Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mammals such as humans are exposed to their greatest temperature-related shock at birth. Coming from a protected and thermoneutral environment, the newborn infant is suddenly exposed to “cool” surroundings, where survival depends on self-generation of sufficient heat to keep warm. The development of the ability to regulate body temperature regardless of the temperature of the surroundings (and through this to ensure that activity of the organism is constant and high) apparently was a necessary step in the evolution of so-called higher animals . A requirement for this development was that newborns be endowed with a physiologic ability to produce the heat essential for survival. To accomplish this, an organ was developed without parallel in ectothermic animals, an organ endowed with the function of producing heat exactly when required, not least around the time of birth. This organ is brown adipose tissue.
Thus, in evolutionary terms, brown adipose tissue is a rather new organ. Even in the scientific community, brown adipose tissue is rather new. Its function as a heat producer in the cold and in newborns , was first described only 60 years ago. However, during subsequent years, understanding has grown about the events responsible for the heat production in that tissue, and at present, researchers are actively elucidating mechanisms underlying the recruitment processes in brown adipose tissue (i.e., those processes that, during perinatal development, are responsible for the growth and differentiation of the tissue).
One of the most important accomplishments in research on brown adipose tissue thermogenesis has been the recognition that the ability to produce heat resides in the fact that brown adipose tissue mitochondria are endowed with a unique protein—the original uncoupling protein-1 (UCP1), also known as thermogenin . This protein functions as a regulated protonophore through the mitochondrial membrane. The identification of this protein consolidated, in many ways, research on brown adipose tissue. Thus, a description of the function and fate of this protein must be a central issue in a review on the perinatal recruitment of brown adipose tissue.
Although it was earlier accepted that brown adipose tissue was found only in young mammals, it has been convincingly demonstrated (reviewed by Nedergaard and colleagues ) that brown adipose tissue is indeed present in most adults, where it has a thermogenic function and is regulated just as in experimental animals. These realizations came from investigations using positron emission tomography (PET) with 18 F-fluorodeoxyglucose (FDG) as the radioactive tracer. Extensive uptake of FDG occurred occasionally in some fatty depots. It became apparent that if patients were feeling cold at the time of the examination, then areas in the neck and supraclavicular area took up significant amounts of tracer. Retrospective studies from hospital archives showed random occurrence of uptake in these depots as the environmental conditions were not controlled. More recent studies have used healthy subjects under controlled conditions and show correlations between FDG uptake in brown adipose tissue and nonshivering thermogenic capacity, with chronic environmental temperature, , with degree of obesity, and inversely with aging. While this method provides significant information, it is inappropriate to use frequently in healthy subjects. The only other available methods to determine the presence of brown adipose tissue (but not its acute activity) are invasive and therefore also inappropriate for extensive studies. Magnetic resonance imaging and infrared thermography techniques are now being evaluated and will hopefully provide more suitable means of study in the near future.
Retrospective analyses of PET scans of young children have also been performed from hospital archive material. However, although such investigations can provide some information on tissue abundance, , they have obviously not been performed under optimal conditions for brown adipose tissue detection, so there is a large information deficit here. There are reports suggesting certain levels of abundance in children, but these are unable to give reliable information, since the environmental temperature has not been suitable for optimizing the visibility of brown adipose tissue. Thus, these give only minimal levels and should not be viewed otherwise. The ethical aspects of using PET to study children preclude attaining further information until a reliable alternative method becomes available.
Experiments describing the function of brown adipose tissue in the perinatal animal have generally been performed in rat fetuses and rat pups, and if not specifically stated, the animal referred to here is the rat.
Significant differences in perinatal development of brown fat (and many other characteristics) have been documented among newborns of different species. Broadly speaking, three different groups of newborns can be distinguished: altricial newborns, so-called true immature newborns, and precocial newborns.
The altricial (literally, nest-dependent) newborns are often born as members of litters of more than five pups. They are poorly developed at birth, have no fur, are blind, and move poorly, if at all. To keep warm, they huddle together. As discussed later on, development of brown adipose tissue in these newborns is a slow process that starts just before birth and reaches its maximum some days after birth. The main driving force for this recruitment is probably the (comparatively) low environmental temperature coupled with the attempt by these newborns to activate processes to oppose hypothermia. Rat and mouse pups belong to this group. The morphologic and ultrastructural development of the tissue in these animals has been described.
