Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Similar to cancer itself, the factors that drive cancer pain evolve and change with progression of the disease. Surgery, radiation therapy, and chemotherapy are used to remove or kill cancer cells, and all can induce pain and/or dysfunction of sensory and sympathetic nerve fibers. In cases in which the cancer continues to grow or relapse occurs, cancer cells and their associated stromal cells generate ongoing pain by releasing algogenic substances, including protons, bradykinin, endothelins, prostaglandins, proteases, and tyrosine kinase activators. With disease progression, tumor growth can directly injure nerve fibers and thereby give rise to neuropathic pain. Additionally, recent studies have demonstrated that cancer and its associated stromal cells release tyrosine kinase activators, including nerve growth factor, which can induce active and highly pathological sprouting and neuroma formation by sensory and sympathetic nerve fibers. This structural reorganization of sensory and sympathetic nerve fibers along with cellular and neurochemical reorganization in the spinal cord and brain may contribute to breakthrough pain. Incorporating this new understanding of cancer pain into novel therapies to treat cancer pain may increase the survival, quality of life, and functional status of cancer patients and survivors.
In 2010 cancer will be diagnosed in more than 12 million people worldwide (excluding non-melanoma skin cancer), and 8 million individuals will die of cancer ( ). Cancer incidence rates are stable or slightly falling in developed countries, whereas in developing countries, these rates are increasing because smoking, obesity, and increased life expectancy have led to a rapid rise in the incidence of cancer ( , , ). Additionally, as detection and treatment of most cancers have dramatically improved, survival rates have increased so that even patients with metastatic cancer are surviving years to decades beyond their initial diagnosis ( ).
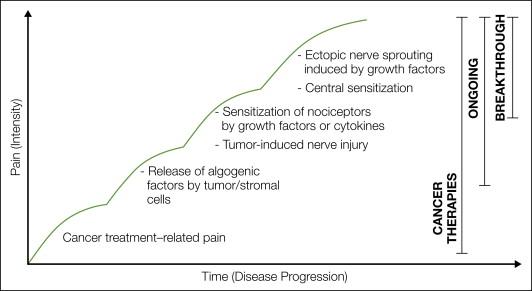
Cancer-associated pain is one of the most common initial symptoms that results in the diagnosis of cancer. Although pain can be present at any time during the course of the disease, it generally increases with disease progression such that 75–90% of patients with metastatic or advanced-stage cancer will experience significant cancer pain ( , ). Figure 72-1 outlines some of the mechanisms that drive cancer pain, including cancer therapy, factors released from tumors that sensitize or excite primary afferent neurons, tumor-induced nerve injury, and tumor-induced nerve sprouting and neuroma formation ( , , , ).
In patients with cancer, undergoing surgery or receiving the full dose of radiation therapy or chemotherapy is one of the most significant factors in determining patient survival ( ). However, peripheral nerve neurotoxicity and the accompanying pain are major side effects of radiation therapy and many of the most commonly used antineoplastic agents, including the taxanes (e.g., paclitaxel, docetaxel), vinca alkaloids (e.g., vincristine, vinblastine), platinum-based compounds (e.g., cisplatin and oxaliplatin), and proteasome inhibitors (e.g., bortezomib, disulfiram) ( , , , , ). If the chemotherapy-induced peripheral neuropathy becomes severe enough, the oncologist or patient may reduce or cease chemotherapy treatment, which decreases the survival rate of the patient and the likelihood that the patient will be disease free.
In cases in which the tumor is inoperable or relapse occurs, cancer and its associated stromal cells can induce significant pain. This pain can arise from the original site of the cancer (i.e., pancreatic cancer, head and neck cancer, osteosarcoma) ( , , ) or from distant sites (such as bone, liver, and lung), where common cancers such as breast, prostate, kidney, and lung cancer avidly metastasize ( ). The original quality of the tumor-induced pain is usually described as dull in character, constant, and gradually increasing in intensity with time ( ) (see Fig. 72-1 ). If the disease continues, a second type of cancer pain known as “breakthrough” or severe “incident pain” can emerge ( ). Incident or breakthrough pain, which is defined as a transitory flare of extreme pain superimposed on an otherwise stable pain pattern in patients treated with opioids ( ), can occur spontaneously or with movement or weight-bearing of a tumor-bearing organ or tissue ( ). Since breakthrough pain is frequently acute and unpredictable in onset, this pain can be severe, debilitating, and difficult to fully control ( , ).
