Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Male predominance (2:1 to 3:1)
Age ranges from second decade to elderly, with most cases occurring in fourth and fifth decades
Occurs most commonly in skull bones, including mandible, maxilla, frontal sinuses, ethmoid sinuses, paranasal sinuses, orbital bones, and calvarium; rarely involves the clavicles and long bones
May be asymptomatic or, if in sinuses, may present with signs of obstruction, including sinusitis and nasal discharges
Orbital tumors may produce diplopia, exophthalmos, and blindness
Radiodense, circumscribed surface, or intramedullary mass usually without destructive features
Nodular or dome-shaped, dense cortical bone
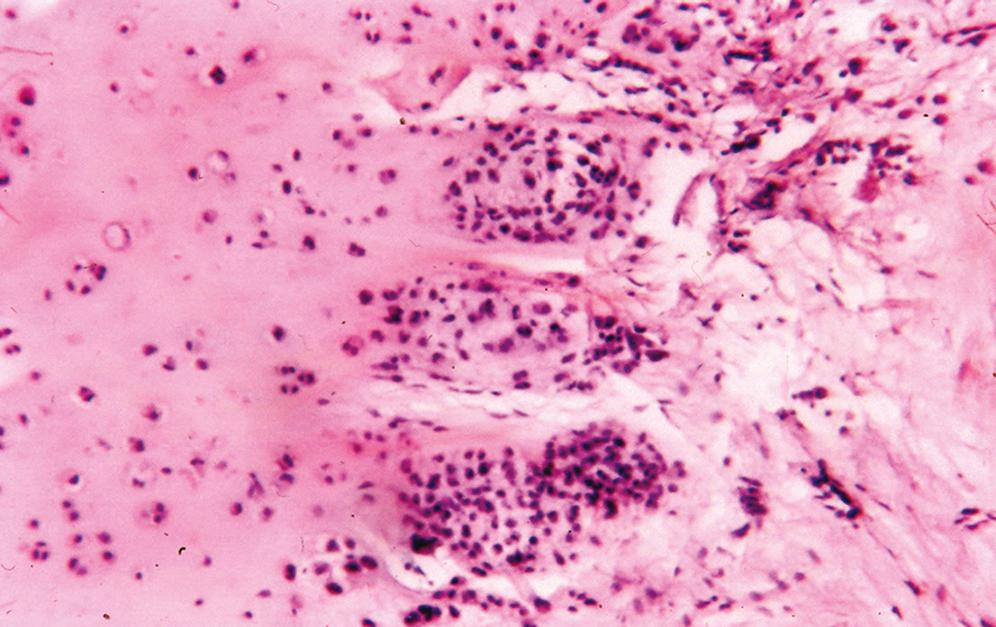
Consists of dense lamellar bone with or without haversian canals and usually without a medullary component ( Figure 16.1 )
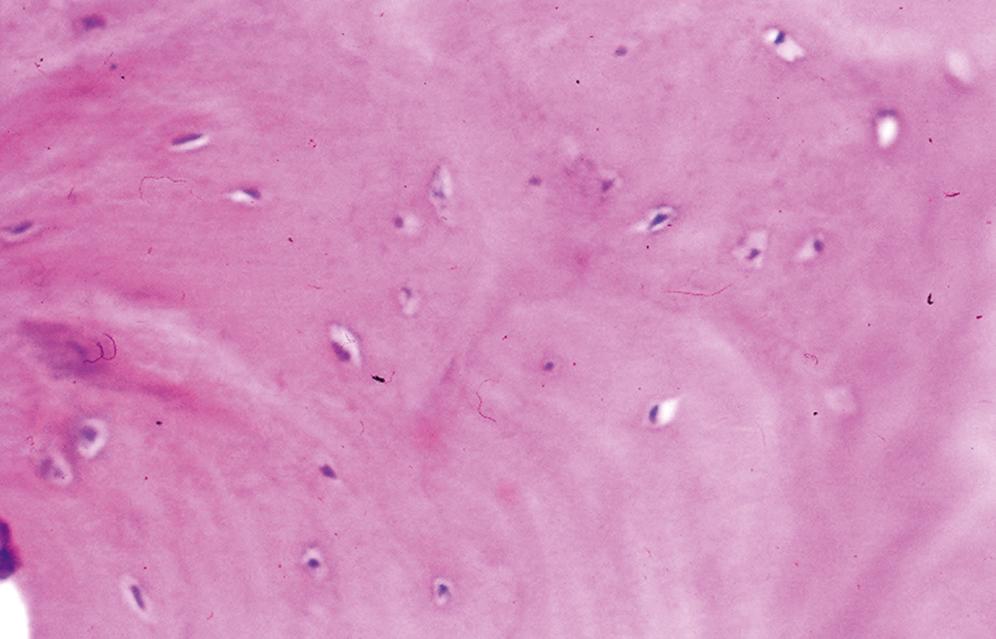
When a medullary component is present, it is represented by hematopoietic tissue or fibroadipose tissue; the process extends up to uninvolved bone and does not blend in with the adjacent normal bone
Noncontributory
Noncontributory
Lamellar bone with prominent osteoblastic rimming
Osteoma may have focal areas of reactive bone with similar features
Tumor osteoid is arranged in parallel arrays and separated by a hypocellular fibroblastic stroma
Asymptomatic, nodular, radiodense tumor involving craniofacial bones and composed of mature osteoid is typically an osteoma
Gardner syndrome (colonic polyposis, fibromatoses, osteomas, and epidermal cysts of skin) should be considered in the presence of multiple osteomas or osteomas of long bones
If surgically removed, recurrences rarely develop; no reported cases of malignant transformation
Male-to-female ratio of 3:1
Usually occurs in second or third decade
Most commonly occurs in the leg, usually in the proximal femur
May involve tibia, vertebra (arch more so than body), and small bones of foot and hand
Typically intracortical tumors
Classic clinical presentation includes progressive pain that is greater at night and is relieved by aspirin
Depending on the site, other symptoms may develop
Vertebrae: peripheral nerve compression and painful scoliosis owing to muscle spasms (symptoms of intravertebral disk disease)
Upper and lower extremities: peritumoral muscular atrophy
Epiphyseal tumors: skeletal asymmetry, arthritis, and joint effusions
Routine radiographs reveal a small, round, central area of radiolucency (nidus) surrounded by sclerosis
Nidus is usually cortical in location and may exhibit central ossification
When plain radiographs fail to reveal the tumor (about 25%), tomograms, bone scans, computed tomography (CT), or magnetic resonance imaging (MRI) may be necessary
Dense sclerotic bone surrounds a central nidus that is round, red, soft, and friable; nidus may be granular if ossified
Typically less than 1 cm
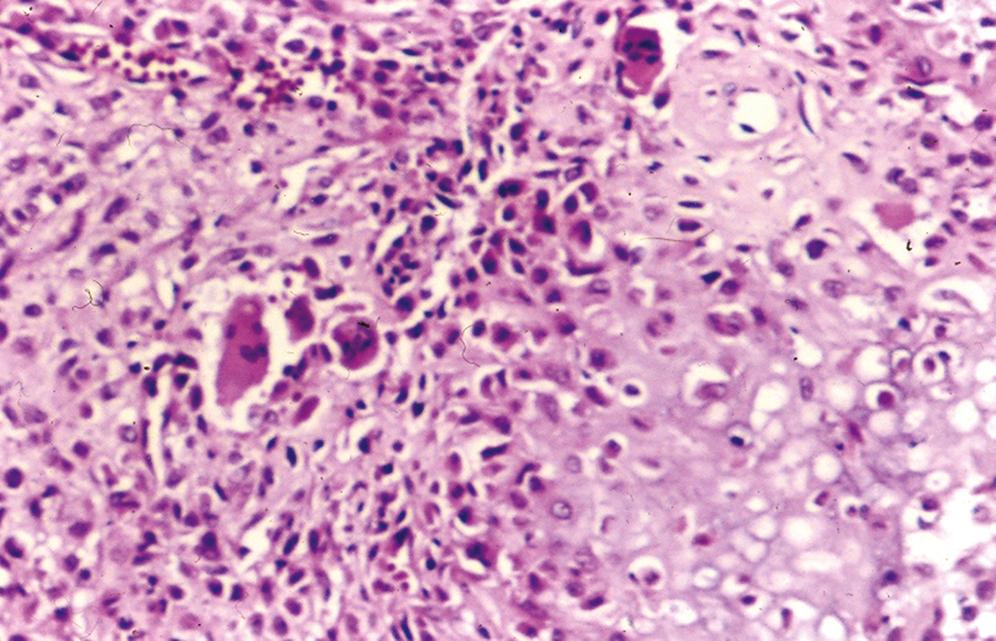
Central nidus is composed of interlacing thin bone trabeculae or woven bone with variable degrees of mineralization ( Figure 16.2 )
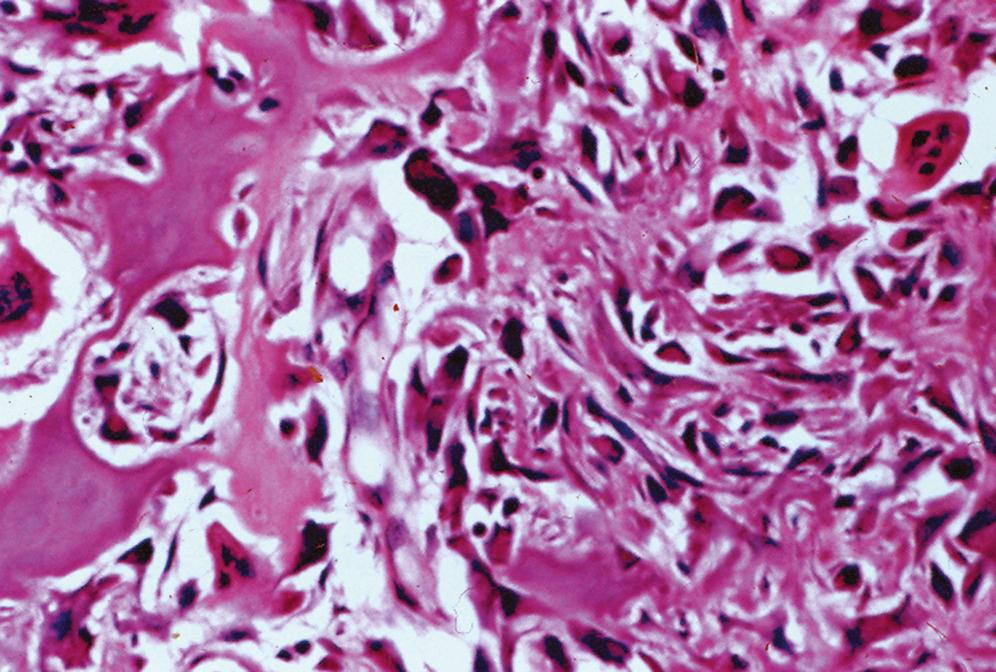
Trabeculae may vary in thickness
Prominent benign osteoblastic rimming of the trabeculae and multinucleated osteoclast-like giant cells are present within intervening fibrovascular stroma
Outside the nidus is an abrupt zone of fibrovascular tissue surrounded by sclerotic compact lamellar bone
No cartilage in the tumor unless there has been a fracture at the tumor site
No hematopoietic tissue or adipose tissue within the tumor
In short decalcified biopsies, FosB proto-oncogene, AP-1 Transcription Factor Subunit (FOS) immunohis- tochemistry can be used to diagnose osteoid osteoma and osteoblastoma, as overexpression is seen in the majority, while being rare in their mimics
Osteoid osteoma is shown to harbor FOS (87%) and FosB proto-oncogene, AP-1 Transcription Factor Subunit (FOSB) (3%) rearrangements
Preoperative tetracycline allows osteoblastic incorporation in the nidus, which is fluorescent under ultraviolet light
Preoperative intravenous technetium-99 m with specimen autoradiography is another technique that may be used to identify a small nidus when curettage is used
May express c-fos and c-jun by immunohistochemical analysis; some cases have demonstrated partial deletion of the long arm of chromosome 22 (22q13.1)
Lack a central nidus
Prominent acute inflammatory cell infiltrate
Pain is usually not as severe
Tumor size is usually much greater, and there is evidence of progressive growth
Lacks a peripheral rim of fibrovascular tissue
Exhibits variable mineralization and thickness of woven osteoid trabeculae, whereas the nidus of an osteoid osteoma shows a pattern of central maturation toward a more calcified and thicker woven osteoid trabecula
Lacks the fibrovascular stroma and osteoblastic rimming of osteoid osteoma
May exhibit chondroid or fibrous differentiation
Zonal pattern with central, more mature, denser bone and peripheral woven bone
Cartilage with endochondral ossification may be present
Pain is related to the presence of unmyelinated nerve fibers in the fibrovascular stroma of the nidus, production of prostaglandin E 2 , and production of prostacyclin
Clinical pain may precede radiographic evidence of osteoid osteoma
When osteoid osteoma is present in the small bones of the hands and feet, patients are typically treated for an inflammatory process (osteomyelitis, arthritis) first
Intra-articular tumors may produce chronic villous synovitis similar to rheumatoid arthritis
Prostaglandin receptors have been identified within bone, and it has been postulated that prostaglandins may also contribute to the formation of osteoid osteoma
Few reports of spontaneous regression of osteoid osteomas
Treatment is surgical removal
Male predominance, with a male-to-female ratio of 2:1 to 3:1
Occurs in first through fourth decades, with most occurring in second and third decades
Predilection for the vertebral column (arch) and sacrum followed by the mandible and craniofacial bones; the next most common sites are the extremities, where it follows a distribution similar to that of osteoid osteoma
Typically intramedullary
Localized pain may be present, but not with the intensity of an osteoid osteoma
Vertebral tumors may produce scoliosis, muscle atrophy, and neurologic deficits
Round, well-demarcated, expansile, radiolucent zone with a peripheral rim of sclerosis (sclerosis may not be as extensive as in osteoid osteoma)
Central radiolucent zone (nidus) is greater than 1.5 cm; central stippled calcifications may be present
