Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The musculoskeletal system is composed of the skeleton, the muscles and accessory tissues, which together allow movement of the body.
The bones of the skeleton are living tissue, and they can be found in a variety of shapes and sizes depending on their function. The skeleton is composed of approximately 206 individual bones that articulate with each other via a network of joints. Joints vary in their structure and function, with some joints allowing a wide range of movements, whilst other joints are virtually fixed and designed for stability.
The skeletal muscles provide power to move and support the skeleton, often linking two bones across a joint. Most of them are attached to the skeleton via tendons , which transmit the muscular contraction to the bones which move at the joints. The skeletal muscles of the face attach not only to the skull but also to other facial muscles and the skin, and contraction of these muscles changes the facial expression. The muscles that act on the skeleton are all under voluntary control and contain the contractile proteins, actin and myosin.
Knowledge of the musculoskeletal system is vital for medical practitioners because one-third of general practitioner (GP) consultations involve the musculoskeletal system. Conditions that affect the musculoskeletal system include osteoarthritis, which affects two-thirds of individuals older than 75 years of age and 20% of the total population, and back pain, which affects 2.5 million people every day in United Kingdom and accounts for 6% of all GP consultations. Approximately 400 000 of us take time off work because of back pain. Musculoskeletal disorders therefore cost the UK economy considerable sums each year.
The integumentary system (integument means covering) is formed by the skin plus associated glands, hair and nails. It covers the entire body and has four main roles:
Protection: it protects the body from the external environment.
Homeostasis: it controls body temperature (see also Ch. 1 ).
Perception: the skin contains receptors for pain, temperature and touch (see Ch. 8 ).
Metabolism: synthesis of vitamin D (see Ch. 10 ).
Generalised connective tissue forms an extracellular matrix inside the body, separating and supporting the organs, and is composed predominantly of collagen and elastin.
Different imaging techniques form an important part of being able to visualise what is in the body.
Radiographs are the earliest form of imaging techniques and are produced by X-rays . Standard radiographs will normally involve two views: anteroposterior (AP) and lateral. Higher-energy X-rays are used in medical imaging as they can easily pass through soft tissue, but are stopped by hard tissue. X-rays interact with tissue in different ways but visualisation is possible through photoabsorption.
Photoabsorption takes place when the X-rays meet high-density material, particularly where high atomic number elements are involved. Bones have a high calcium content and calcium also has a high atomic number, facilitating absorption of the X-rays. Therefore, bone will appear white on an X-ray, whereas soft tissue is less dense and allows more X-rays through and appears grey; air will appear black.
Traditionally medical X-rays were performed using photographic film that could be developed. Today most X-rays are digital X-rays, which use an electronic detector instead of film, and the image is processed by a computer. This provides the advantage of images being stored digitally and viewed on the screen immediately without processing.
Dual-energy X-ray absorptiometry ( DEXA scanning ) uses two different low-energy X-ray sources to measure bone density. This technique is particularly useful in the diagnosis of osteoporosis. DEXA scans can also be used to measure total body composition to calculate the total body fat to lean muscle mass.
Computed tomography (CT) or computed axial tomography (CAT) scans are computer-generated images from multiple X-ray beams at different angles that can be used to build a cross-sectional image of the body. The multiple transverse images can then be used to produce a three-dimensional image that can be examined in coronal and sagittal planes, as well as the transverse plane. In addition, a three-dimensional image can be reconstructed from the individual transverse scans. CT scans also provide a greater grey scale separation, which allows soft tissue types to be more easily differentiated.
Magnetic resonance imaging (MRI) is based on the principle that certain atomic nuclei can absorb and emit radiofrequency energy when placed in an external magnetic field. The human body contains a large amount of water that contains two hydrogen nuclei (protons) which are magnetised and aligned when the human body is located within a large magnetic scanner. Varying the magnetic field leads the nuclei to spin at a particular frequency (resonance), which then stops when the magnetic field is switched off. The rate at which the protons return to their original relaxed state varies according to the tissue and the spatial organisation of the nuclei, and this is used to inform the final image. Fourier transformation is a mathematical technique used to produce the image. Because of their high water content, MRI scans are very useful in examining soft tissues, such as nervous tissue, muscles and ligaments, and also in detecting tumours.
Ultrasound waves are particularly useful for imaging soft tissue. Different frequencies facilitate different penetration into tissue, the tissue reflecting the sound waves in ways that provide fine differentiation within variable tissue types. The technique can be applied to many areas of medicine, including in foetal medicine to examine the developing foetus, in echocardiography to examine the heart structure and in Doppler ultrasound to detect and observe the movement of blood.
Bone scans . The radio-isotope technetium ( 99m Tc) is bound to bisphosphonates which are taken up by osteoblasts on injection. The technique is very sensitive in the detection of bone-rebuilding activity and is therefore useful in the examination of fractures and tumours that invade bone. Detection is with a gamma camera.
Positron emission tomography (PET) scans. This technique uses molecules, such as glucose analogues that are labelled with a short-life radionuclide, as a radiotracer, and injected into the body. The radionuclides emit positrons, releasing gamma rays as they decay, and their distribution within body tissue can be examined. This technique is particularly useful to diagnose and monitor cancer as rapidly growing cells utilise the sugar, phosphorylating it by the action of hexokinase, which effectively then traps the labelled compound within the cell, producing an intense signal. It is also used to examine the activity of different areas of the brain.
Skeletal tissues are modified connective tissues in which the fibres are often organised and condensed to produce strong or rigid tissues. The skeleton is mainly composed of bones which form a framework for the rest of the body tissues. Bone is a complex and dynamic living tissue. It is continually being broken down and replaced by new bone. The adult skeleton also contains cartilage, which is more elastic than bone and forms a semi-rigid part of the skeleton and a protective layer at many joint surfaces. Other fibrous connective tissues form tendons and ligaments, which provide strength without being rigid or elastic.
Cartilage, which consists largely of water, contains two cell types:
Immature chondroblasts that secrete the extracellular matrix of cartilage, composed of collagen fibres, elastin fibres and other proteinaceous components, such as proteoglycans (heavily glycosylated proteins), glycoproteins and water.
Chondrocytes , which are mature cartilage cells, located in spaces, called lacunae . They are derived from chondroblasts that have become trapped within the extracellular matrix.
Cartilage does not contain blood vessels, so all metabolites are exchanged by diffusion. The proteoglycans are negatively charged and attract and hold water in the extracellular matrix, allowing the diffusion of nutrients into the cartilage. Although cartilage has a low metabolic rate, diffusion limits its potential thickness and prevents rapid repair following injury; diffusion is only effective over a distance of approximately 4 mm. The high water content of cartilage ensures that it is resilient and retains its shape under pressure, as water is virtually incompressible.
Three types of cartilage are found in the adult body:
Hyaline ( glassy ) cartilage covers the ends of synovial joints ( articular cartilage , see later), connects the ribs to the sternum ( costal cartilage ), forms the larynx and part of the nose and reinforces the trachea and bronchi. Osteoarthritis is caused by degeneration of cartilage at the joints.