The immature (not to be confused with premature) neonate is not able to respond to the environmental temperature at birth and is truly poikilothermic. In species with immature newborns, the recruitment process in brown adipose tissue starts only when the central control system has developed, after which processes occur that seem similar to those elicited immediately after birth in the altricial newborns. Hamster pups and possibly the newborns of marsupials , belong to this group. (However, it is unlikely that marsupials at any stage of development have thermogenically active brown adipose tissue, despite the presence of a UCP1 ortholog.)
The precocial newborns are normally born singly or in small litters. They are very well developed, their eyes are open, they are furred, and they can walk around shortly after birth. These newborns are born with well-developed brown adipose tissue, which tends to atrophy postnatally. It is unknown how this intrauterine recruitment of brown adipose tissue is accomplished. Certain small newborns, such as the guinea pig, belong to this group, but more obvious members are the newborns of many larger species, such as lambs, goats, calves, , and musk oxen.
Classification of the human infant (or other primate infants ) into one or the other of these groups is not self-evident. Although born in a somewhat developed state, the human infant has several traits of immaturity and probably is most akin to the altricial newborns—specifically, human perinatal brown adipose tissue development has similarities to that found in the newborn rat pup.
Experiments concerning the function of most body organs are helped from a practical standpoint by the fact that most organs are anatomically well-defined entities with a single localization in the body and with structurally homogeneous and stable cells. Unfortunately, such characteristics do not pertain to brown adipose tissue.
Brown adipose tissue is found in many depots within the body; six major depots have been identified in the human infant and constitute 90% of the total brown fat. These include the perirenal depots, interscapular and cervical depots, and periaortic depots. The remaining 10% is found in seven other sites. ,
Distinguishing between white and brown fat is not straightforward. When activated, brown adipose tissue can be distinguished from white by its ability to express UCP1. However, because the potential ability to express UCP1 in a given cell (in a given physiologic situation) may not have been invoked at the moment of study, it is likely that each anatomically defined depot contains cells that are genuinely “white,” as well as cells that are only visually disguised as white but that still carry the potential to be brown-like. Leptin is well expressed in white adipose tissue, but it is not expressed in recruited (i.e., activated) brown adipose tissue. However, it is expressed in nonactive brown adipose tissue, and its expression in both adipose tissues is inhibited by sympathetic stimulation.
Morphologically, even epididymal white adipose tissue can be altered by an intense cold stress to visually resemble brown adipose tissue and to have brown fat-like mitochondria. , However, not even under these extreme circumstances was it originally possible to detect UCP1 as a protein immunologically. More recently, the use of quantitative polymerase chain reaction has allowed the detection of UCP1 messenger RNA (mRNA) in epididymal fat depots, although its functional significance is unclear and thermogenically limited. Thus, within this depot, apparently mainly “true” white fat cells are present, with few potentially brown-like precursors. By contrast, in the parallel female tissue, the parametrial fat pad, good evidence has emerged for the presence of genuine brown-like fat cells (i.e., those containing UCP1).
In certain species, the subcutaneous adipose tissue (which is generally believed to have storage and insulatory properties) can be or become brown-like. As indicated by a functional analysis of the mitochondria in the subcutaneous adipose tissue of newborn seals and in dog pups treated with sympathomimetics, the presence of UCP1 in subcutaneous tissue can be readily discerned. In these species, the subcutaneous adipose tissue could even work as an “electric blanket.” Even in mouse strains where UCP1 is fairly well expressed in inguinal adipose depots, the total thermogenic capacity is nonetheless only a fraction of that in the total brown depots. Thus, although certain fat depots apparently lack a significant capacity for conversion to brown-like fat, most depots have this potential to some degree, and UCP1 may be expressed under certain physiologic or pharmacologic circumstances.
As a general rule, more visible brown adipose tissue depots are present in the newborn than in the adult, even the cold-acclimated adult. It has been stated that in some newborn species, all adipose tissue is brown, and these depots are converted to white adipose tissue in the adult. However, in cell biologic terms, the meaning of this statement is vague. It is now clear that the adipocytes in certain classic brown depots have a close lineage relation with a skeletal muscle lineage. However, the brown-like depots, also termed beige or brite (brown-like in white), may or may not be muscle-related depending on localization. Some brown-like depots are related to smooth muscle. Certainly, the behavior of certain depots that look white in the adult is functionally close to that of brown adipose tissue in the newborn, and the fat in these depots retains some potential to again become brown-like.