Tumor may be surrounded by an area of new bone formation
About one fourth may exhibit cortical destruction with periosteal new bone formation, suggesting a malignant tumor (osteosarcoma)
Secondary aneurysmal cyst formation may be present
Features similar to osteoid osteoma; however, these tumors are larger (>1.5 cm)
Central nidus is red, soft, and friable; if calcified, the nidus may be yellow and gritty
Cortical bone may be destroyed or thin, and there may be hemorrhagic cysts within the nidus, representing secondary aneurysmal cyst formation
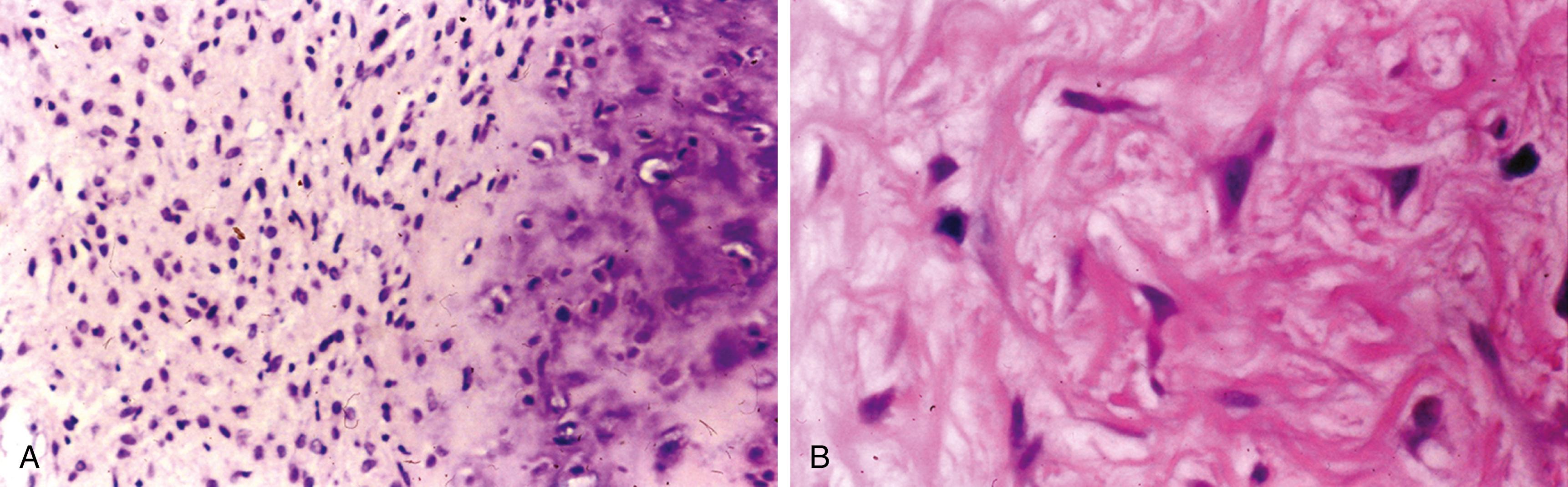
Irregular interlacing network of osteoid with prominent osteoblastic rimming and features of woven bone ( Figure 16.3 )
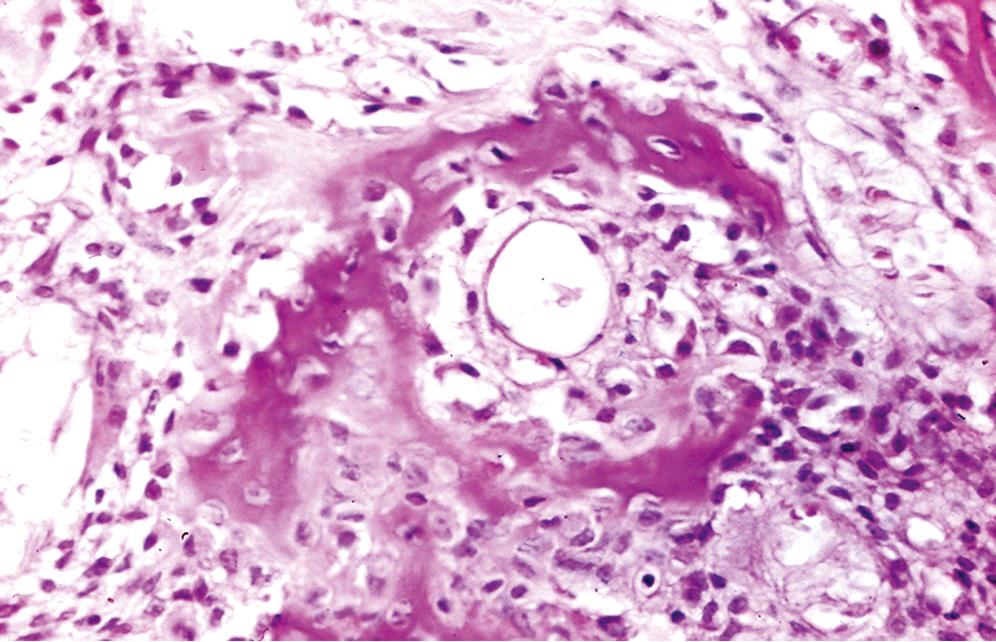
Osteoid may be fine and lacelike with variable mineralization
Osteoblasts have benign cytologic features
Osteoblasts may exhibit abundant mitotic activity but no atypical forms
Osteoid is separated by fibrovascular stroma containing multinucleated osteoclast-like giant cells
Appears well circumscribed, with tumor osteoid merging with adjacent uninvolved bone
Large blood lakes representing secondary aneurysmal cystic changes may be seen
Cartilage is usually not present in the tumor, although rare cases have been reported
Osteoblasts may have epithelioid features represented by large cells with abundant eosinophilic cytoplasm and enlarged nuclei containing large nucleoli
When epithelioid cells exceed 75% of the osteoblast population, the diagnosis of aggressive osteoblastoma should be made, which denotes an increased risk for recurrence, although no cases of metastases are reported
Rare tumors may contain bizarre, cytologically atypical multinucleated giant cells without mitotic activity (these tumors may be designated bizarre osteoblastoma or pseudomalignant osteoblastoma )
In short decalcified biopsies, FOS immunohistochemistry shows as overexpression in the majority
Osteoid osteoma and osteoblastoma are shown to harbor FOS (87%) and FOSB (3%) rearrangements
Usually smaller than 1 cm; clinically, the pain is of greater intensity
Periphery of tumor contains a fibrovascular rim
Nidus exhibits a more zonal pattern with central maturation and less variability in the thickness and degree of mineralization of the osteoid
No evidence of progressive growth
Usually involves the epiphyses of long bones
Rare in vertebrae, but when they occur in a vertebra, the body and not the arch is usually involved
Giant cells in giant cell tumors are larger and contain more nuclei
Often composed of sheets of giant cells
Giant cell tumors contain mononuclear stromal cells
Similar presentation and radiographic findings as osteoblastoma and also tend to involve the vertebra
Small foci of reactive osteoid may be present in aneurysmal bone cysts, which should not be confused with osteoblastoma
Radiographically, osteosarcoma is poorly circumscribed with cortical destruction and evidence of periosteal reactive bone
Permeative pattern of growth at the periphery
Stroma of osteosarcoma is sarcomatoid with cytologic atypia and atypical mitoses
Sheets or aggregates of atypical osteoblasts are present in osteosarcoma, in contrast to a single rim of osteoblasts around osteoid in osteoid osteoma
In short decalcified biopsies, FOS immunohistochemistry shows no overexpression
About one fourth of the cases of osteoblastoma exhibit radiographic evidence suggesting a malignant tumor (osteosarcoma); differentiation from an osteoblastic osteosarcoma can be difficult (see “Differential Diagnosis”)
In short decalcified biopsies, FOS immunohistochemistry can be used to diagnose osteoid osteoma and osteoblastoma, as overexpression is seen in the majority, while being rare in their mimics
Slight male predominance, with a male-to-female ratio of 1.5:1
Bimodal age distribution, with most cases occurring in second decade; a second, smaller peak occurs in patients older than 50 years
Represents the fourth most common cause of malignancy in the pediatric age group
Patients with hereditary retinoblastoma are at increased risk for developing an osteosarcoma
Other conditions that may be associated with the development of osteosarcoma: Li-Fraumeni syndrome, Ollier disease, osteoblastoma, fibrous dysplasia, Paget disease of bone, hereditary multiple exostosis, previous radiation or chemotherapy, hypoplastic or aplasia of thumbs, Werner syndrome, and Rothmund-Thomson syndrome
Occurs in parts of the skeleton with the highest growth rates
Predilection for the distal femur, proximal tibia, and proximal humerus
Approximately 50% of cases occur in the region of the knee
Typically presents with a history of short-term (several weeks to several months), mild, intermittent pain
Affected area may be swollen and tender to palpation, and the overlying skin may exhibit telangiectasia and be warm
Serum alkaline phosphatase may be elevated
Classically shows a large lytic, sclerotic, or mixed lytic-sclerotic mass arising in medullary bone of the metaphysis that extends through the cortex and creates a soft tissue mass
Variable mineralization within the tumor, which causes cloudy opacities
Outer cortical surface exhibits prominent periosteal reaction represented by Codman triangle, sunbursts, or onion-skinning
CT and MRI are used for staging (intramedullary involvement, presence of skip lesions in marrow, and soft tissue involvement)
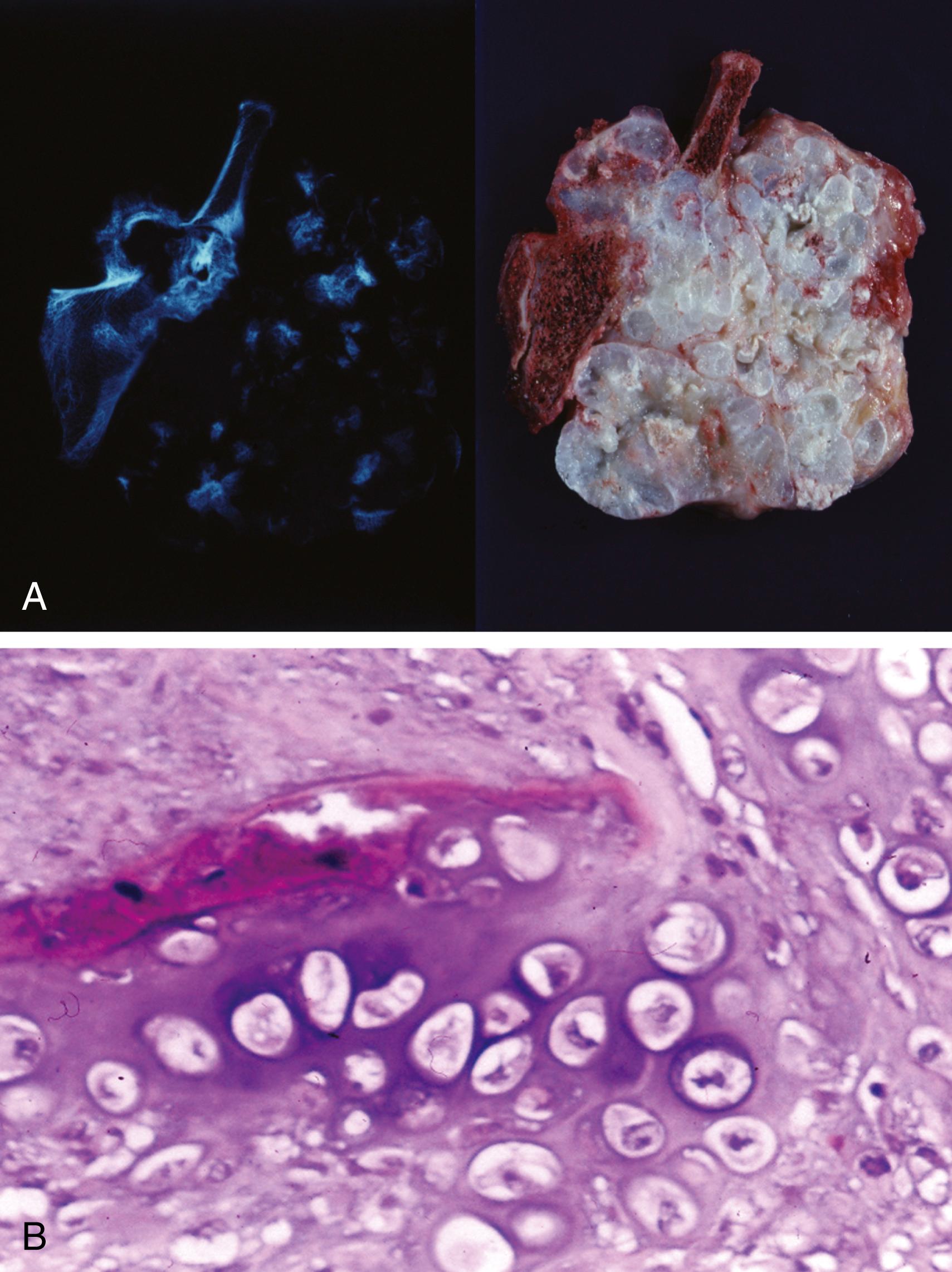
Resected specimens exhibit an intramedullary metaphyseal mass that has usually penetrated through the cortex and invades into soft tissue ( Figure 16.4A )
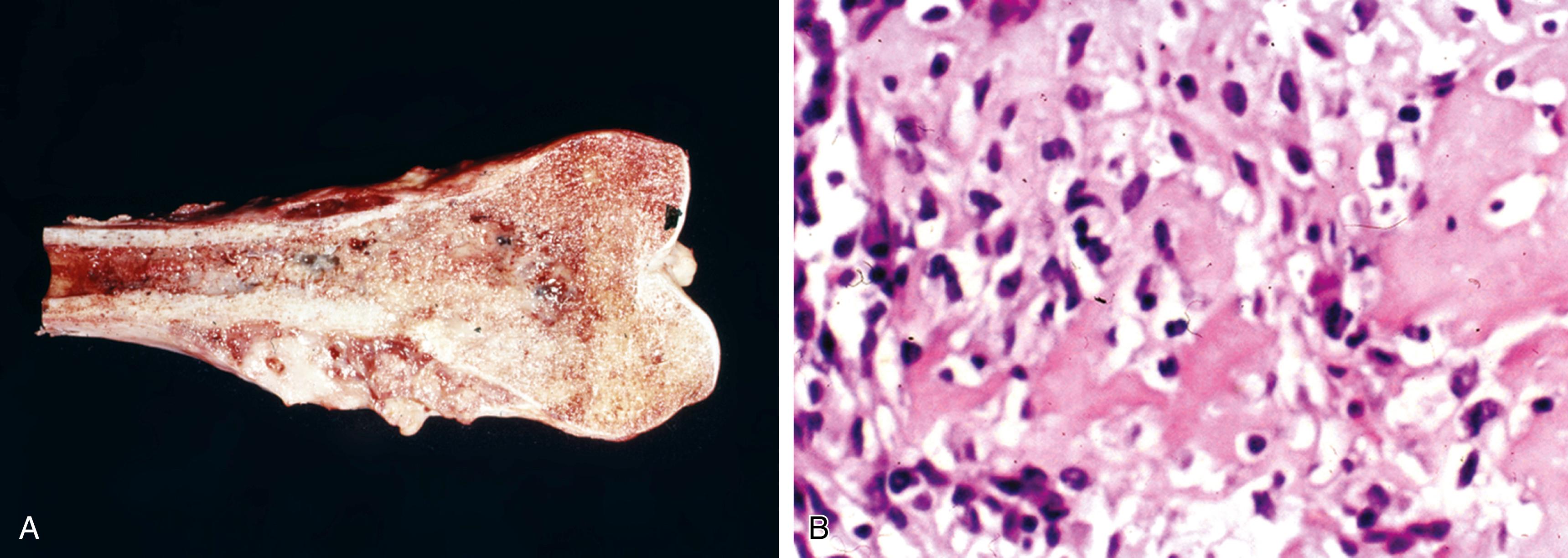
Marrow extension of the tumor proximally is usually seen, and there may be skip lesions in which normal marrow separates islands of tumor
Gross characteristics of the tumor are heterogeneous and variable, depending on the stromal component
Highly ossified areas are yellow to white and hard
Chondroid areas are lobulated, translucent, and light gray to white
Osteoblastic areas are firm, white to yellow, and sometimes gritty
Fibroblastic areas are soft and fleshy
Tumor may contain areas of necrosis, hemorrhage, and cystic changes
Microscopic features may vary considerably in different areas of a tumor
Tumor is basically composed of sarcomatous, spindle-shaped cells exhibiting evidence of tumor osteoid production (see Figure 16.4B )
Sarcomatous stroma is hypercellular and may exhibit osteoblastic, chondroblastic, fibroblastic, or malignant fibrous histiocytoma-like differentiation
