Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients with cancer pain typically have a complex pain syndrome composed of multiple physical causes. The median number of the individual pains per patient is four with a range of one to seven. In addition, there is likely to be a substantial affective component consisting of anger, anxiety, and depression. Careful evaluation and identification of the separate pains with a clear diagnosis of their underlying cause should be sought and treatment subsequently individualized for each of them. Pharmacological intervention is based on the use of regular analgesics according to the principles of the analgesic ladder of the World Health Organization, which starts with simple analgesics such as paracetamol or non-steroidal anti-inflammatory drugs, escalates to weak opioids such as codeine, and proceeds to strong opioids when the former are ineffective. With this approach, 60% of patients will require step 3, strong opioid medication, and 76% will achieve good pain relief without further intervention. Morphine remains the drug of choice taken orally; oxycodone and hydromorphone are suitable alternatives. Transdermal buprenorphine or fentanyl and subcutaneous diamorphine, oxycodone, or hydromorphone provide alternative routes of administration according to the patient’s circumstances. Appropriate use of adjuvant analgesics, including antidepressants, steroids, anticonvulsants, and muscle relaxants, is a further important component of management. Non-pharmacological methods and, according to the primary tumor, intervention with radiotherapy, surgery, or systemic cancer therapy also have a role. The most common use of radiotherapy is for bone metastases; at least 50% pain relief is achieved in 60% of patients. Similar results for more widespread bone pain are achieved with wide-field external beam radiation therapy and radioisotope therapy.
Patients with advanced cancer are in a dynamic state with changing pathology and therefore symptoms vary from day to day. Careful and close assessment plus reassessment along with adjustments in medications is required throughout, which implies both discontinuation of ineffective measures and introduction of new measures when needed. In addition, clear and concise aims for each treatment intervention need to be defined with the patient. A realistic view of the degree of pain relief that can be achieved may be important, as well as sometimes acceptance that some degree of pain is likely to remain in some circumstances despite the best treatment schedules.
Cancer pain, as discussed in Chapter 72 , is a complex chronic pain that often has multiple physical components invoking different pain mechanisms, along with a significant affective component. It is therefore to be expected that treatment of patients with cancer pain should be multimodal and incorporate optimal combinations of analgesics and adjuvant analgesic drugs, psychological and social support, and specific cancer treatments. This is based on careful assessment of the individual components of a patient’s pain and individualization of the treatment program. A further important principle in the management of cancer pain is that the disease is a dynamic process that will evolve from day to day and week to week. This demands that all treatment programs be kept under careful review and different treatment modalities be introduced as the situation changes from that of a relatively fit ambulant patient to one at the end of life.
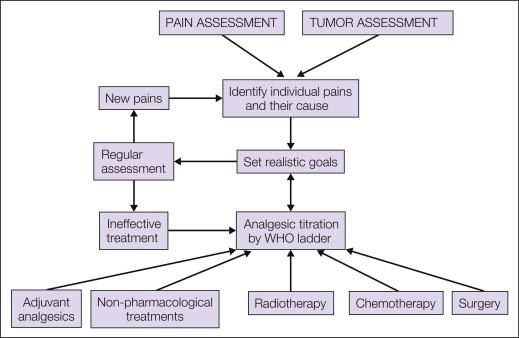
Management decisions should be based on a careful evaluation of the underlying pain, as described in Chapter 73 , to define its components and the specific underlying cause of each of them. A careful psychological and social assessment of the patient should be included. In addition, the underlying disease requires assessment to determine whether the malignancy is potentially curable, whether it is advanced but amenable to palliative treatment when medium-term survival (i.e., several months) is expected, or whether the patient is approaching the end of life. An overview of management is presented in Figure 75-1 .
When embarking on the treatment of cancer pain it is important to establish a dialogue with patients and their caregivers and to clearly define expectations from treatment. This avoids unrealistic hopes compounding the already complex emotional response to advanced cancer and its underlying symptoms. As a simple rule of thumb it is usually possible to define four levels of pain relief that can be aimed for:
Pain controlled during the night to enable rest and sleep
Pain controlled at rest during the day
Pain controlled with limited mobility
Pain controlled with full mobility
It is also important to have clear expectations with regard to the concept of “control” of pain and to understand what the patient understands by relief of pain and what the patient’s expectations are. Very few patients will require no analgesics and record pain scores of zero. The expectation in this population is that regular analgesics with or without adjuvant analgesics will be required; other specific interventions may also be necessary, but nonetheless, there may still be episodes of discomfort or a need for changes in lifestyle to ensure that pain is not a significant component of their experience.
