Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer is characterized by genetic and epigenetic instability leading to both unique and sometimes common mutations and “ectopic” overexpression of genes not normally expressed in the tissue of origin. Cancer-specific proteins arising as a result of genetic mutations appear to provide the most potent antigens visible to the T-cell arm of the immune system. Because the number of cancer-specific antigens expressed varies greatly among individual patients and among cancer histologies, cancer immunogenicity varies substantially.
As cancers develop, they are sculpted by “immune pressure” to eliminate antigens, or diminish the degree to which antigenic peptides are processed or presented to the immune system, through a process termed editing. Multiple cells and molecular interactions in the tumor microenvironment also inhibit antitumor immune responses, including regulatory T cells and myeloid-derived suppressor cells. Tumor-induced immunosuppression is also mediated by signaling CTLA-4 and PD-1, inhibitory receptors on T cells.
Inhibition of CTLA-4 and/or PD-1 signaling on T cells enhances antitumor immunity by unleashing naturally induced adaptive antitumor immune responses that have undergone active suppression. Clinical trials using antibodies that block CTLA-4 have demonstrated impressive effects in melanoma, and clinical trials using antibodies that block PD-1 signaling have demonstrated impressive effects in many cancers, including melanoma, non–small cell lung cancer, renal cell carcinoma, bladder carcinoma, head and neck cancer, Hodgkin lymphoma, Merkel cell carcinoma, and others.
Synthetic biology can be used to engineer immunotherapies toward antigens differentially expressed on cancer versus normal tissues, and such therapeutics do not require inherent tumor immunogenicity to be effective. Examples of such therapeutics are monoclonal antibodies, bispecific antibodies, and T cells engineered to express chimeric antigen receptors. T cells expressing a chimeric antigen receptor targeting CD19 have demonstrated impressive effects against B-cell malignancies.
At least two features of the immune system make it a unique therapeutic tool against cancer. First, the diversity of receptors in the adaptive immune system (T-cell receptor [TCR] for T cells and antibodies made by B cells) offers unparalleled capacity for target specificity, far greater than any synthetic drug library. Second, diverse cell-killing weaponry from both the innate immune system and cytotoxic T cells offers the potential to kill any cell, once appropriately recognized. Central to the concept of successful cancer immunotherapy are the dual tenets that tumor cells express an antigenic target distinct from their normal cellular counterparts and that the immune system is capable of recognizing these antigenic differences.
The notion that the immune system can be used as an anticancer therapy emerged from experiments in animal models of carcinogen-induced cancer. It was demonstrated that a number of experimentally induced tumors could be rejected on transplantation into syngeneic immunocompetent animals. Extensive studies by Prehn on the phenomenon of tumor rejection suggested that the most potent tumor rejection antigens were unique to the individual tumor. These studies led to the hypothesis that the immune system may be harnessed to eliminate cancer cells while sparing normal tissue. This chapter reviews the biology of tumor–immune system interactions and discusses how scientific insights from immunology have translated into novel strategies for harnessing the immune response to treat cancer, highlighting recent developments in T-cell checkpoint-blocking antibodies, adoptive therapies with engineered T cells, and tumor vaccines.
Genetic instability, a hallmark of cancer, is a primary generator of tumor-specific antigens. On average, cancers express between 50 and 1000 missense mutations in coding regions, roughly 20% of which create neoantigenic peptides presented by at least one of the individual's human leukocyte antigen (HLA) alleles and thus recognized by T cells. In addition, deletions, amplifications, and chromosomal rearrangements can result in new genetic sequences resulting from juxtaposition of coding sequences not normally contiguous in untransformed cells. The vast majority of these mutations occur in intracellular proteins, and therefore the “neoantigens” they encode would not be readily targeted by antibodies. However, the major histocompatibility complex (MHC) presentation system for T-cell recognition renders peptides derived from all cellular proteins available on the cell surface as peptide MHC complexes capable of being recognized by T cells. In accordance with the original findings of Prehn, the vast majority of tumor-specific antigens derived from genetic mutations are unique to individual tumors. As a result, antigen-specific immunotherapies targeted at truly tumor-specific antigens are most commonly patient specific. However, some tumor-specific mutations are shared (e.g., KRAS codon 12 G->A in colon and pancreatic cancers; BRAF V600E found in melanomas; p53 codon 249 G->T mutation found in hepatocellular carcinomas) and could serve as potential immunotherapy targets.