White fibrocartilage has less matrix and more collagen than other cartilage, which makes it more compressible and able to resist high pressures. It is found in areas of high stress such as between the vertebrae ( intervertebral disc ) and in the knee joint ( meniscus ).
Elastic ( yellow ) cartilage is found in only two places: (1) the external ear, where it forms the pinna and the external auditory canal, the Eustachian tube , and (2) the throat, where it forms the epiglottis . Elastic cartilage contains high levels of elastic fibres, which gives these tissues a large degree of flexibility.
Hyaline and elastic cartilage, but not the articular cartilage within synovial joints, are surrounded by a fibrous layer, the perichondrium , containing fibroblasts along with type I collagen in the outer layer and chondroblasts in the inner layer. It is continuous with the periosteal bone and the surface of surrounding connective tissue and forms the periosteum as it becomes vascularised, lining the outside surface of all bones. In the embryo (at 6 weeks) the skeleton is composed of hyaline cartilage and fibrous tissue formed by mesenchymal cells. These are then converted to bone by endochondral ossification and intramembranous ossification, respectively (see later).
Bone is classified as a connective tissue because it shows the characteristics of all connective tissues: relatively acellular, with osteogenic (bone-generating) cells widely separated within an abundant extracellular matrix.
In bone, the matrix is composed of approximately:
25% water
25% organic protein fibres
50% crystallised mineral salts.
Most (90%–95%) of the organic component of bone consists of collagen fibres , 90% of which are type I.
Approximately 50% of bone is composed of inorganic crystals made up of the mineral hydroxyapatite , which itself is composed of calcium phosphate, calcium carbonate, calcium fluoride, calcium hydroxide and citrate.
There are four types of cells in bone ( Fig. 9.1 ) :
Osteogenic progenitor cells
Osteoblasts
Osteocytes
Osteoclasts.
The osteogenic progenitor cells are the precursors of the osteoblasts and are derived from mesenchymal cells. The osteogenic progenitor cells are found in the periosteum and endosteum (lining the medullary cavity) of the bone and also in the canals within the bone that contain blood vessels (see later) and differentiate into osteoblasts.
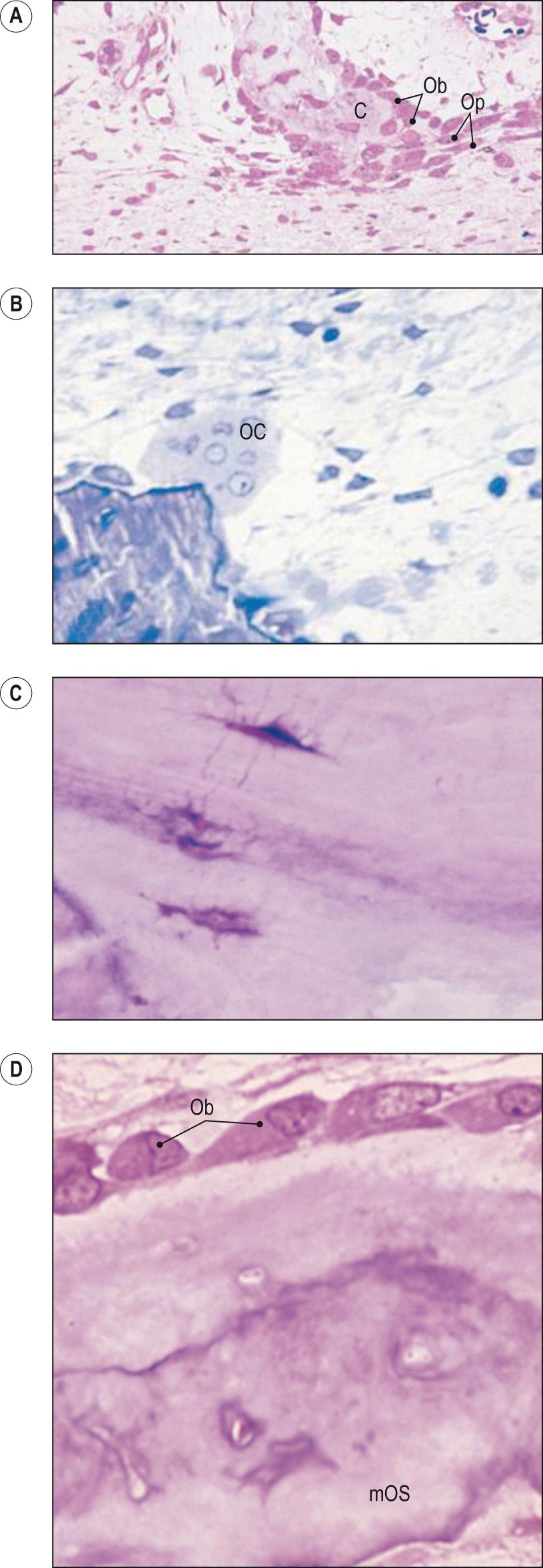
Osteoblasts are the cells that make new bone matrix. They synthesise and secrete collagen fibres and other organic components, in particular alkaline phosphatase (ALP), which dephosphorylates many molecules, initiating the calcification of the matrix by laying down deposits of calcium phosphate.
In the presence of normal liver function, increased serum ALP is a strong indicator of increased osteoblast activity and is useful in the diagnosis of Paget disease , in which increased bone resorption is compensated for by increased osteoblast activity.
Osteocytes are found in the more mature bone and were once osteoblasts, but have now become surrounded and entrapped in their own matrix. They no longer secrete matrix, and their role is to maintain the daily cellular activities of the bone tissue, and to sense the stresses placed on the bone matrix. These activities include the exchange of nutrients and waste products with the blood.
The final type of cell found in bone is the osteoclast whose role is the removal of old bone. Historically it was believed that osteoclasts were formed from osteoblasts but they have been shown to have different lineage, with osteoclasts originating from monocyte-macrophage precursors. They are very large, multinucleated and concentrated in the endosteum (the layer of connective tissue lining the bone medullary cavity). The plasma membrane of the osteoclast facing the bone surface has a deep ruffled border and releases powerful lysosomal enzymes and acids that digest and dissolve the protein and mineral matrix. The removal of old bone matrix is usually balanced with the osteoblasts' production of new bone (see later).
The bone matrix is unlike other connective tissues because it contains lots of inorganic salts, the main one being hydroxyapatite , which is mainly calcium phosphate, but there is also some calcium carbonate and small amounts of other calcium salts, along with magnesium hydroxide, fluoride and sulphate. These are all deposited on the framework of collagen fibres secreted by the osteoblasts. As the minerals crystallise they harden the tissue, in a process called ossification .
The hardness of the bone depends on the amount and type of the crystallised salts. Bone must remain slightly flexible to be able to withstand the forces it is subjected to daily and this is dependent on the presence of collagen fibres which provide the bone with tensile strength. Calcification only takes place in the presence of the collagen fibres, with mineral salts first crystallising in the microscopic spaces between the collagen fibres ( Clinical box 9.1 ). Once these spaces have been filled, more mineral crystals accumulate around the collagen fibres.
