (melanoma), PSA, PAP, PSMA (prostate cancer), CEA (colorectal cancer), Mesothelin (multiple cancers) |
| Overexpression | ERBB2 (HER2/Neu) (breast cancer and some ovarian, colorectal, lung, gastric cancers), Muc1 and WT1 (multiple cancers) |
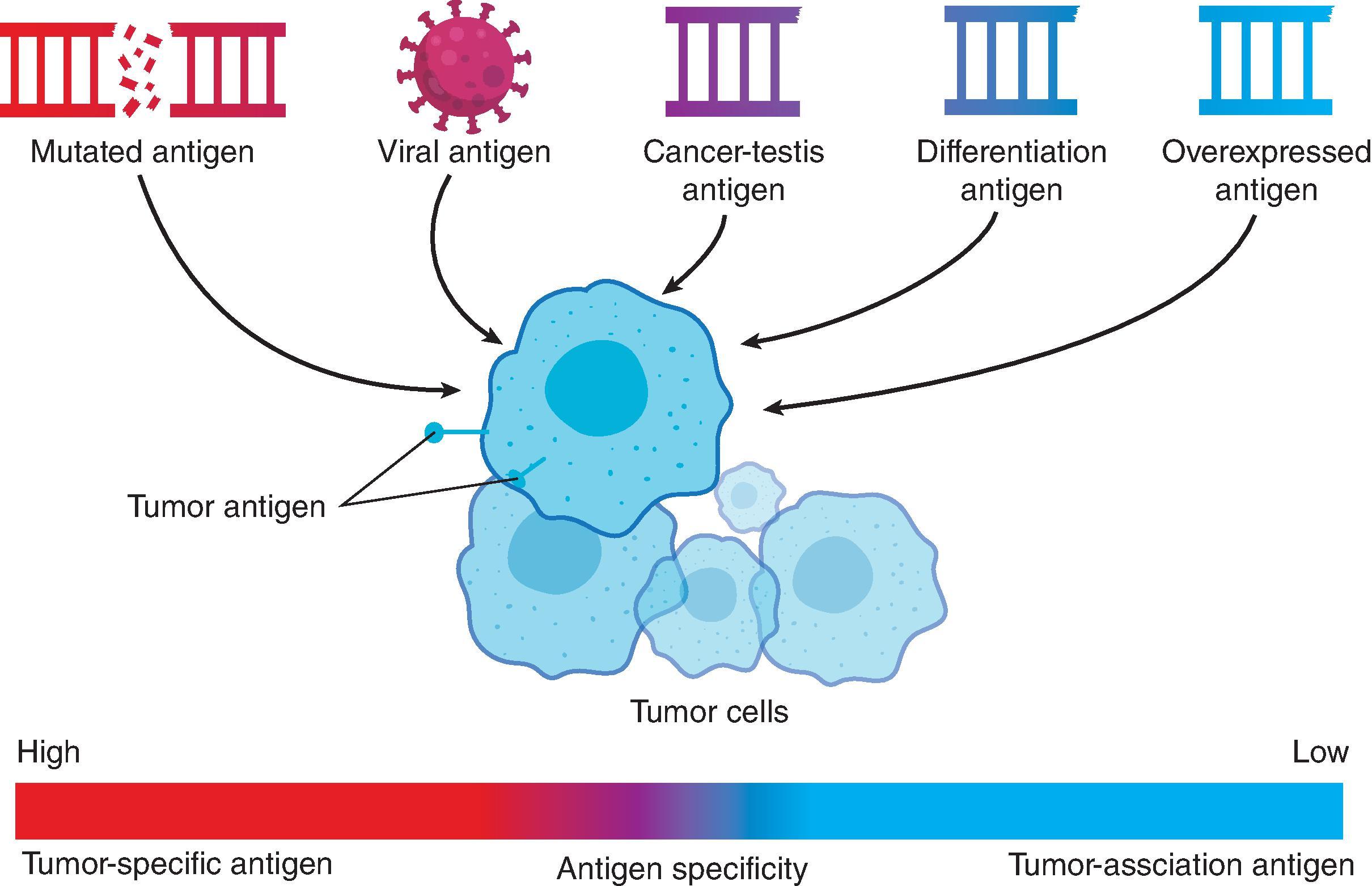
TSAs are not encoded by the healthy host genome and are comprised of oncogenic viral antigens (for tumors caused by oncogenic viruses) and neoantigens (altered gene products) that result from point mutations or a rearrangement of a gene-coding sequence. Oncogenic viral antigens (i.e., E6 and E7 of Human Papilloma Virus [HPV]) are not self-antigens and can act as tumor antigens required by the tumor cells for their transformed phenotype. , Neoantigens are generated by nonsynonymous somatic mutations resulting from point mutations, internal deletions (or frame shift mutations), and recombination events (chromosomal translocations and viral insertional mutagenesis) in cancer cells without an intact mismatch repair system . To induce robust antitumor immune responses, neoantigens must be properly presented by MHC class I and II molecules to generate durable T-cell activity. However, surface presentation of the neoantigen is not sufficient for immunogenicity. Other factors such as binding affinity, stability, abundance, cross-reactivity, foreignness of the neoantigen determine its immunogenicity. It is important to note, that not all neoantigens possess the same immunogenicity, which implicates a neoantigen-dependent component to the process of immunoediting. First, only a subset of mutations leads to peptides that can bind to a patient's HLA molecules for presentation to T cells. Second, clonal neoantigens are considered to be more potent than subclonal ones as they are expressed on most if not all cancer cells and required for cancer cell survival and fitness. Thus, targeting these clonal neoantigens should reduce the probability that escaping tumor clones develop and may make ideal targets for cancer vaccine.