Cells usually have obvious cytologic malignant features, including brisk mitotic activity with atypical forms
Some cells may exhibit epithelioid features
Tumor osteoid is represented by eosinophilic, amorphous, fibrillary deposits between individual tumor cells or small aggregates of tumor cells
Early tumor osteoid forms a lacelike pattern around tumor cells, whereas the more advanced type is mineralized and has the appearance of woven tumor bone
As tumor cells become incorporated with tumor osteoid, they tend to become smaller; this feature is regarded as normalization
Some tumors exhibit prominent chondroblastic differentiation requiring careful search for tumor osteoid
Fibroblastic areas may exhibit a herringbone pattern; diligent search for tumor osteoid is sometimes required
Some tumors may have large numbers of osteoclast-like giant cells and are designated as giant cell–rich osteosarcoma
Some tumors may contain foci rich in vascular structures with an hemangiopericytoma-like pattern
Small cell variant
May have features suggestive of Ewing sarcoma/primitive neuroectodermal tumor (PNET), mesenchymal chondrosarcoma, and lymphoma and require immunohistochemistry for differentiation
Presence of tumor osteoid
Rare cases of small cell variant share genetic features of Ewing sarcoma/PNET
Preoperative chemotherapy may result in tumor necrosis represented by acellular tumor osteoid, acellular chondroid tissue, fibrosis, or hyalinized vascular stroma; preoperative chemotherapy is considered effective when greater than 90% of the tumor is necrotic
SATB2: recently discovered osteoblast transcription factor (nuclear stain) critical for osteoblast lineage commitment
SATB2 immunohistochemistry can be useful in the diagnosis of osteosarcoma when histologic features of matrix are equivocal (i.e., osteoid vs hyalinized collagen) and when biopsy only samples tumor with undifferentiated appearance
DNA ploidy analysis usually shows prominent aneuploid clones
Conversion from pretreatment aneuploidy to predominant diploidy after chemotherapy correlates with subtotal or total necrosis of the tumor
One case of the small cell variant of osteosarcoma reportedly demonstrated chromosome translocation t(11;22)(q24;q12) typical of Ewing sarcoma/PNET; however, subsequent studies have not replicated this result
Hereditary form shows a loss of function of the RB gene; in nonhereditary form, there may be mutation of the TP53 gene (about 20% of cases)
Callus woven bone or osteoid exhibits a parallel pattern with prominent osteoblastic rimming
Absence of nuclear atypia and abnormal mitoses in callus
Cartilage with endochondral ossification is present in callus
Radiographic findings may mimic osteosarcoma
Readily differentiated using histologic features
Lacks atypical mitoses, infiltrative pattern, and destructive growth pattern
Giant cell tumors usually affect skeletally mature patients with closed epiphyses
Usually involve the epiphyses and extend toward the articular cartilage
Mononuclear stromal cells without atypia or abnormal mitotic activity
Radiographic findings can help in differentiating these two entities
Low-grade chondrosarcoma with areas of ossification may mimic osteosarcoma, whereas chondroblastic osteosarcoma usually contains a high-grade cartilaginous component
Dedifferentiated chondrosarcoma contains an osteoblastic osteosarcoma component but retains low-grade chondrosarcoma foci
Clear cell chondrosarcomas may produce bone, thus imitating osteosarcoma
Presence of clear cells and typical epiphyseal location of clear cell chondrosarcoma help differentiate these two entities
Typically occurs in older patients
Lacks tumor osteoid formation
No production of tumor osteoid
Small cell variant of osteosarcoma will have tumor osteoid
Immunohistochemistry may be helpful in differentiating these tumors (leukocyte common antigen [LCA] is positive in lymphoma, S-100 protein is positive in mesenchymal chondrosarcoma, CD99 is positive in Ewing sarcoma/PNET, SATB2 is positive in osteosarcomas)
Prostate and mammary carcinomas can elicit a prominent osteoblastic reaction
Epithelial markers and specific tumor markers by immunohistochemistry can help differentiate metastatic carcinoma
Osteosarcoma is the fourth most common malignant tumor found in adolescents; the three most common ones in descending order are leukemia, brain tumors, and lymphoma
If pain has been present for more than 1 year, the diagnosis of osteosarcoma is unlikely
About half of cases of primary osteosarcomas of bone occur in the knee region; osteosarcomas of the hands and feet are rare
Initial clinical presentation of osteosarcoma as a pathologic fracture is rare
Elevated serum alkaline phosphatase levels typically occur in tumors with prominent osteoblastic patterns but may also be elevated in other conditions such as osteoblastoma, osteomyelitis, and callus; a posttherapy increase in serum alkaline phosphatase suggests metastatic disease or recurrence
Most osteosarcomas exhibit diagnostic features on routine radiographs, whereas occasionally they may exhibit deceptively benign radiographic features
Rare cases of epiphyseal osteosarcoma may exhibit radiographic features of clear cell chondrosarcoma or chondroblastoma
A radiologically malignant metaphyseal tumor in 10- to 30-year-olds is most likely osteosarcoma
Rare osteosarcomas contain cytologically benign-appearing stromal giant cells that hide the sarcomatous component; careful search is necessary to identify the sarcomatous component and tumor osteoid, which is usually found in a perivascular location
Osteosarcomas of craniofacial bones, ribs, and vertebrae are usually related to Paget disease or radiation and typically occur in older individuals
Male-to-female ratio is 2:1
Most occur in second decade
Accounts for about 4% of all osteosarcomas
Similar distribution as conventional intramedullary osteosarcoma
Predominantly affects distal femur, proximal tibia, and proximal humerus
Similar symptoms to conventional osteosarcoma, except it is more likely to present as a pathologic fracture (25% of cases)
Recognizable as a completely lytic lesion involving the metaphysis with infiltrating destructive margins
May cause cortical expansion of the bone
Periosteal new bone formation may be represented by onion-skinning or Codman triangle
Some cases may exhibit benign features and mimic an aneurysmal bone cyst
Hemorrhagic mass that may be multicystic and necrotic
No areas of fleshy, sarcoma-like tissue or sclerotic areas