The treatments available for the management of patients with cancer pain are outlined in Figure 75-1 . Most patients will require pharmacological intervention with regular analgesics with or without adjuvant analgesics. Anticancer therapy may be indicated in specific scenarios, for example, bone metastasis requiring radiotherapy or a pathological fracture of a long bone requiring surgery. In some selected cases, non-pharmacological interventional pain management will be necessary. All patients, however, are likely to benefit from psychological support during their illness, and optimal management of cancer pain requires a large multidisciplinary team that includes oncologists, palliative care physicians, specialist nurses, pain specialists, anesthetists, surgeons, psychologists, physiotherapists, occupational therapists, and spiritual counselors.
Analgesics have pain-relieving activity and work on the physiological mechanisms of pain. Adjuvant analgesics are drugs that have a primary indication for conditions other than pain but will be analgesic in certain painful conditions, for which they can modify the underlying pain process.
The use of analgesics for cancer pain is well established and follows clear guidelines based on the serial introduction of drugs with increasing analgesic potency titrated to pain relief as described by the World Health Organization (WHO) analgesic ladder ( ) illustrated in Figure 75-2 . The ladder defines a number of fundamental principles:
Analgesics should be given regularly at appropriate dose intervals to maintain therapeutic serum levels. This enables prevention of pain rather than awaiting its inevitable return.
Analgesic potency should be escalated sequentially according to the WHO analgesic ladder.
Alongside regular drug administration there should be ready access to appropriate medication in an appropriate dose for breakthrough pain. The regular need for breakthrough doses of analgesic should herald a review of the regular medication.
The side effects of analgesics, in particular, constipation and nausea, should be anticipated and the patient given prophylactic medication to prevent them.
Careful and regular monitoring is essential. Drugs that are no longer effective should be withdrawn and substituted by others to minimize the number of drugs that may be required at any time.
Pain is “whatever the experiencing person says it is, existing whenever the experiencing person says it does” ( ). Patients should have ready access to analgesics and should not feel that they have to “earn” their analgesics.
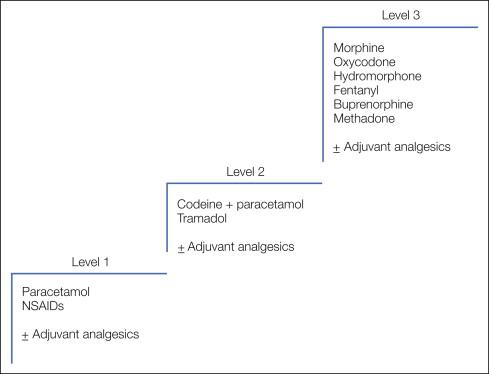
The WHO analgesic ladder defines stepwise changes in medication through a hierarchy of increasingly potent analgesic drugs. This approach enables a simple stepwise escalation in analgesic potency until relief of pain is achieved. Although this approach has not been subject to validation in large randomized controlled trials, several large descriptive studies provide level III evidence of its efficacy. The largest of these included 2118 patients managed with strict adherence to the analgesic ladder. Step 1 analgesics were used on 11%, step 2 on 31%, and step 3 on 49% of treatment days. Seventy-six percent had good and 12% had satisfactory pain relief, whereas the remainder required additional measures such as neurolytic procedures to achieve pain control ( ). Further evidence in support of a formal escalation program comes from a randomized study involving 81 cancer patients in which allocation to a formal “multilevel treatment algorithm” was compared with “standard-practice” pain control. A significant reduction in “usual pain intensity” was seen in the structured algorithm group ( ).