Heat production may result from shivering or nonshivering thermogenesis. In the newborn infant and in the adult animal, the first resource used for extra heat production is nonshivering thermogenesis. Only after this capacity is used does shivering begin. In an adult living in a warm environment, with little active brown adipose tissue, shivering is initially the dominant source of extra heat production. However, in the infant, the ability to shiver sufficiently to produce substantial amounts of heat does not seem to be fully developed, so brown adipose tissue is the sole source of heat production.
Most heat produced during nonshivering thermogenesis results directly from the activity in brown adipose tissue of the unique mitochondrial UCP1 (i.e., thermogenin); in the absence of this protein, no heat is produced in brown fat cells. , Heat production is governed by signals from the hypothalamus. These signals are relayed through the sympathetic nervous system and transmitted to the cell as a norepinephrine stimulus leading to a series of events within the cell (for an overview, Fig. 32.1 ). Sympathetic innervation to the tissue is dense. One system of fibers, which contains co-stored neuropeptide Y, innervates the numerous blood vessels entering the tissue, and another system innervates the adipocytes. The neuropeptides calcitonin gene-related peptide (CGRP) and substance P also are found in the tissue, the latter in afferent nerves; however, the localization of CGRP is currently not completely understood, and the functions of these neuropeptides are unknown.
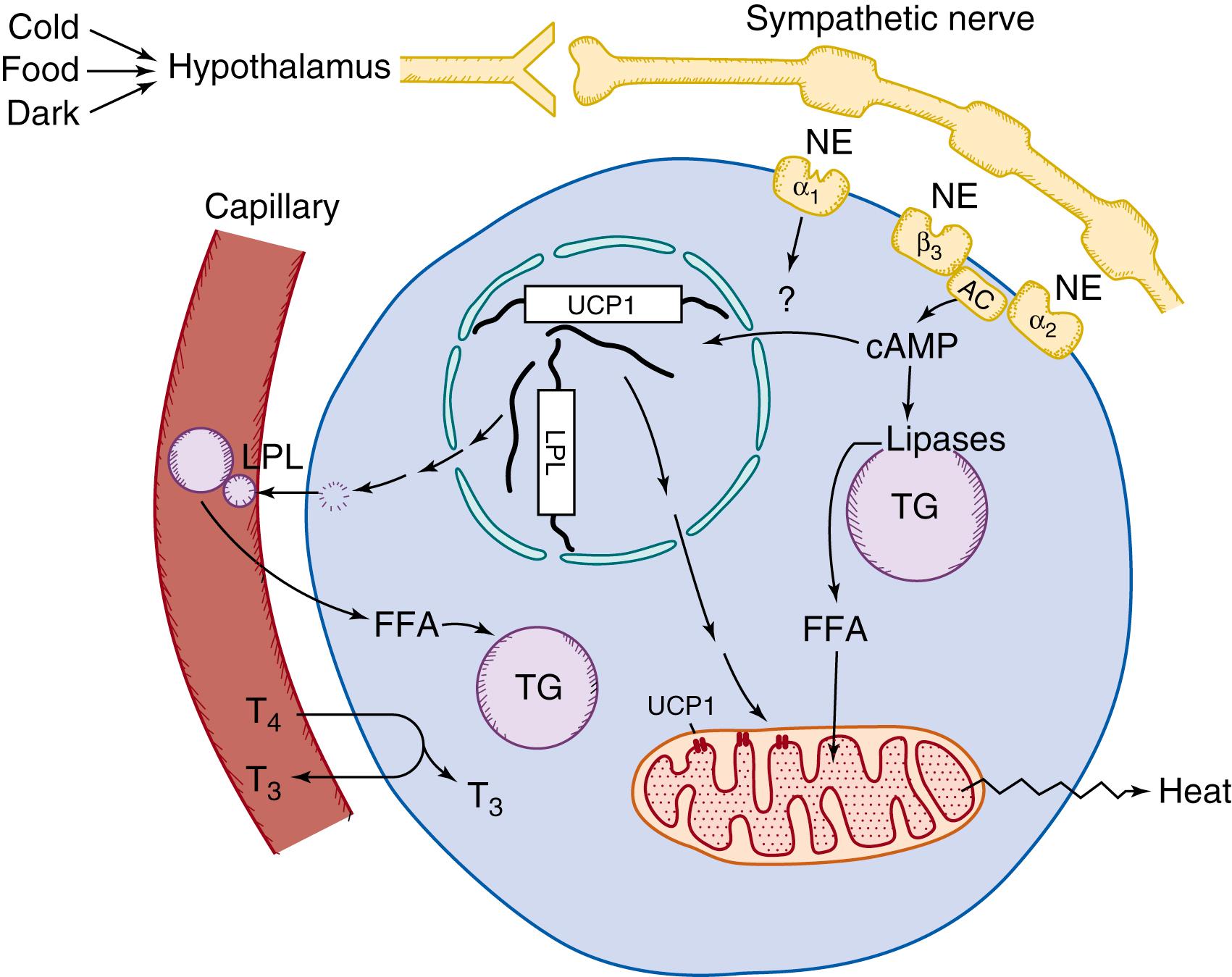