Multiple cystlike spaces resembling an aneurysmal bone cyst, except that the septa of the cysts contain stromal cells (mononuclear and multinucleated) with cytologically malignant features intermixed with benign osteoclast-like giant cells ( Figure 16.5 )
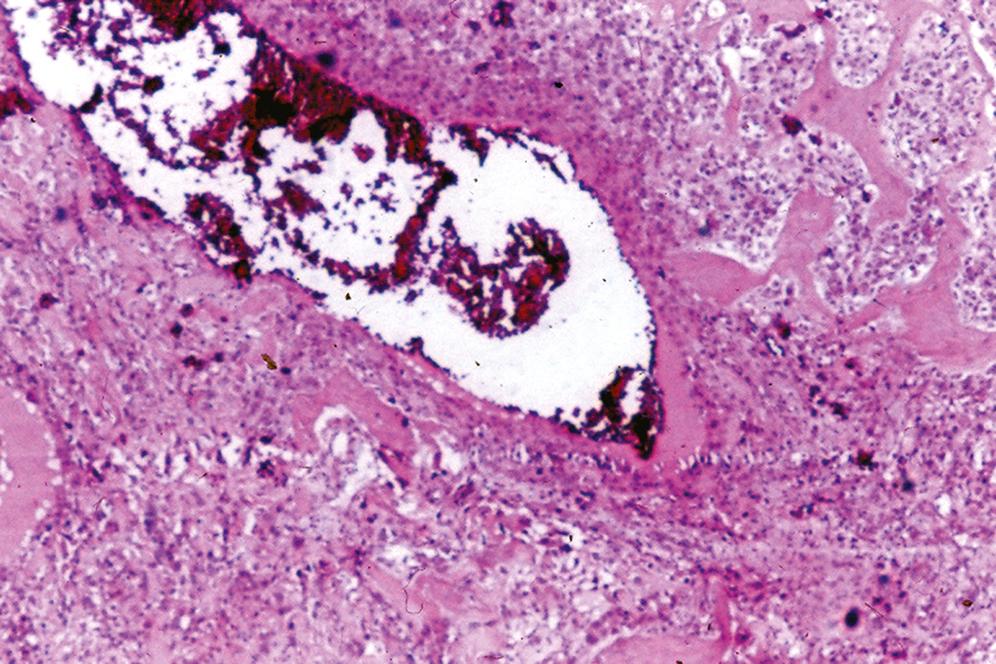
Mitotic features are present, including atypical forms
Sometimes the malignant stromal cells are floating in the center of the hemorrhagic cysts; identification of the stromal cells may be difficult, requiring multiple sections
Tumor osteoid can be difficult to identify; it is usually focal and found in a delicate lacelike pattern
SATB2 immunohistochemistry can be useful in the diagnosis when histologic features of matrix are equivocal (i.e., osteoid vs. hyalinized collagen) and when biopsy only samples tumor with undifferentiated appearance
Noncontributory
Stroma may be cellular but typically lacks cytologic atypia and atypical mitoses; may contain reactive bone with atypical osteoblasts
Definitive cytologic malignant features and atypical mitoses are absent
Radiographically, these tumors are not purely lytic
Intramedullary osteosarcoma may contain focal telangiectatic areas, which should not be overinterpreted
Telangiectatic osteosarcoma is frequently the type of osteosarcoma associated with long-term Paget disease
Better prognosis than conventional intramedullary osteosarcoma
When a diagnosis of aneurysmal bone cyst is being considered, all tissue should be evaluated histologically for evidence of malignant stroma to rule out telangiectatic osteosarcoma
Also known as “juxtacortical osteosarcoma”
Slight female predominance, with a male-to-female ratio of 1:1.5
Occurs predominantly in third decade
Involves metaphyses of long bones with approximately three fourths of cases involving the distal posterior femur, with the proximal tibia as the second most common site
Clinically presents as a painless mass of long duration (slow growing); pain may occur late in the course but is not typical initially
May also present as an inability to flex the knee
Radiodense, bosselated, or mushroom-shaped mass arising on the surface of a bone (outside of periosteum); in long-term lesions, tumor may encircle the bone
A separate lucent zone between the tumor and the cortex known as a string sign may be seen
No evidence of periosteal bone reaction
Peripheral lucent areas may represent a cartilaginous cap
Central lucent areas may represent high-grade sarcoma or dedifferentiated tumors
CT or MRI may be necessary to visualize lucent areas
Well-ossified mass that appears attached to the cortical surface of the bone
Cartilaginous cap may be present and there may be soft foci, which should be sampled; these foci may represent high-grade sarcomatous regions or dedifferentiated tumor
Tumor osteoid is arranged in parallel arrays and separated by a hypocellular fibroblastic stroma that exhibits minimal cytologic atypia and minimal mitotic activity without atypical forms ( Figure 16.6 )
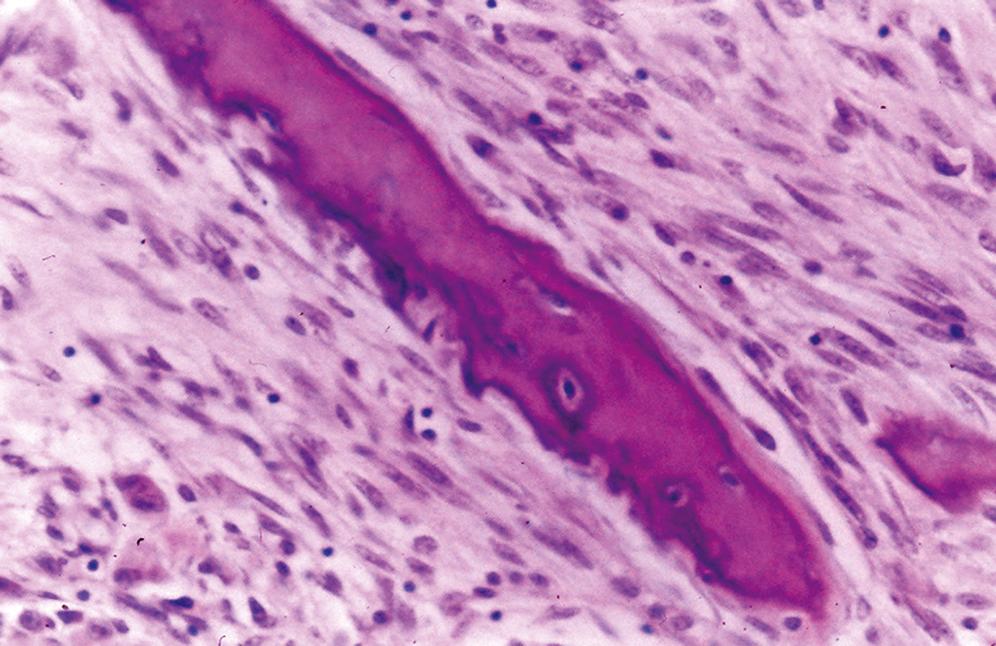
Islands of cartilaginous tissue and a cartilaginous cap may be present
Chondrocytes are atypical and do not exhibit orderly arrangement
Atypia is mild and reminiscent of chondrocytic atypia seen in enchondromas
No evidence of periosteal new bone formation
Areas of dedifferentiated high-grade sarcoma may be seen (about 15% of cases)
No fatty or hematopoietic marrow is seen in association with the tumor
SATB2 immunohistochemistry can be useful
Cytogenetic studies: a ring chromosome may be seen
Medullary spaces contain adipose tissue or marrow hematopoietic tissue
Maturation toward lamellar bone and marrow adipose tissue begins peripherally and extends centrally in this proliferative process, which is the reverse in parosteal osteosarcoma
These are cytologically high-grade tumors that lack residual low-grade areas
Abundant cartilage is present
Higher-grade osseous component and evidence of periosteal reaction
Symptoms may last up to 10 years
Typically affects an older age group compared with intramedullary osteosarcoma
It is not uncommon for these patients to have a history of recurrence of a previously diagnosed osteochondroma
Radiologic and histologic evidence of periosteal new bone formation is absent
Central lucent areas identified on CT scan or MRI may represent high-grade sarcomatous areas or regions of dedifferentiation
Children may exhibit radiographic lesions that mimic parosteal osteosarcoma of the distal femur; histologically, they have features of fibrous cortical defect
Slight male predominance, with a male-to-female ratio of 1.7:1
Typically occurs in second to third decades (later than a conventional osteosarcoma appears and sooner than a parosteal osteosarcoma appears)
Most occur in the diaphysis and metaphysis of the tibia and femur
Patients present with pain, swelling, and tenderness; symptoms often present for less than 1 year
Rare (<2% of all osteosarcomas)
Represented by a surface radiolucent tumor containing a spiculated pattern of calcifications that are oriented perpendicular to the long axis of the primary bone
May see cortical thickening or erosion
Periosteal reaction may be present
No medullary involvement
Lobulated surface mass having a cartilaginous appearance
Cortical erosion may be seen, but the tumor does not extend into the medullary cavity
Malignant osteoid must be present (may only be focal), but the predominant pattern of tumor is represented by lobulated chondromatous tissue with cytologic features of grade 2 or 3 chondrosarcoma
Tumor is located on the surface of the bone and may extend into soft tissue
High-grade anaplastic sarcomatous spindle cell component may separate lobules of the malignant chondroid component
Periosteal bone formation may be present, and there may be cortical erosion, but the tumor does not involve the medullary cavity
SATB2 immunohistochemistry can be useful in the diagnosis of osteosarcoma when histologic features of matrix are equivocal (i.e., osteoid vs. hyalinized collagen) and when biopsy only samples tumor with undifferentiated appearance
Cytogenetics: usually diploid
Usually smaller and better defined
Composed of benign chondroid tissue; does not contain malignant tumor osteoid
Radiographically, it contains “popcorn” calcifications
Histologically, it is a low-grade chondrosarcoma containing no tumor osteoid
Radiographically, these tumors are more radiodense
Histologically, this is a low-grade malignant fibro-osseous tumor without chondroid differentiation
This is a higher-grade osteosarcoma involving the medullary cavity