Osteogenesis imperfecta , or brittle bone disease, is a genetic disease that affects the production of collagen type I. Collagen has a triple helix formation and in type I forms tightly packed microfibrils. This collagen is important in forming the organic scaffold of bone. The inheritance pattern is autosomal dominant, and osteogenesis imperfecta is the result of a genetic mutation that results in either less collagen type I being produced (due to a premature stop codon or mutant mRNA) or an abnormal collagen type I protein being made (usually when the mutation substitutes a glycine amino acid with cysteine or alanine). The former glycine is a very small molecule and facilitates the super helix formation of collagen type I which gives it its strength (larger molecules produce abnormal chains which are much weaker). New spontaneous mutations account for 25% of cases.
At least seven types of osteogenesis imperfecta have been identified: type 1 is the most common form of the disease and also the mildest, whereas type 2 is the most severe (often leading to perinatal death), and those with type 3 are often wheelchair bound. Other types are milder. The main symptom is that the bones fracture easily so that X-rays of an affected person usually show evidence of multiple fractures that have healed or are in the process of healing. As collagen type I is found elsewhere in the body in addition to bone, osteogenesis imperfecta also affects other tissues and organs. For example, in the eyes, the sclera (the whites of the eye) have a tendency to be blue rather than white, joints tend to be loose or lax as tendons and ligaments are partly composed of collagen type I, and patients often have low muscle tone, brittle teeth and may suffer from hearing loss. Other features include increased perspiration and easy bruising, as the skin tends to be thin and smooth, as collagen type I is also found in the dermis of the skin.
Osteogenesis imperfecta is usually diagnosed during infancy, as babies with the more severe types are often born with fractures and babies with type 1 often have their first fracture in the first year of life; some milder forms are not diagnosed until the teenage ages or even into adulthood.
Whereas the presence of calcium salts gives bone its great compressional strength, collagen increases its tensile strength (the ability to endure a stretching force). Bone does not, however, have great torsional strength and many fractures result from excessive twisting forces applied to long bones.
Bone is not completely solid and contains many small spaces, some of which provide space for blood vessels to supply the bone cells with nutrients and remove waste products. Other spaces are filled with bone marrow (haemopoietic cells). Bone can be divided into two categories, compact and spongy, depending on the distribution of the spaces. Overall, 80% of the total weight of the skeleton is compact bone and 20% is spongy ( cancellous ) bone , although bones in the axial skeleton are 70% spongy bone.
The skeleton can be divided into two parts:
Axial skeleton , which forms the long axis of the body and contains the skull, vertebral column ( Anatomy box 9.1 ) and ribs
The vertebral column is a rigid yet flexible structure that extends from the base of the skull to the tip of the coccyx ( Fig. AB1 ) . The vertebral column allows trunk movement, helps to maintain an upright posture, supports the weight of the body and head and allows the head to turn. It also protects the spinal cord, which runs down through a canal formed by the vertebrae.
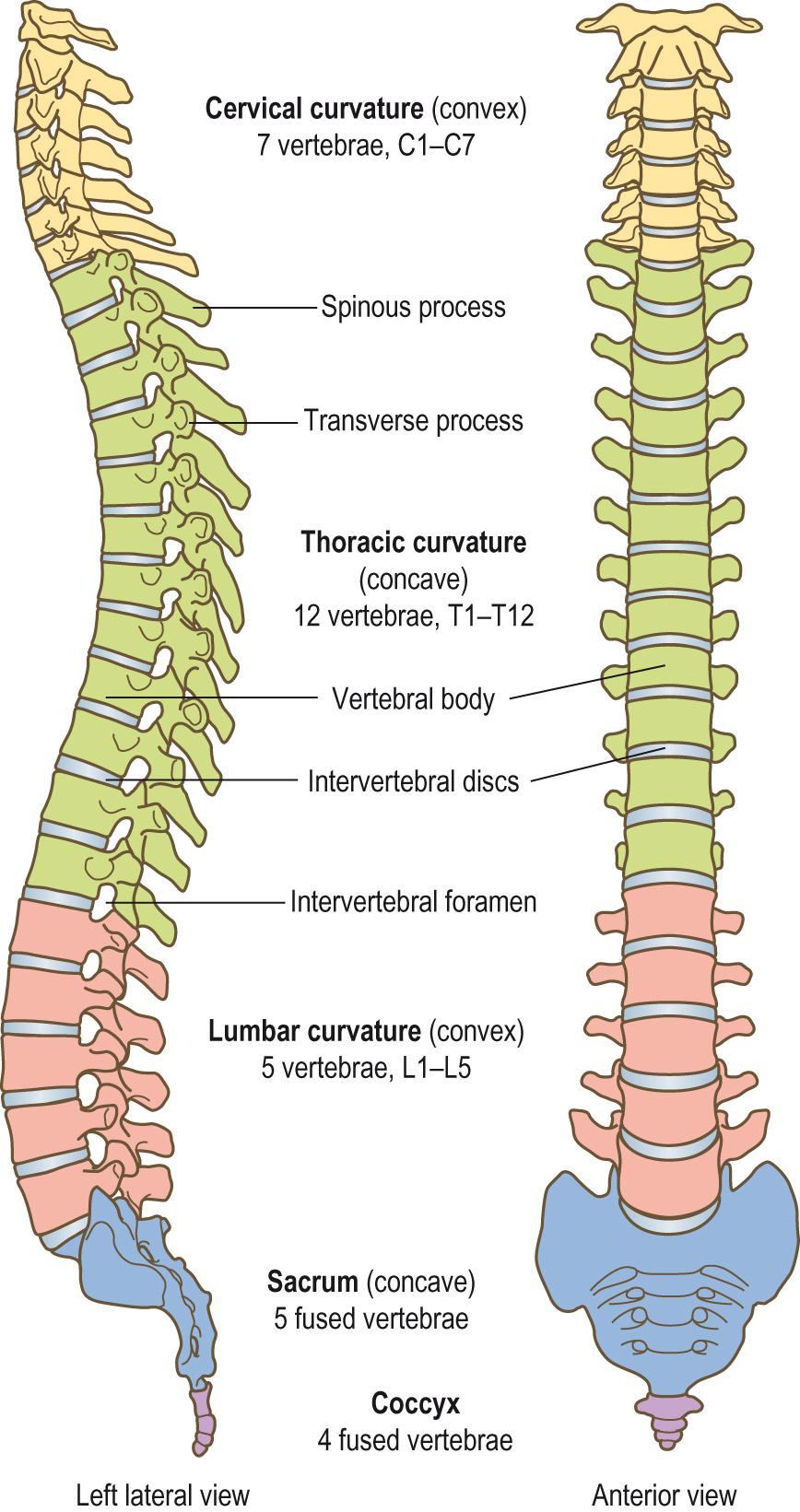
The vertebral column is composed of 24 moveable vertebrae :
7 in the cervical (neck) region (C1–C7) ( Clinical box 9.2 )
12 in the thoracic region (T1–T12)
5 in the lumbar region (L1–L5).