Tumors also manifest global alterations in DNA methylation and chromatin structure resulting in dramatic shifts in gene expression. Tumors often overexpress hundreds of genes relative to their normal counterparts, including genes that are normally completely silent in their normal cellular counterparts. Overexpressed genes in tumor cells are attractive targets for both antibody- and cell-based immunotherapies, because overexpressed genes are shared among many tumors of a given tissue origin or sometimes multiple tumor types. For example, mesothelin, which is targeted by T cells from vaccinated pancreatic cancer patients, is highly expressed in virtually all pancreatic cancers, mesotheliomas, and most ovarian cancers. Whereas mesothelin is expressed at low to moderate levels in the pleural mesothelium, it is not expressed at all in normal pancreatic or ovarian ductal epithelial cells. Another example of tumor-selective expression of epigenetically altered genes is the so-called cancer-testis antigens. These genes are expressed almost exclusively in germ cells of the testis and ovaries, and some appear to encode proteins associated with meiosis. Many cancer-testis antigens are recognized by T cells from nonvaccinated and vaccinated cancer patients. A final category of tumor antigen consists of tissue-specific differentiation antigens shared by tumors of similar histologic origin. Commonly generated melanoma-reactive T cells from melanoma patients recognize melanocyte antigens, including tyrosinase, a melanocyte-specific protein required for melanin synthesis. Although tissue-specific antigens are not truly tumor specific, they are nonetheless potentially effective targets when the tissue is dispensable (e.g., prostate cancer or melanoma).
The immune surveillance hypothesis, first conceived nearly a half century ago, proposed that a fundamental role of the immune system is to survey the body for tumors as it does for infection with pathogens, recognizing and eliminating them based on their expression of tumor-associated antigens (TAAs). In animal models, carcinogen-induced tumors can be divided into those that grow progressively (termed progressor tumors ) and those that are rejected after an initial period of growth (termed regressor tumors ). The phenomenon of regressor tumors was explained based on ongoing immune surveillance of cancer. A corollary to the original immune surveillance hypothesis is that progressor tumors in animals (presumed to represent clinically progressing cancers in humans) fail to be eliminated because they develop active mechanisms of either immune escape or resistance ( Fig. 6.1 ).
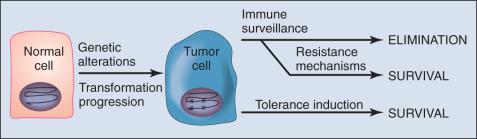
Genetically manipulated mice have provided clear evidence that the immune system can eliminate both carcinogen-induced and spontaneously arising cancers. When profoundly immunodeficient mice were treated with carcinogens or crossed onto a cancer-prone p53 knockout, the incidence of cancers was modestly but significantly increased relative to nonimmunodeficient counterparts when observed over an extended period (greater than 1 year). Further, tumors that arise in immunodeficient animals behave as regressor tumors when transplanted into immunocompetent animals. These findings are consistent with a model wherein tumors that arise in immunodeficient animals would have been eliminated had they arisen in immunocompetent animals.
Epidemiologic studies of patients with heritable immunodeficiencies have also confirmed a significantly increased risk of certain cancers that are distinct from the epithelial cancers commonly observed in normal immunocompetent adults. Although many cancers observed in immunodeficient individuals are associated with viral infections, (e.g., Epstein-Barr virus [EBV]–associated lymphomas, Kaposi sarcoma–associated herpesvirus [KSHV]–associated Kaposi sarcoma, and human papillomavirus [HPV])–associated cervical cancer ), immunodeficient individuals also demonstrate an increased incidence of non–pathogen-associated cancers, particularly melanoma.