The original analgesic ladder used a straightforward three-step escalation from simple non-opioid analgesics to weak opioids to strong opioids, and this general principle has remained unchallenged for many years. The drugs within each step have evolved with the introduction of new agents, but the three-step approach allows these new agents to be appropriately incorporated into clinical practice. There is some evidence that the second step of the ladder may delay patients receiving strong opioids and thus achieving pain control, and some palliative care physicians use a two-step ladder in selected patients ( ). Most adhere to the three-step ladder, but a large randomized controlled trial to compare the two approaches is currently under way.
Paracetamol is the simplest and safest analgesic available at step 1 ( ). Alternatives include aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs), but they may be associated with more side effects. Despite being a widely available painkiller, paracetamol is an effective analgesic, and patients who have not undergone a trial of regular analgesia should have an initial period of regular full-dose paracetamol, 1 g every 6 hours. Paracetamol is predominantly a peripherally acting analgesic with antipyretic activity that works through central and peripheral non-opioid action that has yet to be fully defined. Significant toxicity is rare with therapeutic doses, and the only concern with paracetamol relates to its hepatotoxicity with an acute overdose or longer-term dosing above recommended levels.
NSAIDs, including aspirin, are also appropriate analgesics at step 1 ( ). In addition, they have adjuvant analgesic activity in situations in which their anti-inflammatory action may be of value, such as musculoskeletal pain. The major drawback of NSAIDs relates to their wider toxicity profile. They will be discussed further in the section Adjuvant Analgesics .
At step 2 of the analgesic ladder patients should have a weak opioid added to paracetamol. Many proprietary formulations of paracetamol and a weak opioid are available.
Codeine, usually in the formulation codeine phosphate, is a weak opioid; chemically, it is methyl morphine. When given alone it is a relatively weak analgesic with a number needed to treat (NNT) of 10–20 to achieve 50% pain relief. Its NNT when used as a combination of codeine, 60 mg, and paracetamol, 1 g (as co-codamol 30/500), however, is 2.2 ( ). It is an opioid agonist and is associated with the usual spectrum of opioid-related side effects (i.e., constipation and nausea). It is recognized to have cough-suppressant activity, but clinically relevant respiratory depression is not seen in patients with normal renal function. Its oral bioavailability is around 35% and it is metabolized in the same way as morphine; indeed, approximately 10% of codeine is demethylated to the parent morphine molecule. This may account in part for its analgesic activity ( ).
If co-codamol 30/500, two tablets every 6 hours, is ineffective, there is little value in trying alternative step 2 analgesics. This is an indication that a strong opioid is required.
A semisynthetic derivative of codeine, dihydrocodeine is equipotent to codeine given alone orally. It is the weak opioid component of co-dydramol in combination with paracetamol. It has no advantages over codeine and is inferior to paracetamol and codeine in combination. In single-dose studies, 30 mg of dihydrocodeine was found to have an NNT of at least 8.1 ( ).
Tramadol has both weak opioid agonist activity and effects on noradrenaline and serotonin uptake in the spinal cord. A single 100-mg oral dose of tramadol is equivalent to 1000 mg of paracetamol. A dose of 100 mg has an NNT of 4.6 for at least 50% pain relief. In some countries it has the advantage of being outside the regulatory restrictions of strong opioid prescription, which can facilitate its use in the community. Otherwise, it is no different from the other drugs in this group, has lower efficacy than co-codamol 30/500, and has the same associated potential side effects when a standard dose of 50–100 mg every 6 hours is used ( ).
When full-dose regular step 2 analgesia is ineffective, there is no value in switching to an alternative step 2 analgesic. More than 50% of patients ultimately require regular strong opioids ( ), and even though there are a large number of drugs available in this class, morphine remains the drug of choice for most patients since it is cheap and readily available. There are still restrictions on its use in many parts of the world.
In the management of cancer-related pain, morphine should whenever possible be taken regularly by mouth, although in patients unable to take the drug orally, parenteral formulations are readily available and may be used. Rectal preparations are also available and may be useful occasionally.