Considerable research by several groups of investigators led to the identification of UCP1 as the key enzyme in nonshivering heat production. UCP1, discussed earlier as thermogenin or as the nucleotide-binding protein, the 32-kDa protein of brown fat, or the guanosine diphosphate (GDP)-binding protein, has also been referred to as the proton conductance pathway of brown adipose tissue mitochondria. Much is known today about this protein ( Fig. 32.2A ). It has been sequenced both as a protein and from complementary DNA (cDNA) clones corresponding to the UCP1 mRNA. The amino acid sequence is known for many species of mammals. The amino acid sequence of the human protein is 79% homologous to that of the rat protein, allowing considerable immunologic cross-reactivity between human UCP1 and rodent UCP1. The human UCP1 gene has also been isolated , and its organization analyzed.
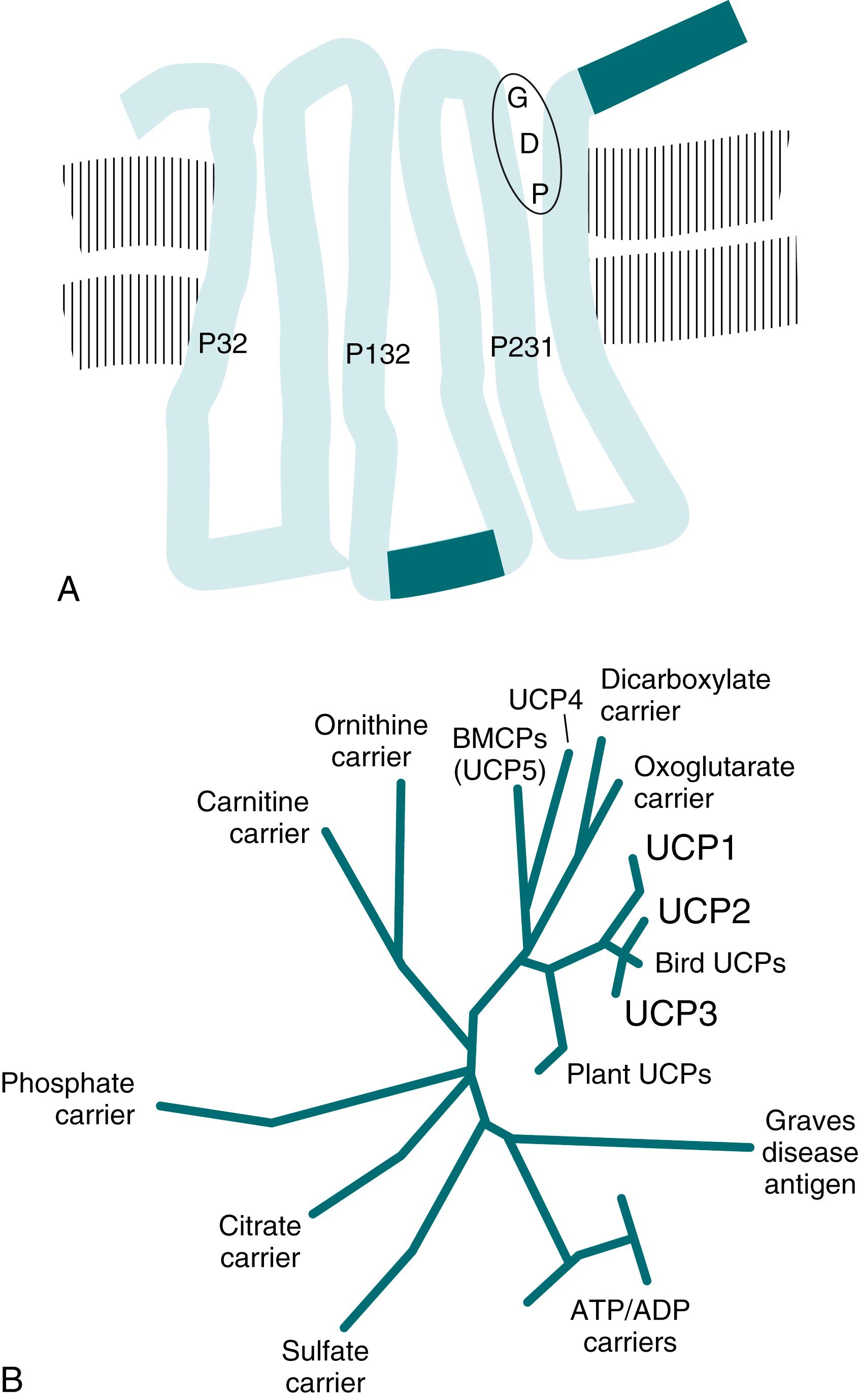
UCP1 is a member of the superfamily of mitochondrial carrier proteins (see Fig. 32.2B ). Most closely related to UCP1 are two more recently identified but evolutionarily more ancient proteins known as UCP2 and UCP3 because of their extensive homology with UCP1. The functions of these proteins remain unclear. However, they are not responsible for any thermoregulatory thermogenesis. Other proteins (e.g., UCP4, UCP5) are not closely related to UCP1; therefore functional relationships cannot be expected.
From analysis of the amino acid sequence, it has been proposed that UCP1 may constitute three membrane-spanning Us (see Fig. 32.2A ). When functionally inserted into the brown fat mitochondrial inner membrane, UCP1 endows these mitochondria with a series of unique properties, including a high rate of respiration in the absence of adenosine diphosphate or uncoupler addition, a high proton conductivity, and a high Cl − conductivity.