Currently, tumor-induced pain is largely managed with an “analgesic ladder” that was originally promulgated by the World Health Organization in 1986 (see Chapter 75 ). This ladder begins with a non-steroidal anti-inflammatory drug (NSAID); if the pain worsens, an NSAID plus a mild opiate; and finally, when the pain becomes severe, an NSAID plus a strong opiate. In addition to this three-step ladder, other adjuvant therapies, including radiation therapy, radioisotopes, nerve blocks, nerve lesions, antiepileptics (e.g., gabapentin, carbamazepine), antidepressants (amitriptyline, imipramine), and steroids, are commonly used to control cancer pain ( ). It should be stressed that most cancer pain can be controlled if it is closely monitored and these therapies are used in a timely and proactive manner. However, the abovementioned therapies all have significant unwanted side effects ( ), and closely monitoring and fully controlling the cancer pain (especially if breakthrough pain is present) can be very time-consuming for the patient, caregiver, and physician ( ). Developing new analgesic therapies that are efficacious and have fewer side effects than current analgesics do and incorporating these advances into mainstream cancer therapy will significantly improve the quality of life and functional status of both the patient and the caregiver.
Given the enormous consequences in terms of human suffering that cancer pain can cause, it was surprising to us when we first began exploring the mechanisms driving cancer pain that no well-established animal model was available for studying any form of cancer pain. However, there were two commonly used in vivo models to study tumor-induced bone destruction. In the first model, tumor cells are injected into the left ventricle of the heart and then spread to multiple sites, including the bone marrow, where they multiply, grow, and destroy the surrounding bone ( , ). Although this model replicates the observation that most tumor cells metastasize to multiple sites, including bone, a major problem with the model is animal-to-animal variability in the sites, size, and extent of the metastasis. Since the tumors frequently metastasize to vital organs such as the lung or liver, the general health of the animal is also variable, which makes behavioral assessment difficult. Additionally, because the tumors frequently metastasize to bone in the vertebral column, tumor growth in the vertebrae can result in collapse of the vertebral column and compression of the spinal cord with resultant spinal dysfunction and paralysis. Given these problems, development of a model of bone cancer pain using intracardiac injection proved difficult at best.
The second major model used to study tumor-induced bone destruction involved the direct injection of lytic sarcoma cells into the intramedullary space of the mouse tibia or femur. The major problem with this model was that since the injection site could not be plugged with conventional sealing agents (because it is a wet, bony surface), the tumor cells rapidly escape and grow avidly in nearby skin and joints, thereby resulting in large extraskeletal tumor masses that not only interfered with behavioral analysis but also destroyed nerves passing though these sites and generated a neuropathic pain state. We chose to adapt and modify this model by plugging the injection hole with a dental amalgam that tightly binds and seals the injection hole in the proximal head of the femur. Plugging of the injection site allowed us to contain the tumor cells within the intramedullary space and prevented invasion of tumor cells into the surrounding soft tissue ( Fig. 72-2 ) ( ). This advance, along with techniques with which we could simultaneously measure bone cancer–induced pain behavior, tumor growth, and tumor-induced bone remodeling has provided us with the first preclinical cancer pain model, which we then used to define the mechanisms that generate and maintain bone cancer pain.