Evidence from murine and human tumors demonstrates the capacity for tumors to induce T-cell tolerance to their antigens. Tolerance induction among tumor antigen–specific T cells can be an active process involving direct antigen recognition or can be associated with failure of antigen recognition by T cells—that is, the immune system “ignores” the tumor. However, tumors do not uniformly tolerize T cells. Ohashi and colleagues observed that lymphocytic choriomeningitis virus (LCMV) GP33–specific TCR transgenic CD8 T cells adoptively transferred into mice expressing pancreatic islet cell tumors that express GP33 manifested evidence for CD8 T-cell activation as a result of antigen cross-presentation in the draining lymph nodes. Despite the activation of tumor-specific T cells, the tumors grew progressively, indicating that the degree of immune activation induced by tumor growth was insufficient to ultimately eliminate the tumors. These results suggest that in some cases T-cell responses are induced to developing tumors, but if the level of immune activation ultimately does not “keep up” with tumor progression, the ultimate result is tumor outgrowth. In the case of the LCMV GP33-specific TCR transgenic mice, because neither anergic nor deletional tolerance was observed, animals treated with the dendritic cell (DC) stimulatory anti-CD40 antibody demonstrated significant slowing of tumor growth. Thus depending on the mechanism of immune escape at play, agents that affect the overall activation state of either antigen-presenting cells or T cells could be used to shift the balance between tumor immune evasion and tumor immune recognition.
T cells retrieved from patients with cancer tend either to be of low affinity for their cognate antigen or to recognize antigens that bind poorly to their presenting HLA (human MHC) molecule, resulting in inefficient recognition by T cells. Furthermore, tumors commonly acquire defects in MHC class I expression to avoid immune destruction. MHC class I proteins display small peptide antigens on the surface of cells and are key for immune recognition by CD8 T cells. For example, activated HRAS leads to reduced levels of messenger RNA (mRNA) transcripts of the transporter associated with antigen processing (TAP), a key protein in the antigen-processing pathway. Repression of proteins involved in the antigen presentation pathway are linked to overexpressed epidermal growth factor receptor (EGFR) or HPV E7 oncoprotein. MHC class I downregulation can also result from MHC gene mutations, defects in other genes in the antigen presentation pathway such as ERAP1 and ERAP2, and epigenetic regulation of TAP genes.
The tumor microenvironment is replete with suppressive mechanisms that dampen antitumor immune immunity ( Fig. 6.2 ). The inhibitory cells, molecules, and signaling pathways found in the tumor microenvironment are not unique to tumors, but rather compose an array of physiologic mechanisms evolved to regulate immune responses to self-antigens and to downmodulate immune and inflammatory responses to foreign antigens so that collateral tissue damage is limited. Tumors co-opt and upregulate mechanisms for resistance to immune attack, and much activity in the field of immuno-oncology is focused on delineating these pathways and developing therapeutics capable of reversing the effects of these mediators.