There are also two immobile composite vertebrae: the sacrum , which is composed of five fused segments (S1–S5); and the coccyx , which is composed of three to five fused vertebrae. This gives a total of 33 ± 1 vertebrae. The number of vertebrae is fairly constant, with an estimated 5% of the population having a slight variation, usually in the thoracic and lumbar regions.
The movements that the vertebral column can perform are:
Anterior, posterior and lateral flexion
Extension
Rotation.
The movement between any two vertebral segments is relatively small, but added together there is a considerable range of movement possible.
The length of the vertebral column is relatively constant, 72–75 cm in adults, with three-quarters of the length made up by the vertebral bodies , and the rest of the length made up by the intervertebral discs that lie between the vertebrae.
In the adult there are four curves in the vertebral column (see Fig. AB1 ).
Two primary curvatures: the thoracic and sacral curves. These curves develop in utero and give the newborn a single concave ‘C’-shaped curve to their backs.
Two secondary curves: the convex cervical and lumbar curves. The cervical curve develops when the baby starts to hold up its head, and the lumbar curve develops when the infant starts to stand up and begins to walk.
Neck pain is a common complaint. Chronic pain, often extending to the shoulders, is commonly associated with sedentary occupations that lead to poor neck posture over prolonged periods (such as computer usage). Cervical spondylosis is the most common degenerative condition affecting the neck, especially at C5–C6. Narrowing of the cervical canal from various causes can lead to cord compression and affect upper limb movement. Whiplash injury is a common cause of persistent neck pain. Classically, the injury occurs as the result of a rear impact into a stationary or slow-moving car, which then is propelled into another object in front. The resultant force on the mass of the car occupant's head leads to a rapid extension, followed by flexion of the cervical spine.
Scoliosis is a lateral curvature of the vertebral column. Most cases are idiopathic but some are due to a developmental defect. The lateral curvature of the spine often results in one shoulder and possibly one side of the pelvis being higher than the other. If the curvature is slight, there may not be any significant defect when standing but the abnormal curve becomes more noticeable on bending over. Severe curves may restrict the lungs and the other internal organs, leading to the need for surgery to fuse the curve, preventing further deterioration.
Kyphosis , also known as ‘dowager's hump’, is an increased thoracic curvature; although more commonly postural, it can also form because of osteoporosis, where the thoracic vertebral bodies collapse, as the result of loss of trabeculae bone mass. The progressive bone loss and collapse of the vertebral bodies can result in an overall loss of height. In severe cases, it may restrict breathing by reducing the capacity for the lungs to expand within the thoracic cage.
An increase in the lumbar curvature is called lordosis and is often associated with weakened trunk muscles. Lordosis can also result from obesity or during the latter stages of pregnancy, when the increase in weight anterior to the vertebral column pulls the lumbar vertebrae forwards. This abnormal curvature tends to be temporary and the vertebral column will revert to its normal curves after the baby is born or if the individual loses weight. If the abnormal curvature is severe enough, then it can cause problems, again by restricting the internal organs, or by impinging on the nerve roots as they leave the spinal cord.
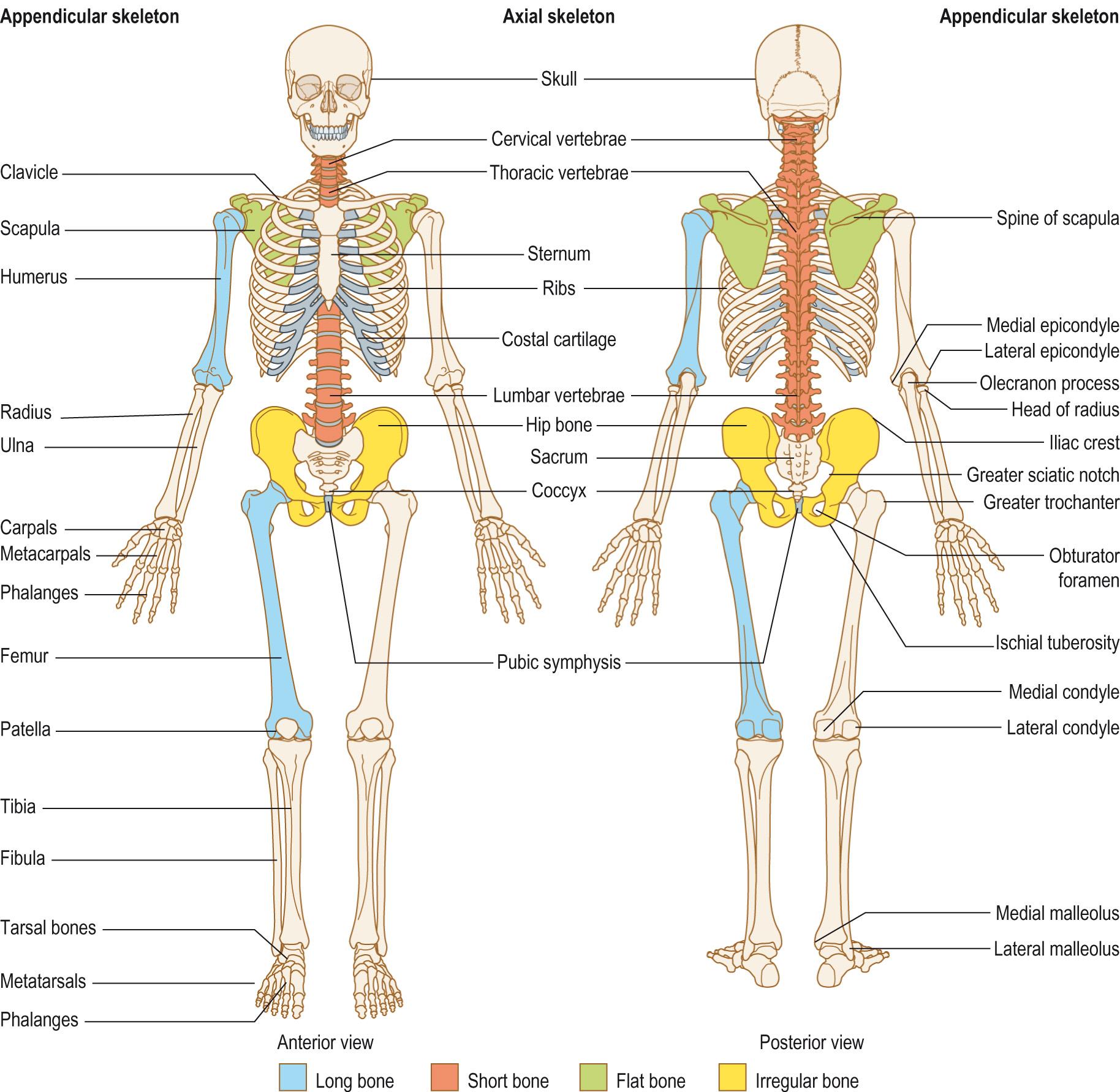
Appendicular skeleton , which consists of the limb bones and their attachments or girdles , the pelvis and scapula and clavicle.