Tumor mutation burden (TMB) is determined by the amount of gene mutations within cancer cells. A higher number of mutations is associated with more candidate peptides. Higher TMB increases the probability of successfully presented neoantigens (more peptides that might have high affinity for the patient's HLA molecules and have sufficient available TCRs to be recognized) and is strongly correlated with the effectiveness of neoantigen-based vaccines. Neoantigens can be patient-specific or common among multiple patients. TMB has become a biomarker for therapeutic cancer vaccines. In general, patients with high TMB are good candidates for personalized neoantigen-based vaccine therapy, while patients with low TMB are better treated better with TAA-based vaccines. With the development of rapid DNA/RNA sequencing and in silico neoantigen prediction, more and more tumor antigens are being found. Higher TMB has also been correlated with greater susceptibility to ICIs since antigen-specific T cells are more common and can be reactivated by checkpoint blockade. ,
Despite the many advantages of TSA neoantigen vaccines, TAA targeted vaccines are still an attractive target, especially when used in combination therapy. TAAs are unaltered self-proteins but are novel to the tissue of origin. They are selectively expressed or overexpressed by cancer cells but are not widely expressed, or expressed at much lower levels, in nonmalignant tissues (for example cancer-testis antigens). , Also, cancer-germline antigens, normally expressed only in immune-privileged germline cells, and cell lineage differentiation antigens, normally not expressed in adult tissues, are considered as TAAs. Because TAAs are self-antigens, unlike neoantigens, the existence of central tolerance hinders the antitumor response of high-affinity T cells against tumor TAAs. Thus, the antigens must be potent enough to “break tolerance.” Overexpression of TAAs may provide an opportunity for a specific T-cell response, as a threshold level of antigen is required for recognition by T cells. Specific antitumor T-cell responses could occur if a cancer cell presents a certain level of MHC-peptide complexes that is higher than the threshold, and normal cells do not.
Moreover, according to the pattern of expression of the parental gene, tumor antigens can be defined as intracellular and extracellular. Most of the mutant proteins are expressed intracellularly and thus are inaccessible to antibodies but may be recognized by T cells if they are appropriately processed. Although the expression of tumor antigens can distinguish tumor cells from healthy cells, antigen-presenting cells (APCs) must still be activated to provide the necessary costimulation for adequate T-cell activation. Small peptides released from proteasomal degradation with suitable size and sequence are transported to the ER where they can bind to nascent MHC class I molecules. Stable peptide-MHC class I complexes will be recognized by CD8 + T cells. In contrast, membrane-bound or surface-expressed proteins are internalized and degraded in endosomes. Then they bind to MHC class II molecules and are subsequently recognized by CD4 + T cells, although surface antigens synthesized in the cell can also be processed in the cytosol to load MHC class I antigens and induce CD8 + T-cell responses. In general, it is believed that to be successful, a cancer vaccine should induce antigen-specific CD8 + cytotoxic T cells, which are responsible for tumor cell lysis, and antigen-specific CD4 + T cells, which produce provide T-cell help and secrete cytokines to enhance CTL activity and B cell responses. However, some cancer vaccines work preferentially through antitumor antibodies.