Morphine should be introduced when regular, full-dose step 2 analgesics are ineffective. The usual starting dose is 10 mg of normal-release morphine every 4 hours. Both liquid and tablet formulations are available, and they should be given according to the patient’s preference. Regular administration every 4 hours necessitates a dose in the middle of the night. The previous practice of giving a double dose as the patient’s last dose of the day is no longer recommended because it is not effective. Other important considerations include the following:
Constipation is almost universal in patients taking regular morphine, and both softening and stimulant laxatives should be given alongside this form of morphine under the principle of regular administration of laxatives to maintain regular bowel function rather than requiring intervention once severe constipation has evolved.
Nausea occurs in 30–50% of patients taking strong opioid drugs, and around one-third of patients will require antiemetics to control nausea while taking regular morphine. Antiemetics should therefore either be given prophylactically on a regular basis or be made readily available to the patient to enable early intervention.
Despite taking regular morphine every 4 hours, breakthrough pain may occur within the 4-hour dosing interval, and therefore rescue medication should be available to all patients taking the same dose of morphine as given every 4 hours ( ). The use of rescue medication regularly should prompt a review of the patient’s analgesic regimen. If the need for breakthrough medication is due to uncontrolled background pain, this is an indication for upward dose titration in which the patient’s previous 24-hour morphine requirement is incorporated into the new regular dosing. Some patients will continue to require rescue medication for pain triggered by movement or voiding their bladder or bowel, for example. If the background pain is controlled, dose escalation in these circumstances can lead to excessive sedation, so patients should be advised to maintain their background analgesic dose and to pre-empt pain by taking rescue medication before a known trigger.
The standard starting dose of morphine is 10 mg every 4 hours; however, the majority of patients will require larger doses, and the correct dose for an individual patient is achieved by careful “dose titration.” This requires close monitoring of the response of the patient’s pain to the medication and serial dose increments until adequate pain control is achieved within the limits of intrusive side effects. The median dose requirements in series of patients that have been published are in the range of 40–60 mg every 4 hours. Patients requiring escalation beyond this point should have their pain carefully reassessed and the role of other pain measures reviewed as detailed elsewhere in this chapter, alongside continued careful dose titration ( ).
The side effects of morphine (and other strong opioids) may be either idiosyncratic or dose related. The majority of patients becomes constipated when taking morphine, and 30–50% will experience nausea and vomiting and, some, a dry mouth. Dose-related side effects increase if the patient’s dose has to be increased to control pain. Such effects include sedation, confusion, vivid dreams, hallucinations, myoclonic jerks, and finally, respiratory depression.
Constipation is universal with opioid medication and should be prevented with the regular use of laxatives. Its main cause is related to smooth muscle relaxation, and therefore a bowel stimulant together with a fecal softener is usually necessary. Proprietary preparations are available that contain both these components in a single formulation, which may be more convenient for the patient. Combinations of senna or bisacodyl with docusate sodium or lactulose are equally effective and do allow differential titration of the softener and stimulant. Opioid-induced constipation should be manageable with oral agents, but sometimes patients will also require rectal measures such as suppositories and enemas.
Methylnaltrexone is a methylated form of the μ-opioid antagonist naltrexone that blocks the peripheral effects of opioids without reversing centrally mediated analgesia. It is administered subcutaneously and leads to 57% of patients having a bowel movement within 4 hours ( ). It is associated with some nausea and abdominal pain. Methylnaltrexone may be useful for some patients with difficult opioid-induced constipation, but most patients should be managed with adequate laxatives given by mouth.
Nausea and vomiting occur in 30–50% of patients starting regular morphine. The majority will respond to regular antiemetics. The mechanism of opioid-induced nausea is predominantly through a central mechanism via the chemoreceptor trigger zone, although peripheral smooth muscle relaxation may also be a component. In the first instance, antiemetics such as haloperidol or cyclizine, which act predominantly centrally, are recommended while recognizing that they may in themselves have additional side effects, in particular, some drowsiness and dry mouth with cyclizine. When these drugs are ineffective, metoclopramide with additional peripheral activity may be of value. If these agents are unhelpful and vomiting continues, subcutaneous levomepromazine may be considered.
Dry mouth has been reported as a morphine-related side effect but will also be compounded by the use of other drugs with anticholinergic activity, for example, cyclizine and antidepressants. This may require attention to oral hygiene and the use of sips of water, ice chips, chewing gum, or artificial saliva.