There are many different types of bone and they can be classified according to their shape ( Fig. 9.2 ) :
Long bones are longer than their width and are the most common. They are mainly formed from compact bone with spongy bone in the centre and at their ends (see later). Examples include the long bones of the arms (humerus, radius and ulna) and legs (femur, tibia and fibula), as well as the small bones of the fingers and toes. The tibia is a particularly important bone as pain in the lower leg is a common complaint ( Clinical box 9.4 ).
Pain originating in the anterior or posterior aspect of the tibia is a common bone pain complaint and can be the result of various causes.
In the anterior aspect:
Osteitis – inflammation of the bone in the metaphyseal areas – seen mainly in children
Bone tumour – a common site for primary bone tumours
Anterior tibial compartment syndrome – a common complication of tibial fractures producing inflammation and ischaemia of the tibialis anterior and extensor hallucis longus muscle, leading to an inability to extend the ankle and big toe. There may be an absent dorsalis pedis pulse and sensory loss due to ischaemia of the deep peroneal nerve. Relief of pressure in the area to prevent muscle necrosis can be a surgical emergency
Stress fracture – also more common in Paget disease (localised bone remodelling).
In the posterior aspect:
Ruptured plantaris tendon – more probably due to tearing of muscle fibres of the gastrocnemius or soleus muscles, rather than the plantaris muscle, causing sudden activity-related pain in the calf
Thrombophlebitis – thrombosis in the superficial calf veins leading to inflammation and pain in the calf is a common sign. Deep vein thrombosis is a common postoperative complication, and it is now recommended that prophylaxis with anticoagulant therapy be used postoperatively.
Short bones : these are cuboidal and contain mainly spongy bone with a surface layer of compact bone. Examples of these are the bones of the wrist and ankles, as well as the sesamoid bones, such as the patella (kneecap), which are found within some tendons.
Flat bones : these are flat, thin and usually slightly curved. They consist of two thin layers of compact bone, surrounding a thin layer of spongy bone. The skull, ribs, sternum (breast bone) and scapula (shoulder blade) are all flat bones.
Irregular bones : these are bones that do not fit the other categories. They are made from spongy bone covered with compact bone. The bones of the vertebrae ( Anatomy box 9.2 ) and pelvis are irregular bones.
The vertebral body is the weight-bearing part of the vertebra; it is a block of bone forming the anterior part of the vertebra ( Fig. AB2 ) . The vertebral bodies become progressively greater in size, especially from T4, reflecting the increased weight that the vertebrae have to carry; the largest vertebral body is that of L5. In the sacrum and coccyx, the size of the vertebral body decreases. Internally, the vertebral body is composed of trabeculae bone that is often subject to osteoporotic-related bone loss leading to the collapse of the vertebral bodies in osteoporosis patients.
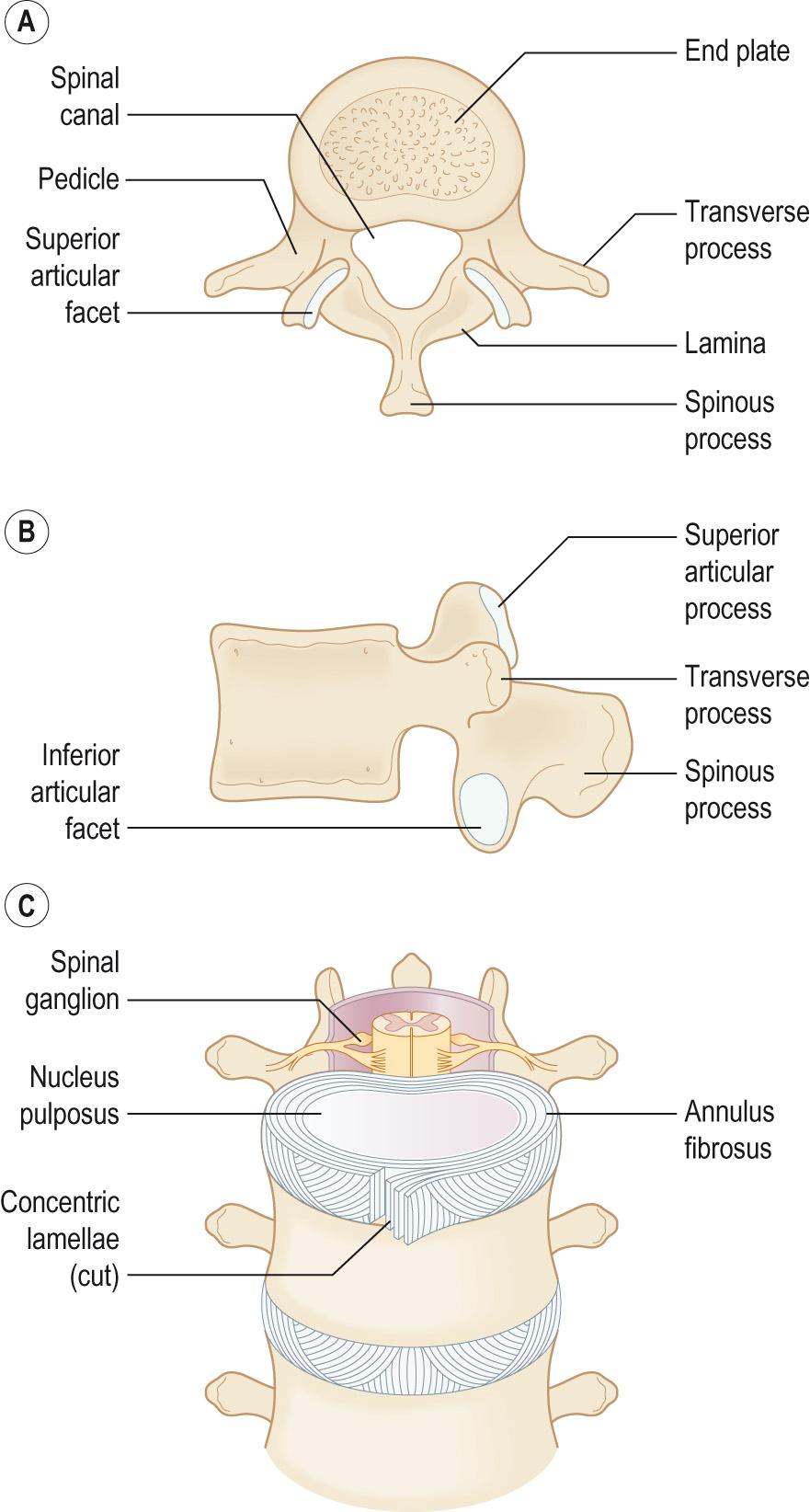
The vertebral arch , or neural arch , is composed of the pedicles and laminae , which form a ring through which the spinal cord passes called the vertebral foramen (spinal canal) ( Fig. AB2 ) . There are notches in the superior and inferior borders of the pedicles called the vertebral notches . These form an intervertebral foramen , through which the nerve roots exit and enter the spinal cord.
Each vertebral arch has seven processes that are attachment sites for muscles and ligaments:
Four articular processes
Two transverse processes
One spinous process.
The vertebrae from different regions show a number of modifications, especially the atlas (C1) and axis (C2) vertebrae of the neck, which form a specialised pivot joint (see later).