The UCP1 gene is found on chromosome 8 in mice and on chromosome 4 in humans. Only one copy of the gene is present.
An interesting functional and evolutionary connection between the exon-intron pattern of the UCP1 gene and the suggested transmembrane structure of the protein has been described. Each of the six proposed membrane-spanning segments is represented by one exon. , , This pattern is also found in the other UCP genes.
The function of UCP1 in the mitochondrial membrane is depicted in simplified form in Fig. 32.3 . UCP1 acts as the equivalent of a proton translocator, allowing dissipation of the proton gradient that has arisen from the functioning of the respiratory chain.
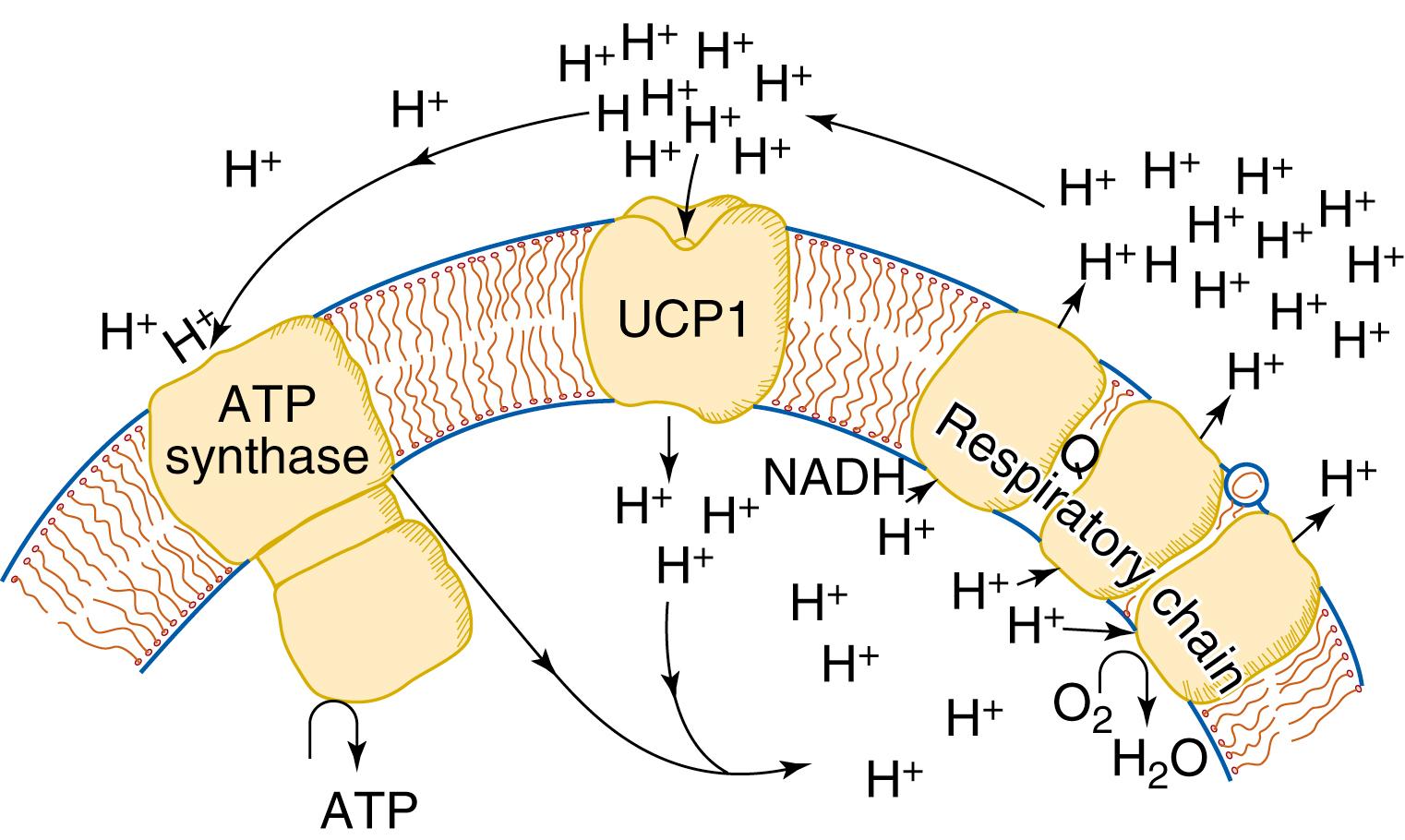
UCP1 cannot be constantly active, because such activity would lead to constant high heat production in the tissue, irrespective of the environmental (or ambient) temperature. In experiments with isolated mitochondria, it has been observed that the activity of UCP1 can be inhibited by the addition of purine nucleotides. , Traditionally, GDP has been the nucleotide of choice for such experiments, and thus UCP1 has been known as the GDP-binding protein . This protein is not related to the G-proteins in signal transduction or to the ribosomal guanosine triphosphate (GTP)-binding proteins. The term may be considered to be physiologically misleading, because adenosine triphosphate (ATP) is as good as or better than GDP for both binding and inhibiting