Drowsiness or a degree of sedation is common when starting morphine or when undergoing dose escalation. It is usually self-limited and best managed by careful explanation and reassurance. As physical tolerance develops, these effects usually regress. Because of this, however, patients should be advised not to drive or undertake other tasks that require similar skills for a few days after initiating or changing the morphine dose ( ). There is no contraindication to such activity once on a stable dose. In patients in whom dose escalation leads to unacceptable side effects, particularly sedation, despite best efforts to control them, a switch to an alternative strong opioid may produce lesser effects. The psychostimulants dextroamphetamine and methylphenidate have been used to manage the sedative side effects and cognitive impairment associated with opioids. A recent systematic review concluded that methylphenidate may have a role in managing opioid-induced sedation or cognitive impairment, or both, in patients for whom opioid dose reduction or switching to an alternative opioid is not possible, but the recommendation is weak because of a poor-quality evidence base ( ).
Cognitive impairment may lead to confusion, and some patients experience particularly vivid and frightening dreams. They may also have visual or auditory hallucinations. These adverse effects often respond to haloperidol given as a single dose of up to 5 mg at night or switching to an alternative opioid.
Myoclonic jerks are manifestations of flexor myoclonus that occur in patients with opioid toxicity, usually in the arms, but they can also occur in the legs. In patients who become drowsy and cognitively impaired while taking opioids, opioid toxicity is often thought to be the cause; however, patients with cancer pain will frequently have many drug- and non–drug-related reasons for their deterioration, such as infection. The presence of myoclonic jerks is helpful in making the diagnosis of opioid toxicity. Patients may need to be cautioned to handle hot drinks with care, and relatives should be reassured that the myoclonus is not due to pain, although patients with severe movement-related pain may find that severe myoclonic jerking precipitates pain. Myoclonus usually settles with time or as the opioid dose is reduced, but if it remains troublesome, benzodiazepines may reduce the myoclonus.
Respiratory depression is often quoted by health care professionals as a concern in patients taking high regular doses of morphine. In practice, “pain is the physiological antagonist” to the respiratory-depressant effects of morphine, and giving patients inadequate doses of analgesics for their pain is an infinitely greater problem than respiratory depression. Systematic evaluation of changes in blood gas findings in patients taking regular morphine for cancer pain reveals no evidence of carbon dioxide retention or other parameters of respiratory failure ( ).
Addiction is also still quoted as a concern with the use of morphine for cancer pain. There is a wealth of clinical experience on the use of regular high-dose morphine by cancer patients that confirms that addiction is extremely rare in this setting ( ). Physical dependence does occur as a physiological response to opioid receptor stimulation with the regular administration of opioid agonists, and hence a slow increase in morphine dose is observed in some longitudinal studies. This is not invariable in clinical practice. Abrupt cessation of morphine in patients who may, for example, undergo a surgical procedure to relieve their pain will result in physical withdrawal symptoms in about 10% of patients. This can also occur with the inadvertent or inexpert administration of an opioid antagonist such as naloxone. If the pain can be managed by a surgical, anesthetic, or neurolytic procedure, gradual withdrawal of morphine over a short period is entirely possible without the development of withdrawal symptoms.
Addiction is a more complex phenomenon related to psychological dependence and habituation alongside physical dependence that results in drug craving and sociological changes to enable continuing and ever-increasing supplies of the drug. This is not a recognized phenomenon in the use of morphine for cancer pain.
Opioid pseudo-addiction has been described and refers to a phenomenon that reflects inadequate use of morphine in a patient suffering severe pain. In this setting, patients will seek to persuade their carers of the severity of their pain and, if denied appropriate analgesics, will go to extreme lengths to seek attention and obtain analgesics, thereby creating a situation of mistrust on both sides; the patient requires what appears to be ever-increasing doses of analgesics for the pain, whereas carers observe abnormal behavior and what they interpret as exaggeration of symptoms to acquire analgesics. This situation should not evolve in modern pain care settings in which appropriate regular use of analgesics according to the analgesic ladder is in operation; sadly, however, it is still seen.