Between C2 to S1 adjacent vertebrae articulate at three joints:
Two synovial facet joints (zygapophyses) between the vertebral arches
One symphysis between the vertebral body and the intervertebral disc.
The zygapophyses are synovial joints of the planar kind and allow limited gliding movements between the vertebrae. Osteoarthritis may attack these joints, and the bony growths that form in this condition may reduce the size of the intervertebral foramen, leading to pressure on the spinal nerves.
The pivot joint between C1 and C2 allows the head to tip forward (the ‘no’ joint), and the atlanto-occipital joint between C1 and the base of the skull is the flexion/extension joint and therefore is the ‘yes’ joint.
Ligaments strengthen and stabilise the vertebral column and stop any excessive movements. The anterior and posterior longitudinal ligaments prevent hyperextension and hyperflexion, respectively. There are also short ligaments joining adjacent vertebrae: the ligamentum flavum . There are also interspinous and supraspinous ligaments and some specialised ligaments around the atlanto-axial joint.
The joints between the vertebral bodies are secondary cartilaginous joints or symphyses, designed for weight bearing and strength. The articulating surfaces of the adjacent vertebrae are connected by an intervertebral disc .
The intervertebral disc is composed of two parts: the annulus fibrosis is a fibrocartilage ring consisting of approximately 20 alternating layers of obliquely orientated collagen fibres surrounding the central gelatinous nucleus pulposus , which is a turgid gel composed of 70%–90% water, proteoglycans and some collagen fibres which allows compression between adjacent vertebrae. When weight is applied to the disc, the nucleus becomes flattened and the annulus fibrosis bulges between the vertebrae.
The water content of the intervertebral discs declines with age so the intervertebral discs are better shock absorbers in the young than in the elderly. This also accounts for some of the loss of height that is experienced with age. If the intervertebral discs reduce in height, the intervertebral foramen also reduces in size, which may lead to entrapment of the spinal nerves ( Clinical box 9.5 ).
A slipped disc is a protrusion of the nucleus pulposus through the annulus fibrosis. The protrusion can press on the spinal nerve roots, causing referred pain. This commonly takes place in the lumbar region, leading to sciatica , where the pain is felt in the lower back, posterior thigh and leg, which is the course of the sciatic nerve. Neurological disturbances can also occur which are segmental and dependent on the level and side of the prolapse. Where the prolapse is large and central and below L1/L2 this can also affect the cauda equina , so called because of the ‘horse tail’ appearance of the nerves at the base of the spinal cord. Bladder incontinence is a common symptom and the condition may become a surgical emergency as it can lead to paraplegia. Approximately 95% of herniations of the nucleus pulposus in the lumbar region take place most commonly at the L5/S1, then L4/L5 levels.
There are superficial, intermediate and deep muscles around the vertebral column. The superficial muscles are located around the back of the neck and shoulders and help to move the shoulders. The intermediate group of muscles is located in the lower neck and thoracic level and may be related to inspiration. The deep back muscles maintain posture and move the vertebral column.
Extreme movements of the vertebral column may lead to back strain. The term ‘strain’ is used to indicate some degree of stretching of the muscles and/or ligaments of the back.
A typical long bone ( Fig. 9.3 ) consists of the following regions:
The diaphysis , which is the shaft of the long bone and is the main portion of the bone
The epiphyses , which are the distal and proximal ends of the bone
The metaphyses , which are the regions in a mature bone where the diaphysis joins the epiphysis. In a growing bone the metaphysis is the region occupied by the epiphyseal growth plate .
Articular cartilage is a thin layer of hyaline cartilage that covers the epiphyses. The articular cartilage reduces the friction at the joints and acts as a shock absorber at freely moveable joints.
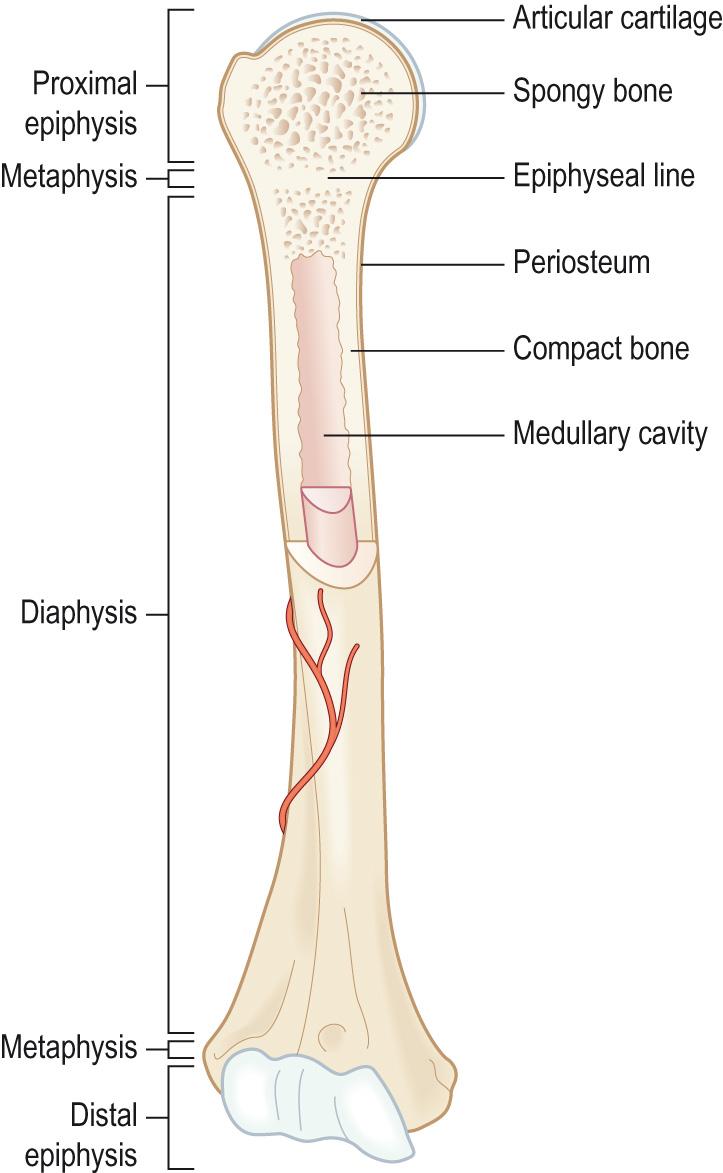
The periosteum is a tough layer of dense irregular connective tissue surrounding the bone surface where it is not covered by the articular cartilage. It contains the osteogenic progenitor cells and as these differentiate into osteoblasts they allow the bone to grow in thickness. The periosteum also helps to protect the bone, assists in fracture repair, helps nourish the bone tissue and serves as an attachment point for tendons and ligaments.
The medullary cavity in the centre of the bones is sometimes called the marrow cavity and is the space within the diaphysis that contains the bone marrow. There are two types of bone marrow found in the medullary cavity:
Red marrow , which produces red and white blood cells and platelets (haemopoietic tissue)
Yellow marrow , which contains fat and connective tissue and produces some white blood cells.