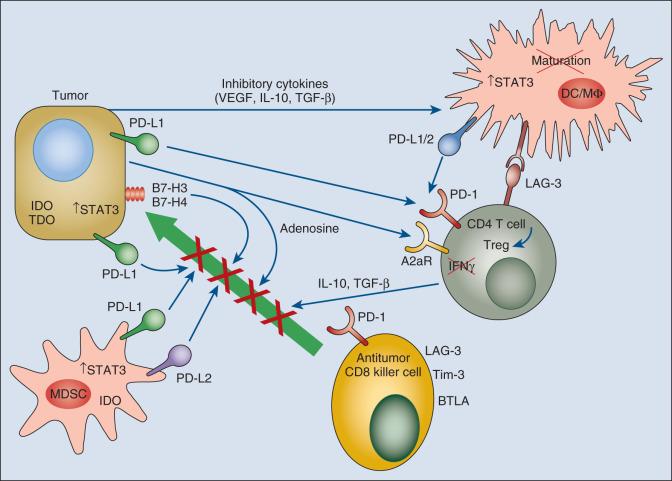
Regulatory T cells (Tregs) maintain tolerance to self-antigens, downregulate immune responses to pathogens, and induce tolerance to tumor antigens. CD4+ Tregs are characterized by expression of Foxp3, a central master regulatory transcription factor. Other Tregs produce inhibitory cytokines such as interleukin (IL)-10 and transforming growth factor–β (TGF-β). In addition, a recently described IL-12 family “hybrid” cytokine, IL-35, consisting of the alpha subunit of IL-12 and the beta subunit of IL-27, is produced by Tregs and suppresses antitumor immunity. Numerous murine studies have demonstrated that Tregs expand in animals with cancer and limit the potency of antitumor immune responses. It is now appreciated that treatment with low-dose cyclophosphamide is a relatively simple and reasonably effective way to temporarily eliminate cycling Tregs. In addition, antibodies neutralizing IL-35 limited tumor growth in multiple preclinical mouse models, but their use alone was not sufficient to eliminate tumors. As new cell membrane molecules that define Tregs are identified, the capacity to block regulatory T-cell activity with antibodies to these molecules presents new opportunities for immunotherapeutic strategies to break tolerance to tumor antigens. Evidence for a role of Tregs in suppressing antitumor immunity in humans includes an extensive study correlating Treg number in resected ovarian cancers with clinical outcome. Patients with greater numbers of CD4+CD25hiFoxp3+ cells had a worse outcome. A number of clinical trials have been performed using a toxin-conjugated IL-2 reagent (denileukin diftitox) that would bind CD25 and selectively kill Tregs. Clinical efficacy of this agent was described in phase III studies of recurrent or refractory cutaneous T-cell lymphoma, and the drug was approved by the US Food and Drug Administration (FDA) for this indication, but the agent is currently not available owing to the manufacturer's decision to prioritize development of a newer improved formulation of the drug.
Immature myeloid cells (iMCs) are a heterogeneous class of cells that include myeloid-derived suppressor cells (MDSCs) as well as tumor-associated macrophages (TAMs). In mice, iMCs and MDSCs are characterized by coexpression of CD11b (considered a macrophage marker) and Gr1 (considered a granulocyte marker) while expressing low or no MHC class II or the CD86 costimulatory molecule. In humans, MDSCs are most commonly described as CD33+ but lack MHC class II expression, as well as markers of mature macrophages, DCs, or granulocytes. A number of molecular species produced by tumors tend to drive iMC and MDSC accumulation, including IL-6, colony stimulating factor 1 (CSF-1), IL-10, and gangliosides. IL-6 and IL-10 are potent inducers of STAT3 signaling, which has been shown to be important in iMC and MDSC persistence and activity. Macrophages have been divided into M1 type (Th1/interferon [IFN] instructed) and M2 type (Th2/IL-4 and IL-13 instructed). M1 macrophages are characterized by expression of genes encoding the nitric oxide (NO)–producing iNOS-2 enzyme and the cytokine IL-12, which amplifies Th1 responses. M1 macrophage activation is thought to be one of the mediators of Th1-orchestrated antitumor immunity, whereas M2 macrophages are thought to be tumor promoting.
Macrophage infiltration into tumors has been largely associated with poor patient prognosis in multiple tumor types. Therapeutic strategies for targeting TAMs include inhibition of macrophage recruitment to the tumor or promotion of their antitumor properties or effector function. Strategies for preventing macrophage accumulation in tumors include disruption of the CCL2/CCR2 axis in phase I clinical trials in patients with advanced-phase solid cancers. The CSF-1/CSF-1R pathway is also being evaluated as a therapeutic strategy to target tumor TAMs, and agents targeting these pathways have been shown to reduce TAM accumulation in tumors or repolarize them into an antitumor phenotype in early-phase clinical trials. Strategies to promote phagocytosis and antigen presentation by TAMs are very promising—for example, by disrupting the CD47-SIRPalpha axis, which has been shown to be effective in preclinical models and is being tested in ongoing clinical trials.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here