respiration. , Because ATP is present in much higher concentrations in the cytosol of brown fat cells than is GDP, ATP is the physiologically relevant nucleotide that binds to UCP1 and inhibits thermogenesis when thermogenesis is not physiologically required.
The affinity of UCP1 for ATP is so high that the binding site would probably always be saturated with ATP and thermogenesis inhibited if this were the only agent that interacted with UCP1. Thus, it is necessary to postulate the existence of a physiologic activator of UCP1. The nature of this “positive” modulator continues to be investigated. A detailed analysis of the problem is outside the scope of this chapter, but a few main points can be summarized here.
Among the many suggestions, the simplest hypothesis for the nature of this modulator is that it is produced during the release of fatty acids, occurring as an effect of stimulation of the cell. Within this framework, several alternatives have been proposed. The positive modulator could be the free fatty acids themselves. , , The most telling experiments that indicate a direct effect of free fatty acids are those of Rial and colleagues. More recent experiments support this theory. , Alternatively, fatty acids have been suggested to have a catalytic function, although in this case a positive modulator may be involved. , Another hypothesis along the same line has suggested that palmitoyl-coenzyme A (CoA) may interact directly with UCP1. , Each postulate has advantages and problems. Although conclusive experiments have been published that the modulator is downstream of lipolysis, general agreement on the mechanism has not yet been reached.