Periosteal osteosarcoma does not involve the medullary cavity
Lacks cartilaginous differentiation
Osteoid component is pleomorphic and high grade
By definition, periosteal osteosarcoma does not involve the medullary cavity
CT scan or MRI may be necessary to rule out medullary involvement
Very rare tumor, with male-to-female ratio of about 3:1
Occurs predominantly in third and fourth decades
Distal and midfemur, proximal humerus, and proximal fibula are most common sites
Pain and swelling are most common symptoms, with duration from less than a year to many years
Similar prognosis to conventional intramedullary osteosarcoma, but poorer prognosis than parosteal osteosarcoma
Exhibits a surface mass with features similar to those of periosteal osteosarcoma, except the mineralization pattern is similar to that of conventional osteosarcoma, revealing a fluffy, cumulus cloud appearance
May be cortical destruction, periosteal reaction, and focal medullary involvement
Large, lobulated surface mass with variable consistency ranging from soft to firm
Should not significantly involve the medullary region
May be hemorrhagic
Histologically high-grade osteosarcoma with features similar to those of conventional intramedullary osteosarcoma, but lacks significant medullary involvement
SATB2 immunohistochemistry can be useful in the diagnosis when histologic features of matrix are equivocal (i.e., osteoid vs. hyalinized collagen) and when biopsy only samples tumor with undifferentiated appearance
Noncontributory
Usually has residual low-grade malignant fibroblastic stromal component
Lacks high-grade anaplastic appearance
Significant medullary component (minimal medullary component in a high-grade surface osteosarcoma)
Radiographically mimics periosteal osteosarcoma, except it has cumulus cloud–like patterns of mineralization
Of all the types of surface osteosarcomas, this has the least favorable prognosis (similar to conventional intramedullary osteosarcoma)
Male-to-female ratio is about 1:1
Most cases occur in third and fourth decades; this variant of osteosarcoma can occur in older age groups
Patients present with a history of pain for many months up to several years; usually no complaint of swelling
Most common sites include mid- and distal femur and proximal and midtibia
Some patients may have been previously diagnosed with fibrous dysplasia
Large, poorly marginated intramedullary mass that either is sclerotic or exhibits trabeculations
Usually no evidence of periosteal reaction
Medullary tumor may extend along the length of the bone to the subarticular bone
May have cortical destruction with formation of a soft tissue mass
Tumors are gritty, gray, medullary masses that may have fibrous and fleshy areas
Cortical destruction may be seen, and the tumor may extend the length of the bone with poor demarcation between tumor and uninvolved medullary bone
Mean size about 9 cm
Similar to parosteal osteosarcoma and can also mimic fibrous dysplasia
Well-differentiated intramedullary fibro-osseous process represented by irregular bony trabeculae separated by fibrous spindly stroma
Spindle cells are fibroblastic-like and have elongated nuclei with nucleoli
Nuclei exhibit minimal atypia and infrequent mitoses; atypical mitoses are rare to absent
Rare chondroid foci may be seen
SATB2 immunohistochemistry may be useful
Noncontributory
Benign nonaggressive radiographic features with no cortical disruption
Histologically, the woven bone in fibrous dysplasia is delicate and curved, in contrast to the coarse tumor osteoid in low-grade central osteosarcoma
Fibrous dysplasia lacks nuclear atypia and mitotic activity
No radiographic evidence of matrix formation
Histologically, the central portion of desmoplastic fibroma will not contain any tumor osteoid
Typically has benign radiographic features
Prominent osteoblastic rimming of bony trabeculae
Nuclear pleomorphism and mitotic activity with atypical forms is greater in this tumor compared with low-grade central osteosarcoma
Parosteal osteosarcoma
Surface location with no medullary involvement, but similar histology
This variant affects older patients more often than traditional osteosarcomas
Not associated with previous radiation therapy or preexisting Paget disease (typical of osteosarcoma seen in elderly patients)
A small number of these tumors may be interpreted as benign radiographically
Histologically similar to parosteal osteosarcoma
Also known as exostosis
Most common benign tumor involving bone
Male-to-female ratio is about 2:1
Most occur in second and third decades, but can present at any age
Majority occur in distal femur, proximal tibia, and humerus; pelvis is also a relatively common site
Extremely rare in craniofacial bones, vertebrae, sacrum, and sternum
Patients present with a longstanding mass that may be painful or asymptomatic
Some lesions are asymptomatic and are identified incidentally on radiographs obtained for other reasons
Pain may be secondary to impingement of a bursa, fracture, or infarction of the lesion
May develop after radiation treatment (more than 1 year) for other malignant processes
Hereditary form (autosomal dominant) is called osteochondromatosis or multiple hereditary exostosis (any bone may be involved except craniofacial bones)
Other hereditary forms with multiple osteochondromas include Langer-Giedion syndrome and DEFECT-11 syndrome
Fewer than 2% of osteochondromas undergo malignant transformation; clinical features suggestive of malignant transformation include pain, rapid growth, large tumor size (>6 cm), and location (axial skeleton)
Radiographs reveal a pedunculated metaphyseal mass projecting from the surface of a bone ( Figure 16.7A )
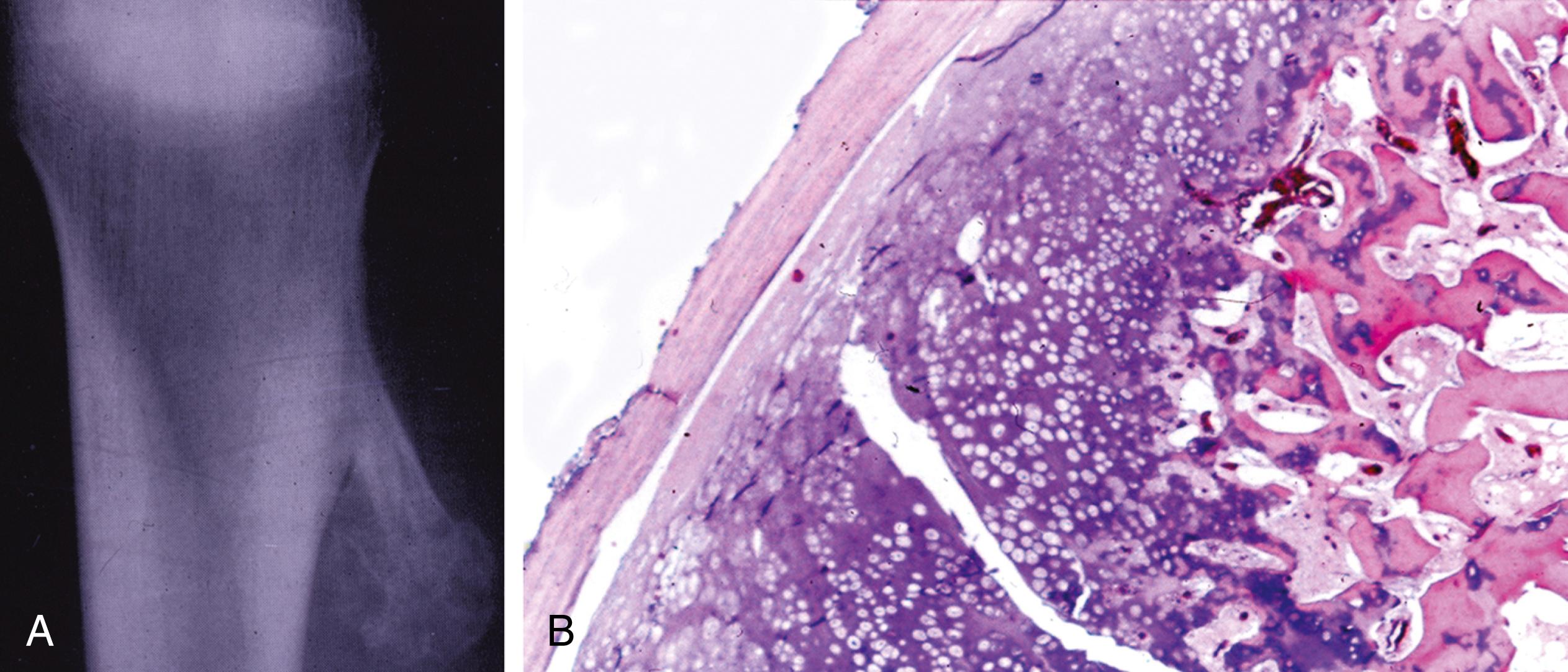
Variable smooth or irregular surface and a variable base (narrow to wide); points toward the diaphysis and away from the nearest epiphysis
Has appearance of mature bone and is continuous with the cortex of the uninvolved adjacent bone
Surface cap represented by cartilage is not identified with routine radiographs unless calcified; MRI is necessary to evaluate the nonmineralized cartilaginous cap
Pedunculated or broad-based mass containing a smooth, thin (<1 cm) cartilaginous cap
In older patients, the cartilaginous cap may be attenuated or absent
Central part of the mass is represented by normal-appearing medullary bone
Outer surface is covered by a thin layer of periosteal fibrous tissue
Cap is represented by hyaline cartilage that contains evenly distributed chondrocytes (see Figure 16.7B )