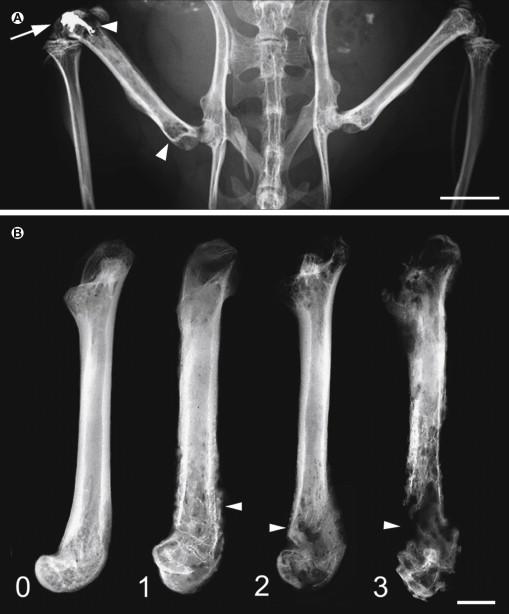
After injection and confinement of primarily osteolytic 2472 murine osteosarcoma tumor cells to the intramedullary space of the mouse femur, the tumor cells grow in a highly reproducible fashion as they proliferate and replace the hematopoietic cells in the bone marrow ( , ). Eventually, the entire marrow space is homogeneously filled with tumor cells and tumor-associated inflammatory/immune cells. In terms of bone remodeling, injection of osteosarcoma cells into the femur induces a dramatic proliferation and hypertrophy of osteoclasts at the tumor–bone interface, with significant bone destruction in both the proximal and distal heads of the femur (see Fig. 72-2 ). In the osteosarcoma model, ongoing pain and movement-evoked pain-related behavior increased in severity with time, and this pain-related behavior correlated with the tumor growth and progressive tumor-induced bone destruction, which mirrors what occurs in patients with primary or metastatic bone cancer. Although sarcoma cells constituted the tumor used in the first bone cancer pain model that we developed, we have since developed other bone cancer pain models using prostate, breast, melanoma, colon, and lung tumors, all of which have provided insight into the similarities and differences by which different tumors drive bone cancer pain ( ).
A tumor is composed of not only cancer cells but also tumor-associated stromal cells. In most tumors, stromal cells far outnumber cancer cells and include endothelial cells, fibroblasts, and a host of inflammatory and immune cells, including macrophages, mast cells, neutrophils, and T lymphocytes ( ) ( Fig. 72-3 ). Both cancer cells and their associated stromal cells secrete a wide variety of factors ( , ), many of which have been shown to sensitize or directly excite primary afferent neurons ( ).
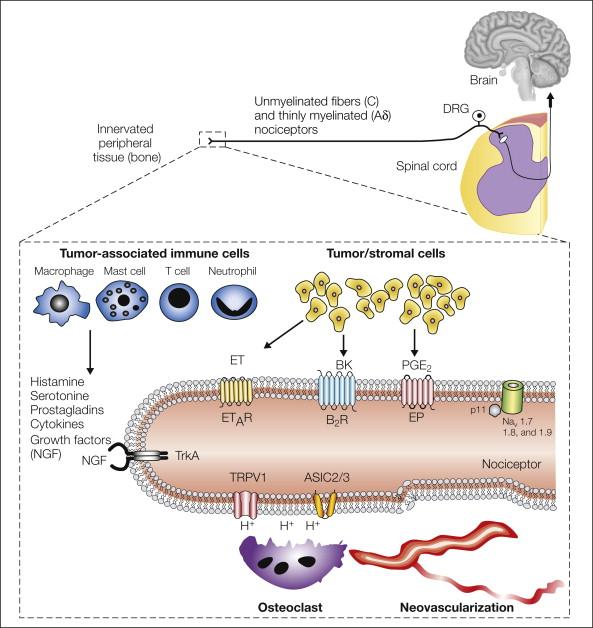
The finding that a subpopulation of sensory neurons express transient receptor potential vanilloid 1 (TRPV1, which is also known as the capsaicin receptor) and the acid-sensing ion channel 3 (ASIC3) and that both these channels respond to acidosis is of significant interest to researchers studying cancer pain ( ) because cancer cells in general have a lower pH (6.8) than normal cells do (pH 7.2) ( ). Importantly, many tumors that metastasize to bone induce a marked proliferation and hypertrophy of osteoclasts. Osteoclasts avidly resorb bone by generating a pH of 2–4 in their resorption bay, which drives the excessive bone resorption that can ultimately lead to fracture of the tumor-bearing bone ( ). To test whether TRPV1 channels were expressed by sensory nerve fibers that innervate tumor-bearing tissue and whether TRPV1 contributed to cancer pain, an in vivo model of bone cancer pain was explored ( ). In these studies it was shown that a subpopulation of nerve fibers that innervate the tumor-bearing bone express TRPV1, that acute or chronic administration of a TRPV1 antagonist attenuates bone cancer pain, and that disruption of the TRPV1 gene results in attenuation of bone cancer pain ( ). Furthermore, administration of a TRPV1 antagonist to TRPV1 null animals with bone cancer resulted in no further reduction in the pain that was already present in the TRPV1 null mice ( ). To date, the results of human clinical trials with TRPV1 or ASIC3 channel antagonists have not been reported in subjects with cancer pain. However, as discussed later, understanding the role that TRPV1 plays in driving bone cancer pain has provided insight into why therapies that inhibit osteoclasts, including bisphosphonates and denosumab (an anti-RANKL fully humanized monoclonal antibody), are efficacious in reducing bone cancer pain ( , , ).