Long-term side effects with morphine are unusual when it is used for cancer pain. Nausea and constipation should be controlled with appropriate adjuvant medication, drowsiness will resolve in most patients, and formal assessment of psychomotor function in cancer patients with established long-term oral morphine treatment has revealed no major differences from controls who took no regular analgesics ( ).
Used by the oral route, given regularly, and taken with appropriate medication to avoid predictable side effects, morphine is an extremely safe analgesic drug when taken in appropriate doses to control chronic cancer pain. Toxicity arises in one of three situations:
Continued upward titration of morphine or other strong opioids may result in toxicity. This may occur (1) because of rapid titration in patients with severe pain, (2) as a result of continued titration in patients with pain that is responding poorly to a given opioid, or (3) because of changes in the pain, such as chemotherapy or radiotherapy interventions leading to a reduction in tumor size and its associated pain and thereby reducing morphine requirements. In all these situations toxicity can usually be easily recognized by increasing drowsiness and cognitive impairment and by myoclonic jerks.
Management of each of these situations is slightly different. (1) If rapid dose titration is necessary, a slight decrease in dose and consideration of the use of adjuvant analgesics and treatment of the side effects, followed by further, more gradual dose escalation, may be sufficient. (2) If the patient has pain responding poorly to a given opioid, switching to an alternative opioid may be helpful. (3) Finally, if the pain has improved because of other treatment interventions, downward titration by increments of up to 25% should allow the correct new morphine dose to be established.
Renal impairment is potentially hazardous in patients taking strong opioids. Morphine is absorbed in the upper part of the small bowel and undergoes glucuronidation in the liver and gut wall to morphine-6-glucuronide and morphine-3-glucuronide. Morphine-3-glucuronide is an inactive metabolite, but morphine-6-glucuronide is an active metabolite that is a potent analgesic ( ). It is excreted renally and therefore accumulates in patients with renal impairment ( ). In the setting of renal impairment, dose intervals should be increased or alternative opioids used. Most other strong opioids also have renally excreted metabolites, but they may accumulate less than morphine-6-glucuronide. Both oxycodone and hydromorphone are often used when the patient’s estimated glomerular filtration rate (eGFR) is 30–60 mL/min (i.e., stages 3 and 4 chronic kidney disease). Opioids with no renally excreted metabolites, such as fentanyl or alfentanil, should be used when the eGFR is less than 30 mL/min. Methadone may also be used in this situation, but generally only by those accustomed to dealing with its variable half-life ( ).
Inappropriate use of modified-release preparations can also lead to drug accumulation. Twelve- and 24-hour modified-release preparations are available and should be used at these dose intervals. More frequent administration will result in drug accumulation, which can be hazardous by allowing levels to be reached beyond those required for analgesia.
Modified-release preparations have obvious advantages for patients requiring regular chronic use of a drug, and they are available for morphine with timed release over a period of 12 and 24 hours. Twice-daily preparations are most commonly used. Randomized controlled trials have confirmed that these preparations achieve analgesia equivalent to the same dose of normal-release morphine every 4 hours and can be substituted on an equivalent total 24-hour dose basis; for example, a patient requiring 20 mg of normal-release morphine every 4 hours will require 60 mg of modified-release morphine every 12 hours. Initial treatment in the hospital is usually with normal-release morphine, and once the patient’s 24-hour dose requirement has been established, treatment can be switched to a modified-release preparation. Randomized controlled trial evidence has shown that no loading dose is required and a simple dose-for-dose switch can be made ( ).
An important principle of using modified-release preparations is that breakthrough medication should be available between the prolonged dosing intervals and be in the form of normal-release morphine at a dose every 4 hours equivalent to that required to achieve the total 24-hour dose. For instance, a patient requiring 120 mg of morphine over a 24-hour period will require a 20-mg breakthrough dose of normal-release morphine. Regular use of normal-release breakthrough medication should lead to adjustment of the total modified-release dose to the new 24-hour dose requirement, provided that breakthrough medication has not been taken to pre-empt movement-related pain, for example.
Modified-release preparations may have a role in initial therapy, with normal-release breakthrough medication used to titrate the dose against a background level. There are limited trial data to suggest that this may be equally effective as titrating a normal-release formulation throughout ( ), but this is common practice when initiating opioids in the community.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here