Nuclei may exhibit atypia and pleomorphism
Junction of the cap and bone mimics the epiphyseal plate and contains linear rows or columns of chondrocytes
Columns undergo endochondral ossification and form bony trabeculae
Medullary spaces between the trabeculae of bone contain adipose tissue and sometimes hematopoietic tissue
Chondrocytic atypia must be evaluated with clinicoradiographic features to determine their significance
Nuclear enlargement, variation in nuclear shape, multinucleation, and formation of chondrocytic clusters that are irregularly shaped may cause some concern but can be found in osteochondromas
Chondrocytic atypia along with clinical features of malignancy (pain, rapidly enlarging tumor, size > 6 cm) and radiographic features of malignancy (irregular thickened cartilaginous cap > 2 cm, radiolucent areas of the cartilaginous cap, extension through periosteum into soft tissue, and bone destruction) are more ominous and concerning for a secondary chondrosarcoma
High mitotic activity is indicative of malignancy
Noncontributory
Chromosome rearrangement of 8q24.1 (EXT1) is found in patients with Langer-Giedion syndrome
Deletions of chromosomal bands 11p11-12 (EXT2) is seen in patients with DEFECT-11 syndrome
The EXT1 and EXT2 gene products add heparan sulfate to proteoglycans and may have tumor suppressor functions
Usually involves small bones of hands and feet
Occurs in third and fourth decades of life
Medullary component of lesion is not in continuity with host bone
Histologically, the cartilage is hypercellular with atypia and multinucleation
Chondroid nodules are separated by a spindle cell proliferation that exhibits mitotic activity (no atypical mitoses or nuclear atypia)
Woven bone with deep basophilia may be present
Clinical findings consist of pain and a rapidly enlarging mass
Radiographic findings consist of thickened (>2 cm), irregular cartilaginous cap, radiolucent zones in cartilaginous cap, extension through periosteum into soft tissue, and evidence of bone destruction
Histologic findings consist of increased cellularity, nuclear atypia represented by enlarged nuclei with open chromatin pattern, multinucleation, and mitotic activity
Fibroblastic stroma is present in the medullary spaces instead of fat and hematopoietic tissue
If a cartilaginous cap is present, it is composed of cytologically low-grade malignant chondrocytes without endochondral ossification
Presence of HEY1-NCOA2 fusion gene
Continuity with the medullary component of the parent bone is not present
Appears to be attached to the surface of the parent bone
Clinical and radiographic findings are important in the evaluation of chondrocytic atypia
Radiographically, the long axis of the stalk points away from the nearest epiphysis
Malignant transformation is rare (<2%)
Cytogenetic abnormalities suggest that osteochondroma represents a true neoplastic process
Male-to-female ratio is about equal; affects all age groups (most occur in second to fifth decades)
Occurs predominantly in the appendicular skeleton, with most occurring in bones of the hands and feet (hands more often affected than feet)
Proximal humerus and femur and distal femur are also affected; rarely found in the pelvis, ribs, sternum, and vertebrae (no reported cases in craniofacial bones)
In general, these tumors are asymptomatic and may be identified on routine radiographs or nuclear scans
Phalangeal tumor may present as a mass
Pain may be a presenting feature in association with a pathologic fracture or trauma to the tumor
Well-defined, predominantly lucent diaphyseal intramedullary mass ( Figure 16.8A )
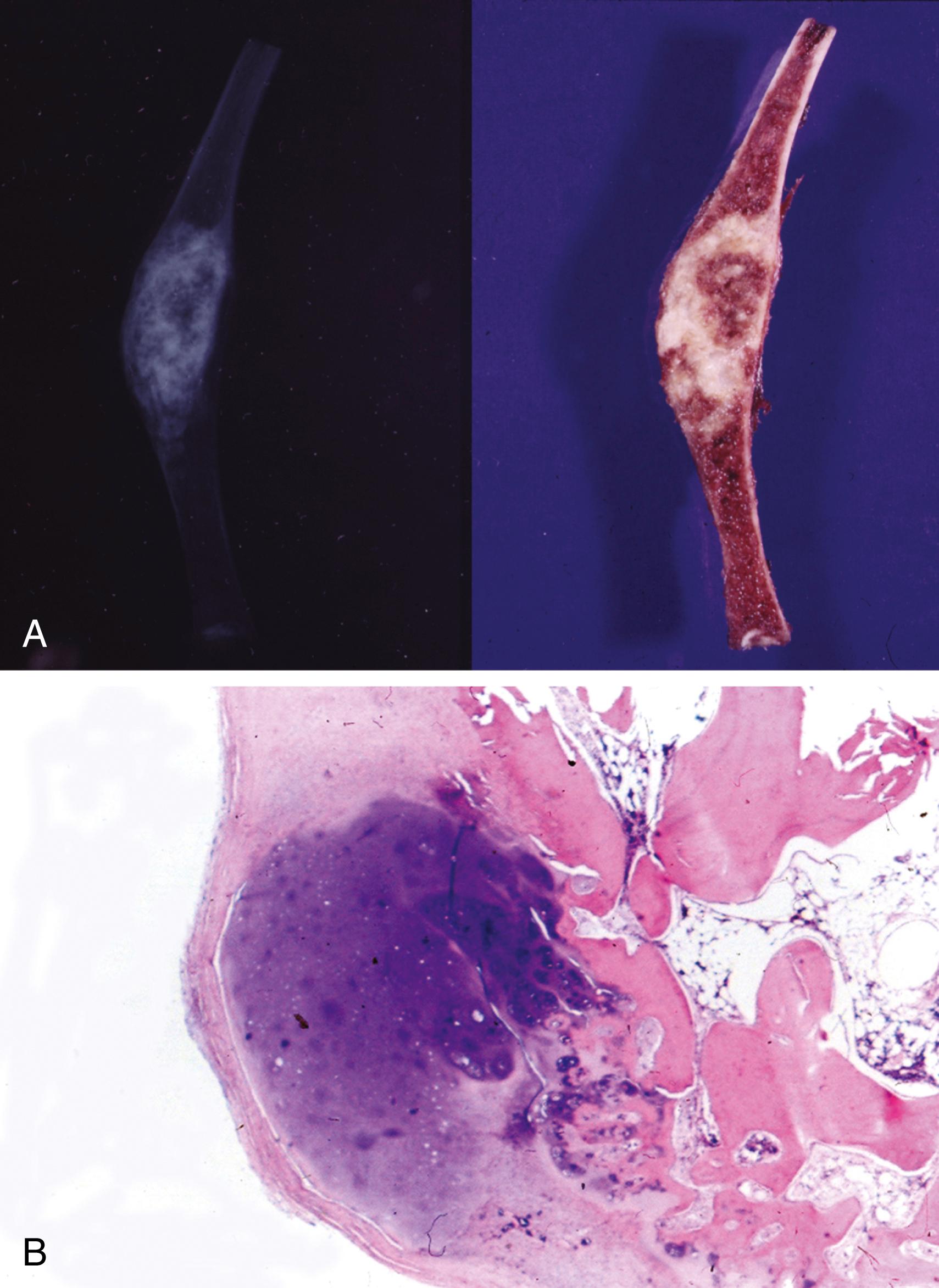
Usually lobulated and sharply demarcated with variable mineralization, stippled, ringlike, or flocculent
Cortical expansion and thinning may be seen, but the cortex should be intact; rare evidence of periosteal reaction
Curettage produces fragments of blue-gray translucent, glistening chondroid tissue intermixed with fragments containing yellow foci representing calcification
Resected specimens consist of a well-circumscribed medullary, confluent, lobulated, cartilaginous mass; periphery of the tumor may be irregular
Composed of lobulated, mature, hyaline-like cartilage (see Figure 16.8B )
Lobules may be separated by marrow hematopoietic tissue or endochondral bone
Chondrocytic cellularity is low and usually evenly distributed
Bland cytologic features with small, slightly hyperchromatic nuclei without pleomorphism; rare multinucleated forms may be present
Nuclei with open chromatin patterns and mitoses are generally absent
Cellularity may be higher in tumors of the hands and feet
Calcifications and endochondral ossification may be present
Myxoid areas should arouse suspicion of malignancy except in tumors of the hands and feet
Mib-1 (Ki-67): in tumors involving bones other than the hands and feet in which malignancy is suspected clinically or radiologically, Mib-1 may be used to demonstrate proliferative activity
Proliferative index is low in enchondroma
Insulin-growth factor II mRNA binding protein (IMP3) is negative in enchondromas that helps to distinguish from chondrosarcomas (IMP3 is overexpressed in 36% of chondrosarcomas)
Enchondromas may exhibit abnormalities of chromosomes 6 and 12
Molecular studies have shown chromosomal rearrangements involving the HMGA2 gene localized to 12q15 in some patients with enchondromas
May clinically mimic enchondroma
Composed of histologically benign chondrocytes with an orderly and regular appearance
Radiographs reveal a ground-glass diaphyseal lesion
Fibro-osseouselementsareseen(absent in enchondroma)
Differentiating this tumor from an enchondroma can be extremely difficult and requires clinical, radiographic, and histologic information
Pain is usually present in low-grade chondrosarcoma; pain is typically absent in enchondroma unless traumatized or pathologically fractured
Radiographic features of low-grade chondrosarcoma include cortical destruction, cortical thickening due to extension of tumor in haversian canals, and a soft tissue mass
Increased cellularity and binucleate chondrocytes are more prominent
Marrow permeation represented by cellular cartilage surrounding mature bone trabeculae and lobules of cartilage separated by fibrous tissue is seen in low-grade chondrosarcoma
Extension of the tumor into haversian canals (not seen in enchondroma)
Prominent myxoid features are not typical of enchondromas