The skeleton is the most common site for distant metastasis from prostate, breast, thyroid, lung, and renal carcinoma ( ). Once tumor cells have metastasized to bone, a cycle of tumor growth, bone destruction, and the formation of woven bone begins and results in significant pain, skeletal fractures, and hypercalcemia ( ). Cancer cells themselves do not destroy bone but rather they and their associated stromal cells express the receptor activator of nuclear factor κB ligand (RANKL), which binds to the receptor RANK expressed by osteoclasts. Activation of the RANKL/RANK pathway promotes the proliferation and hypertrophy of these bone-destroying osteoclasts ( ). Osteoclasts resorb bone by forming a highly acidic resorption “bay” or “pit” between the osteoclast and bone, which can stimulate TRPV1 or ASIC3 channels and drive bone cancer pain ( ). In the past decade multiple studies have shown that two therapies that reduce osteoclast function also significantly reduce bone cancer pain ( , , , , ).
The first and most widely used therapy is the class of compounds known as bisphosphonates, which avidly bind to bone. Once the bisphosphonate has bound to bone, osteoclasts that are resorbing bone generally need to actively take up the breakdown products of bone at the apical (bone facing) surface and transfer these products via transcytosis for release at the distal surface of the osteoclast so that they can be disposed by exocytosis ( ). However, if a bisphosphonate is tightly bound to the bone that is being resorbed, the bisphosphonate will also be taken up by endocytosis ( ). Once internalized, the bisphosphonate interferes either with adenosine triphosphate energy metabolism (non–nitrogen-containing bisphosphonates) or with the mevalonate pathway (nitrogen-containing bisphosphonates), which results first in osteoclast dysfunction and ultimately in osteoclast apoptosis ( , ). Because a significant population of nerve fibers that innervate bone express TRPV1 ( ), one way that bisphosphonates appear to relieve bone pain is by decreasing osteoclast-induced acidosis, which in turn will decrease activation of the ion-sensing TRPV1 or ASIC3 receptors that are expressed by sensory nerve fibers.
Another method that is highly effective in reducing tumor-induced osteoclast bone resorption in both animals and humans is interference in the binding of RANKL to RANK, which is required for osteoclast proliferation and maturation ( ). Within 2 days of administration of therapies that interfere with binding of RANKL to RANK (such as osteoprotegerin or denosumab), there is an almost complete loss of activated osteoclasts, a marked reduction in plasma markers of bone resorption, and significant attenuation of bone cancer pain in a mouse model of bone cancer pain ( ).
Therapies that inhibit osteoclast-induced bone resorption also maintain the mechanical strength of bone even though tumor cells are present in the bone. Thus, in addition to acidosis, excessive tumor-induced osteoclast bone resorption destroys bone and leads to mechanical instability and fracture of bone, which causes mechanical distortion of the nerve fibers innervating the bone ( , ). Thus, following significant weakening or fracture secondary to tumor-induced bone remodeling, significant movement-evoked pain can occur, presumably because of mechanical distortion of the mechanosensitive sensory nerve fibers that innervate the bone. Clearly, pain associated with the fracture is attenuated if the bone is stabilized and repositioned into its normal orientation ( ). Both osteolytic and osteoblastic tumors induce loss of the mechanical strength and stability of mineralized bone ( ), so with significant bone remodeling, normally innocuous mechanical stress can now result in distortion and activation of the mechanosensitive nerve fibers that innervate the bone. Since bisphosphonates and anti-RANKL therapies reduce tumor-induced osteoclast bone remodeling, preserve the mechanical strength of bone, reduce bone fractures, and decrease osteoclast-induced acidosis in both animals and humans, these therapies are highly useful in managing pain resulting from metastasis of cancer to bone.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here