When a promising target antigen is found, the next step is to select the mode of delivery of the antigen. The common vaccine delivery strategies often are divided into cellular, viral vector, or molecular ( Fig. 14.4 ). Also, the dose of the antigen, the speed of antigen release, and route of administration play important roles in vaccine efficacy. For example, skin-resident immune cells, like Langerhans cells, are needed to able to efficiently uptake antigens directly from the skin when injected intradermally. In contrast, the intramuscular injection route typically relies on recruitment of immune cells to the site and APCs to internalize antigens, whereas intravenous injection has the advantage that injected substances can rapidly access secondary lymphoid tissues.
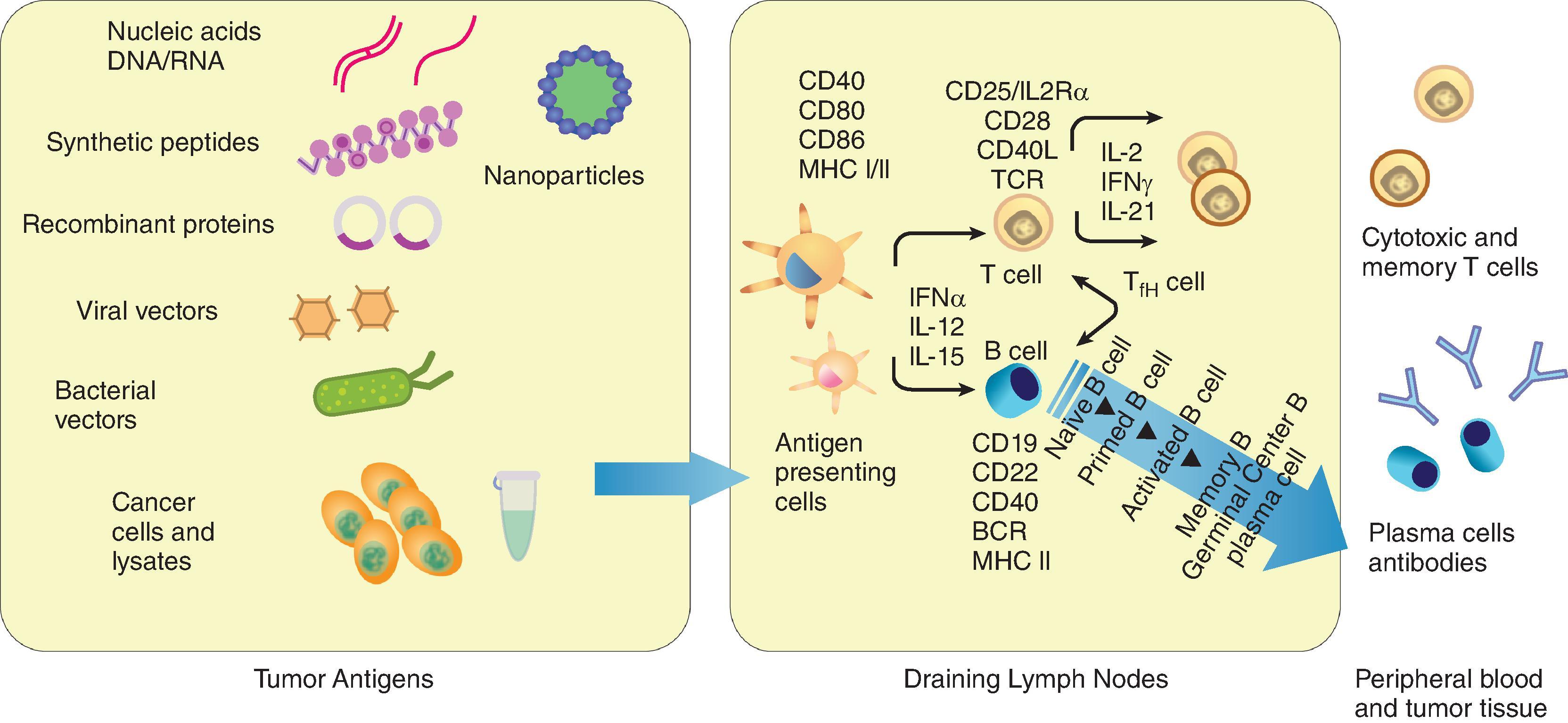
Cellular vaccines, which include whole-tumor cells (autologous or allogeneic) and dendritic cells (DCs), have been explored extensively in both preclinical and clinical settings.