Immunoperoxidase stains for proliferative activity (Mib-1) reveal nuclear positivity in low-grade chondrosarcomas (generally minimal or no staining in enchondromas except for hand and foot tumors)
Insulin-growth factor II mRNA binding protein (IMP3) is negative in enchondromas that helps to distinguish from chondrosarcomas (IMP3 is overexpressed in 36% of chondrosarcomas)
Any cartilaginous neoplasm in the pelvis, ribs, sternum, or vertebrae should be considered a potentially aggressive tumor unless exhibiting completely benign clinical features, benign radiographic features, and benign histologic features
Presence of pain in the absence of trauma or associated pathologic fracture should arouse suspicion of malignancy in a patient with a low-grade chondroid neoplasm
Myxoid features in an enchondroma should raise suspicion of malignancy
Significance of atypical cytologic features, atypical cellularity, and myxoid features in an enchondroma increases as the tumor location gets closer to the axial skeleton
Ollier disease (enchondromatosis) presents with multiple enchondromas and carries a higher risk for malignant transformation
Maffucci syndrome: congenital syndrome consisting of multiple enchondromas and hemangiomas; increased risk for developing chondrosarcomas and malignant vascular tumors (angiosarcoma)
Male-to-female ratio is 2:1
Most cases occur in second and third decades
Proximal humerus, proximal femur, distal femur, and hand bones are most commonly affected
Usually asymptomatic and often found incidentally on routine radiographs
Occurs near tendon insertions and thus may cause functional abnormalities and discomfort related to movement
May occasionally be palpable
Typically a periosteal mass with variable mineralization
May appear lytic or markedly calcified
Erodes the outer cortex but does not extend into the medullary cavity
Periosteal bone formation creating a peripheral buttress causing the tumor to be cup-shaped or crater-like
Cortical, subperiosteal, gray-white lobulated chondroid mass with an outer thin layer of periosteum
Does not extend into the medullary cavity
Yellow calcifications may be present
Similar features to those of an enchondroma composed of hyaline cartilage
May exhibit higher cellularity, increased nuclear atypia, and more multinucleated chondrocytes than enchondromas
Myxoid change may be present
Tumor may appear to extend or push into the medullary cavity, but there is a rim of lamellar bone at the junction of the tumor and medullary cavity
Tumor cells express S-100 protein
Noncontributory
Exhibits radiographic features of an aggressive process and does not have buttressing periosteal new bone at the peripheral margins
May extend into soft tissue and may show variable cytologic atypia
Radiographically, this tumor exhibits perpendicular feathery calcifications and lacks peripheral buttressing
Composed of tumor osteoid and has immature mesenchymal stroma between lobules of cartilage
Periosteal tumor that creates a cup- or crater-shaped mass extending into the superficial cortex but not into the medullary canal
Composed of hyaline cartilage similar to enchondroma
Male-to-female ratio is 1.5:1
Most cases occur in second decade; 95% occur between ages 5 and 25 years
Predilection for epiphyses of bones
Typically involves long bones in skeletally immature patients
Common sites include distal femur, proximal tibia, and proximal humerus
Other sites include acetabular area, iliac crest of pelvis, ribs, scapulae, spine, tarsal bones, base of skull, and temporal bone
Usually presents with pain over months to years; may have muscle wasting, arthralgia of the adjacent joint, and joint effusion
Rare (<1% of primary neoplasms involving bone)
Circumscribed, well-demarcated epiphyseal lytic mass with a rim of sclerotic bone
Calcifications vary from focal stippling to coarse trabecular patterns
Periosteal reaction is variable, but never to the degree seen in malignant neoplasms
Curettage reveals friable and gritty red tissue with yellow foci of calcifications
Resected specimens reveal a well-circumscribed, epiphyseal gray mass containing regional calcification, hemorrhage, and cystic changes
Usually measure 3 to 6 cm in greatest dimension
May have bluish-gray areas representing chondroid matrix
Rim of sclerotic bone surrounds the tumor
Composed of immature cells with features of fetal chondroblasts (stromal cells), multinucleated giant cells, and chondroid matrix ( Figure 16.10 )
Stromal cells are round and have distinct cell membranes
Contain predominantly eosinophilic cytoplasm and a centrally placed round nucleus, which gives a fried-egg appearance to the cell
Nuclei contain grooves or clefts, and chromatin patterns are finely granular and evenly distributed
Infrequent mitotic figures without atypical forms are seen
Regional areas where cells do not have well-demarcated borders and form syncytia may be seen
Often have areas in which the cells contain larger atypical nuclei
Multinucleated giant cells containing numerous nuclei (more than 20) are scattered throughout the tumor
Variable amounts of chondroid matrix, which may contain stromal cells
Calcification is an important histologic feature and has two patterns
Most common pattern is represented by linear calcifications surrounding stromal cells, imparting a chicken-wire appearance
Other pattern includes coarse calcifications of the chondroid matrix
May also have areas of spindle-shaped cells, secondary aneurysmal cystic changes, myxoid changes, and cystic changes containing eosinophilic amorphous material
H3F3 K36M mutant antibody is a sensitive and specific marker
S-100 protein: stromal cells are positive
May be helpful to identify the scant numbers of stromal cells in tumors with prominent secondary aneurysmal cystic changes
95% of chondroblastomas harbor a p.K36M mutation in either H3F3A (chromosome 1) or H3F3B (chromosome 17), with the majority involving H3F3B
Usually involves the metaphyses
Lacks calcifications and has more prominent lobulated myxoid stroma
Usually occurs in skeletally mature patients
Stromal cells with nuclear grooves are absent (negative for S-100 protein)
Lacks chondroid matrix and calcification
May radiographically and cytologically (nuclear grooves) mimic chondroblastoma
Contains eosinophils and lacks chondroid matrix and calcifications
Chondroblastoma with prominent secondary aneurysmal bone cyst formation may mimic a primary aneurysmal bone cyst
S-100 protein may be useful in identifying stromal cells in chondroblastoma
Usually seen in older patients
Composed of cells with clear-staining cytoplasm