The two types of bone marrow are interconvertible. At birth, there is only red bone marrow present and as the person grows the red marrow in many of the bones is replaced by yellow marrow. By adulthood, only approximately half of the bone marrow is red. The change from red to yellow is due to a decrease in the level of the haemopoietic stimulant, erythropoietin , the hormone that regulates red cell mitosis and differentiation, which reduces with age such that in the elderly only approximately 30% of the bone marrow is red bone marrow. Red bone marrow is found mostly in the ribs, sternum (breastbone), scapulae (shoulder blades), clavicles (collar bones), pelvis (hip bones), skull and vertebrae.
The endosteum is a membrane that lines the medullary cavity and contains bone-forming cells; it is the equivalent of the periosteum surrounding the outside of the bone. It serves as the site of formation for new bone and contains the osteogenic precursor cells.
Compact bone forms the outer layer of all bones, where it provides support and protection to the spongy bone in the centre and resists the stresses produced by weight and movement. It is formed of collagen which is impregnated with inorganic calcium salts, giving its exterior hardness. Compact bone is organised into osteons , sometimes called Haversian systems . In the centre of each osteon there is a central canal ( Haversian canal ) that runs longitudinally through the bone; the blood and lymph vessels and nerves run in these central canals. Around the central canal the bone is arranged into concentric layers ( lamellae ), which are rings of calcified matrix. Osteocytes lie within spaces ( lacunae ) between the layers. Lacunae are connected via small channels called canaliculi in which finger-like projections of the osteocytes extend. The canaliculi form a complex branching network of small channels that connect with canaliculi and also with the central canal. This allows for the movement of blood-borne nutrients and oxygen to diffuse through the bone and for waste products to diffuse back to the blood vessels. The blood vessels of the central canal are connected to the periosteal vessels on the surface of the bone by perforating ( Volkmann ) canals , which also connect with the internal medullary cavity ( Fig. 9.4 ) .
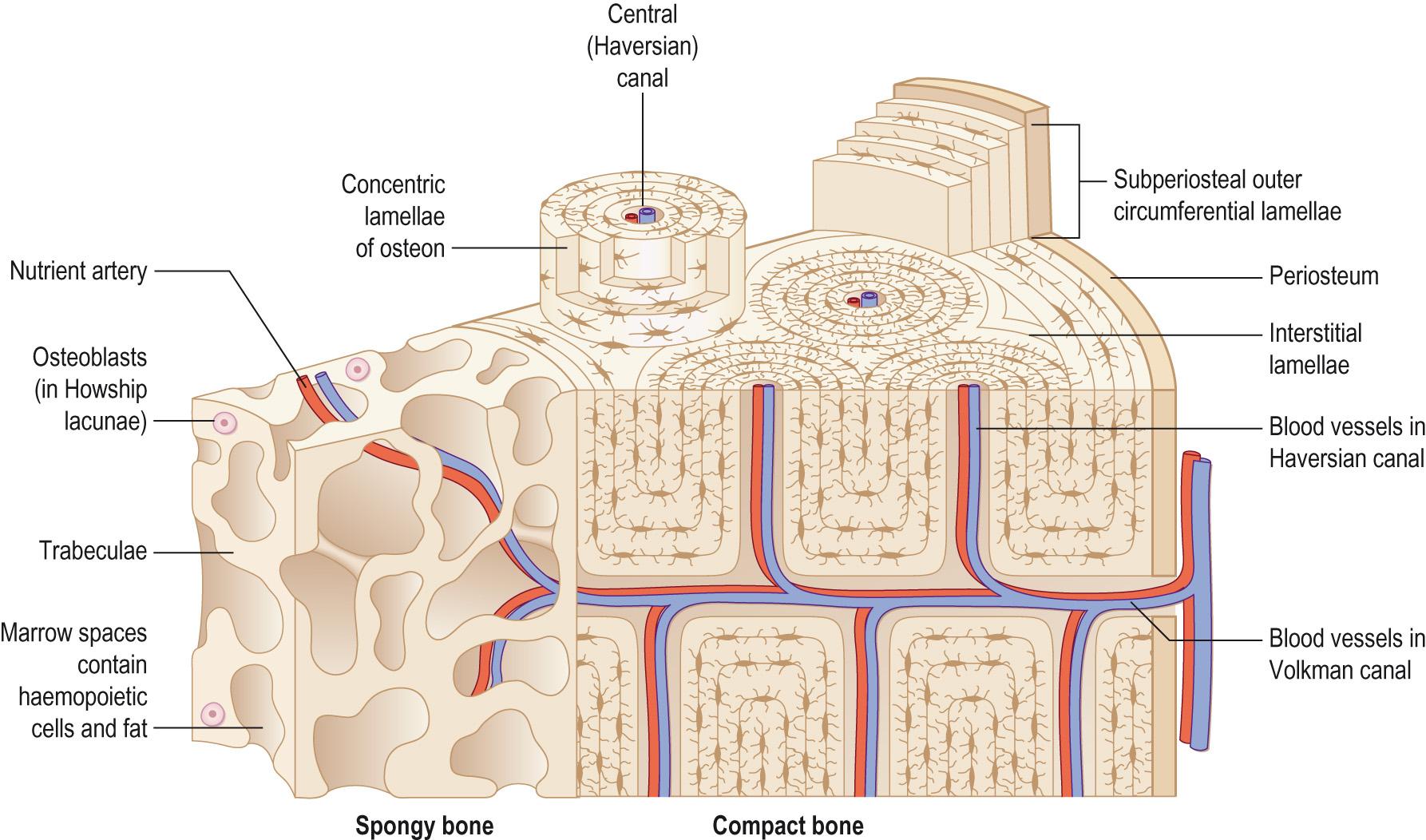
The osteons in compact bone are all aligned in the same direction along the lines of stress. In the diaphysis of a long bone, for example, they run parallel to the long axis of the bone. This allows the diaphysis to resist bending or fracturing even when considerable force is applied from either end. The stresses on bone are not constant. The organisation of the osteons is dynamic in response to new stresses made on the bone, and is part of the normal destruction of old bone and the formation of new bone matrix. The response of bone to new stresses can be seen by the presence of interstitial lamellae between the osteons; these are the remnants of old osteons that have been partly broken down.
Spongy bone does not contain true osteons and consists of lamellae arranged into an irregular lattice of thin interconnecting struts ( trabeculae ). The spaces between the trabeculae are filled with red or yellow bone marrow, which is responsible for the production of blood cells. Within each trabecula osteocytes lie in lacunae with radiating canaliculi, much like in the osteons of the compact bone. The osteocytes in the spongy bone trabeculae receive their nutrients directly from the blood circulating through the medullary cavity (see Fig. 9.4 ).
Spongy bone makes up the majority of bony tissue in the short, flat and irregularly shaped bones and the epiphyses of the long bones, and lines the medullary cavity of the diaphysis of the long bones.
The orientation of the trabeculae in spongy bone is along the lines of stress, like the osteons in compact bone. This characteristic helps bone resist stresses and the transfer of force without breaking. Spongy bone is located where bones are not heavily stressed or where the stresses are applied from many directions, as this type of bone has both flexibility and strength. Spongy bone has a higher rate of turnover than compact bone, and so responds to the changing stresses placed on it faster than compact bone does. For this reason osteoporosis is more evident in bones that have a high proportion of spongy bone, such as the vertebrae and neck and head of the femur.