In general, a major advantage of whole-tumor cell lysate vaccines is that they offer a broad range of antigens (both for TSAs or TAAs), including antigens that are yet not defined. Such antigens could potentially stimulate a strong and broad polyclonal T-cell response, preventing the emergence of aggressive immuno-resistant tumor variants. Moreover, allogeneic whole-tumor cell lysate vaccines, including two or three established and well-characterized human cancer cell lines of a various types, can be universally applied as they are not restricted to specific haplotypes of MHC molecules. However, autologous tumor cell lysates are preferred over allogeneic tumor cell lysates, as they likely contain a full repertoire of patient-specific unique point mutations or fusion gene products, including those critical for ones crucial for tumor rejection. In addition to the broad antigenic repertoire contained in whole tumor cell lysates, this approach also promotes and ensures the balanced activation of both CD4 + and CD8 + T-cell responses, which is essential for efficient tumor clearance and is not always provided by the peptide-based vaccine approach. However, whole-tumor cell lysate contains tumor antigens, self-antigens that could lead to autoimmunity, and other signaling molecules that could dampen the immune response. These suppressive signaling molecules include: IL-10, TGF-beta (inhibiting T-cell function), vascular endothelial growth factor (VEGF) (suppressing differentiation and maturation of DCs), soluble FAS ligand (inducing T-cell apoptosis), and MHC class I chain-related protein A (MICA) and MHC class I chain-related protein B (MICB) (inhibiting NKG2D-mediated killing). Irradiation of tumor cells, repeated freeze-thaw cycles, hyperthermia and high hydrostatic pressure (HPP), and hypochlorous acid (HOCI) oxidation strategies have been used to increase immunogenicity of whole tumor cells. ,
DCs, attractive candidates for developing cellular vaccines, serve as potent APCs that engulf, process, and present tumor antigens, and also have high expression of MHC and costimulatory molecules. , DC-based vaccines are mainly based on antigen loading on DCs. First, the DCs are generated in vitro from monocytes (CD14 + ) (called monocyte-derived DCs or MDDCs) or hematopoietic progenitor cells (CD34 + ) by activating them with a combination of different TLR ligands (CpG, Poly I:C, and LPS) and cytokines (IL-2, IL-15, and IL-7). Next, tumor antigens can be loaded onto DCs through direct pulsing with peptides (TSA or TAA), electroporation (DNA or RNA), viral transduction (lentiviral or adenoviral), fusion with tumor cells or pulsing with cell lysate. Activated and antigen-loaded DC vaccines, injected back to the patients intravenously, intradermally, or subcutaneously, not only directly prime T cells when functioning as APCs, but also serve as antigen donor cells that transfer antigens to endogenous cross-presenting DCs.
In addition, in situ targeting approaches to activate immune cells, including DCs, can target antigens for uptake by DCs or make use of antigens from dead or dying cancer cells in the tumor environment produced by other mechanisms of tumor cell killing. This approach includes administration of agents such as fms-like tyrosine kinase 3 ligand (FLTL3), specific cytokines (GM-CSF, IL-12, IL-15, and IL-2), and targeting the DCs with antibodies (CD40 receptor agonists or activators of CD40 and STING agonists) that are conjugated with the antigen of interest or activated in the tumor to pick up tumor antigens from dead cells. This in situ vaccination approach has a potential risk of inducing autoreactivity while increasing responsiveness to the therapeutic cancer vaccine.
Different viral vectors have been used to construct recombinant vaccines to induce antitumor immune responses to TAA or TSA. These vaccines have the advantage of inducing both antibody (B cell) and cytotoxic T-cell-mediated immune responses. Because of the intracellular expression of the inserted transgene of tumor antigen, it allows processing and presentation of the antigen by both MHC class I and II molecules. In addition, the viral vector may be constructed to express a transmembrane antigen on the surface of the infected cell for B cell recognition. Viral vaccines can become oncolytic by genetically engineering them to selectively infect cancer cells, disseminate, and eradicate cancer cells. Moreover, the antigen gene expressed by the viral vector can be modified to enhance specifically MHC class I or II presentation of the tumor antigen for optimal recognition by CD8 + or CD4 + T cells. For example, insertion of an ER signal sequence added to the NH 2 terminus of the tumor antigens circumvents the requirement of proteasomal degradation and transport. It has been shown that the tumor antigen-expressing transgene becomes more immunogenic when inserted in a viral vector because of TLR activation and viral-induced local inflammatory responses. However, there is a risk of virus neutralization by the host immune response to the viral vector, which could hinder repeated use (i.e., high level of preexisting antiviral immunity against adenoviral vectors). However, strategies to increase the efficacy by injecting different vectors for priming and boost have provided promising results in some cancers. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here