Contains chondrocytic cells with cytologic malignant features
Tends to be more heavily calcified than chondroblastoma
May rarely involve the epiphyses and mimic chondroblastoma
Contains tumor osteoid
Three common epiphyseal tumors are giant cell tumor, clear cell chondrosarcoma, and chondroblastoma
Rare cases of metastasizing chondroblastoma have been reported without consistent, definitive, and predictable histopathologic features
Aneurysmal lesions of tarsal bones may have minute foci of chondroblastic stroma
Extremely rare benign tumor
Male-to-female ratio is 1.5:1
Most cases occur in second and third decades, 80% before the age of 40 years
Typically occur in the metaphyses of long bones in the lower extremity; most common sites are the distal femur and proximal tibia; may also involve the pelvis or small bones of the feet
Patients usually present with a long-term history of pain
Eccentric, expansile, lobulated metaphyseal mass that sometimes extends to the epiphysis
Long axis of the tumor runs parallel to the long axis of the parent bone
Well-demarcated, completely lytic mass with scalloped margins
Calcifications on radiographs are rare
Pelvic tumors have a multiloculated soap-bubble appearance
Secondary aneurysmal bone cystic changes may be present
Sharply circumscribed, lobulated, soft, gray-white tumor, usually 3 to 8 cm in greatest dimension
Hemorrhagic and cystic areas may be present
Myxoid areas may be seen, but are usually not prominent
At low magnification, the tumor has a lobulated appearance
Lobulated areas are myxoid and composed of spindled or stellate cells
The lobules are hypocellular centrally and hypercellular at the periphery
In the hypercellular peripheral areas, the cells have features of chondroblasts
In about 30% of cases, bizarre cells with pleomorphic hyperchromatic nuclei are present and may suggest malignancy; however, there is no mitotic activity
Matrix of the lobules may have chondroid or myxoid characteristics
Lobules are separated by fibrous tissue containing vessels, multinucleated giant cells, and occasionally osteoid
Secondary aneurysmal bone cyst changes and foci of necrosis may be seen
Rare to absent mitotic activity
Solid cellular areas with features of chondroblastoma may be present
Although not seen on radiographs, calcifications are extensive histologically and interfere with appreciation of a lobular architecture
Longstanding tumors may show hyalinization
S-100 protein is helpful in demonstrating the presence of chondroid differentiation
Smooth muscle actin (SMA) and CD34 positivity can be seen in nonchondroid areas
Recurrent anomalies of the long arm of chromosome 6 (6q25), in particular the pericentromeric inversion inv(6)p25q13), have been described
Typically involves the epiphyses
Calcifications seen both radiographically and histologically (chicken-wire appearance)
Most occur in older patients and predominantly in the axial skeleton
Radiographically, these tumors are poorly circumscribed and contain calcifications; may demonstrate cortical thickening or cortical destruction if high grade
May cause a soft tissue mass
Demonstrates marrow extension with chondroid tissue surrounding bony trabeculae
High-grade tumors may mimic chondromyxoid fibroma but exhibit abundant mitotic activity not seen in the latter
Multinucleated giant cells and aneurysmal bone cyst changes are usually not present in chondrosarcoma
Use low power to appreciate the lobulated or nodular architecture
In curettage, the fibrous septa may be fragmented and go unnoticed histologically
Mitotic activity is not prominent, but if present it supports a diagnosis of chondrosarcoma
Male-to-female ratio is 1.5:1
Usually seen in older adults; most patients are older than 50 years; rare in patients younger than 45 years
Predilection for the trunk, with pelvis, ribs, proximal femur, and proximal humerus also affected
Childhood tumors oftentimes involve the extremities
Dull pain at rest that is often worse at night; symptom duration is typically several months to years
Radiolucent mass with variable calcifications ranging from ring-shaped or punctate calcifications to markedly calcified lesions
Cortex is thin with endosteal scalloping and erosion through the cortex
Prominent cortical thickening, representing extension into haversian canals, may be seen
Periosteal reaction is minimal or absent
Nodular mass with blue-gray, glistening, translucent tissue resembling cartilage ( Figure 16.12A )
Areas of yellow calcifications at the periphery
May have foci of necrosis, hemorrhage, or myxoid degeneration
Presence of fleshy tissue indicates a high-grade tumor
Significant histologic variation; diagnosis of low-grade tumors requires clinical and radiographic features to correlate with histology
Large amounts of chondroid matrix with variable cellularity; no tumor osteoid
Chondrocytes within the matrix form clusters and are swollen as a result of cytoplasmic vacuolization
Nuclei are mildly pleomorphic, and multinucleated forms are present in variable numbers
Cells with chondroid differentiation containing nuclei with open chromatin patterns, nucleoli, and mitoses are indicative of malignancy
The following are criteria for grading based on cellularity and cytology
Grade 1: cellularity is low, chondrocytes have small, dark nuclei, and multinucleated forms are rare; no mitotic activity, small foci of necrosis
Grade 2: cellularity is increased mainly at the periphery of lobules, myxoid change is present, chondrocytes have more abundant cytoplasm with mildly pleomorphic nuclei, and multinucleated forms are more common; mitoses are rare, foci of necrosis are present
Grade 3: cellularity is increased with sparse chondroid matrix; nuclei are large and pleomorphic and contain nucleoli; mitoses are present, and necrosis may be prominent
Features suggestive of malignancy (hypercellularity, hyperchromasia, binucleated forms, and myxoid changes) may be seen in benign chondroid processes of the hands and feet; must have clinical and radiologic data before rendering diagnosis
S-100 protein positive in grades 1 and 2 tumors; grade 3 tumors may be negative in poorly differentiated areas
Insulin-growth factor II mRNA binding protein (IMP3) is overexpressed in 36% of chondrosarcomas
DNA ploidy analysis may have prognostic significance
Grade 1 tumors are diploid
Grade 2 tumors may be diploid or aneuploid
Grade 3 tumors are aneuploid
Gains of the long arms of chromosomes 20 and 8 (20q+ and 8q+) are oftentimes seen
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here