Under certain conditions, apparently no direct correlation has been established between the amount of UCP1 (e.g., as measured immunologically) and the activity of UCP1 (as estimated by GDP binding or proton or Cl – translocation). When the level of activity is found to be lower than expected, the phenomenon has been termed masking of UCP1. UCP1 is thus typically masked when it is inactive (as is probably the case in utero) but becomes unmasked when called into function. Accordingly, it is reasonable to speculate that the rapid increase in GDP-binding capacity seen shortly after birth in guinea pigs may be due to this unmasking mechanism.
The total amount of UCP1 in the newborn is expected to be the rate-limiting factor for nonshivering thermogenesis. In newborns and adults, the amount of UCP1 is under precise regulation. Although a significant temporal delay between a change in UCP1 mRNA and the ensuing increase in the amount of UCP1 has been documented, the simplest explanation is that the mRNA amount determines the protein amount.
The major stimulus for UCP1 gene expression is from the sympathetic nervous system through the release of norepinephrine. It is well recognized that β-adrenergic receptors are involved in this regulation. Furthermore, some evidence indicates that simultaneous α-stimulation is necessary in vivo. Perhaps this α-stimulation is of a permissive nature.
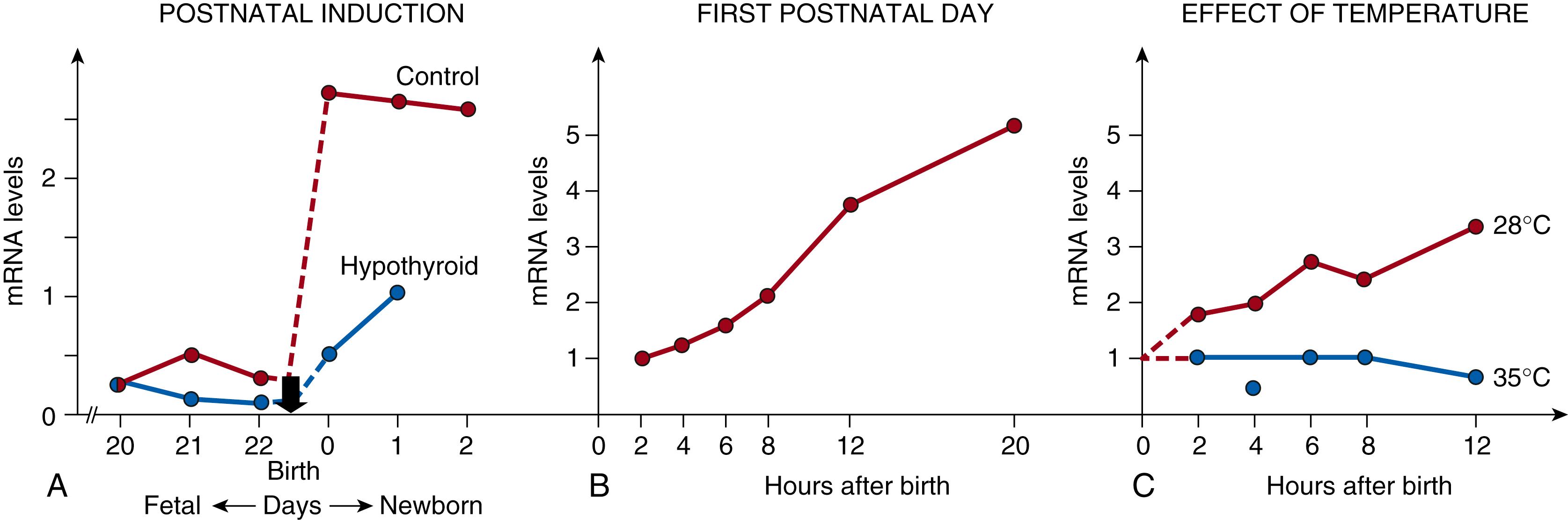
The amount of UCP1 increases around the time of birth (in mice and rats). , This increase is caused by a concomitant increase in the amount of UCP1 mRNA ( Fig. 32.4A and B). The cause of the postnatal recruitment in brown adipose tissue (and increase in UCP1 mRNA) could be either ontogenic or an effect of the cold stress experienced by the pups at birth. No postnatal increase in UCP1 mRNA occurs in the absence of a postnatal cold stress (see Fig. 32.4C ); therefore, in these species, the postnatal brown adipose tissue recruitment is presumably a response to the cold stress experienced by the newborn. This correlation indicates that the increase is mediated by sympathetic activation, as in adults.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here