Spongy bone reduces the weight of the skeleton, so that the muscles acting on the skeleton do not have to work as hard. In the adult the spongy bone and its red bone marrow is the only site of haemopoiesis, especially the spongy bone of the pelvis (hip), ribs, sternum (breast bone), vertebrae and the ends of the long bones.
Certain regions of bone contain large quantities of red bone marrow and these regions have a very good blood supply that passes from the periosteum into the interior of the bone. The periosteal arteries are accompanied by nerves and they enter the diaphysis through the perforating Volkmann canals. They supply the periosteum and the compact bone. Near the centre of the diaphysis there is a large nutrient artery that passes obliquely through the compact bone through a hole – the nutrient foramen. When the nutrient artery reaches the medullary cavity it divides into proximal and distal branches, which supply both the inner layers of compact bone and spongy bone of the diaphysis and the red marrow as far as the epiphyseal growth plates (or metaphyseal line). The number of nutrient foramina varies from bone to bone – the tibia has only one nutrient artery, whereas the femur has many; this variation is due to the size of the bone and the relative amounts of red bone marrow that the bone has. The ends of the bone are supplied by the metaphyseal and epiphyseal arteries . These arteries arise from those that supply the joint. Both the metaphyseal and epiphyseal arteries also enter the bone and supply the red bone marrow in their respective regions.
There are usually one or two nutrient veins that accompany the artery in the diaphysis, and there are many epiphyseal and metaphyseal veins which also exit with the respective arteries. Finally, there are also periosteal veins that drain blood from the periosteum.
Nerves accompany the blood vessels of bone. The periosteum has a rich supply of sensory nerves, some of which transmit pain sensations. These nerves are sensitive to tearing or tension and explain the severe pain from a fracture or a bone tumour.
Bony tissue is formed by one of two processes during embryonic development:
Endochondral ossification: the replacement of hyaline cartilage with bone tissue
Intramembranous ossification: the direct ossification of the mesenchymal cells.
Ossification begins during the sixth or seventh week of human development; once they are fully formed, the bones produced by these two processes are indistinguishable in structure.
The majority of the bones in the body are formed from cartilage by endochondral ossification. Hyaline cartilage forms an initial model of the future bone from mesenchymal cells that differentiate into chondroblasts. Once the hyaline model of the bone has formed, osteoblasts gradually replace the cartilage with bone matrix, which is then ossified. The process is most clearly seen in the long bones of the arms and legs ( Fig. 9.5 ) .
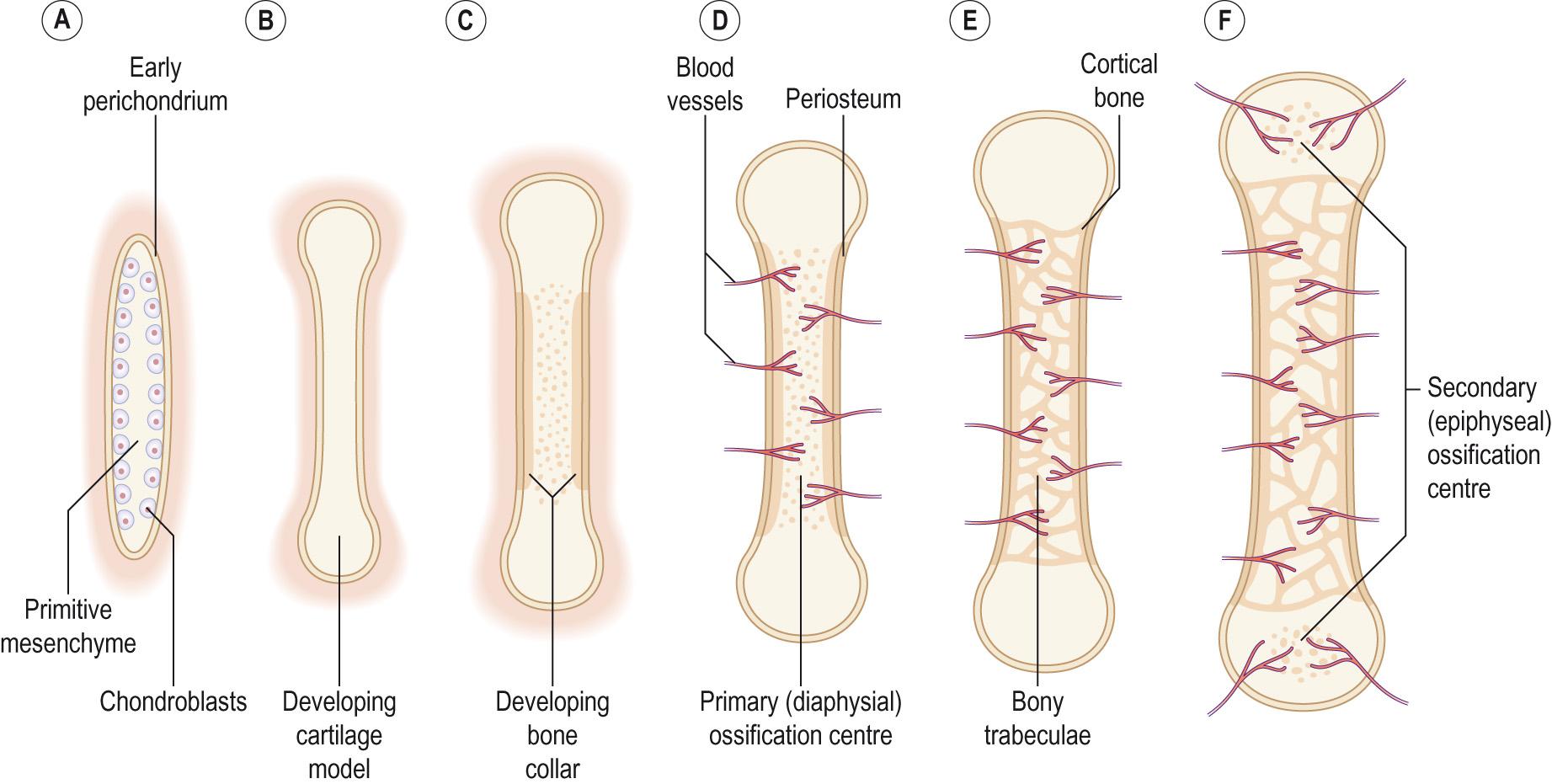
The process begins with mesenchymal cells at the site of the future bone condensing into a rough approximation of the bones. These mesenchymal cells differentiate into chondroblasts (precursors of cartilage) under the influence of various factors in the environment. The chondroblasts then begin to secrete a cartilage matrix around themselves, with a membrane (the perichondrium ) forming around the cartilage model containing the chondroblast precursors. As more cartilage matrix is produced, the chondroblasts become buried in the matrix, and they mature into chondrocytes .
As the foetus grows so does the cartilage model. The chondrocytes can divide and the new chondrocytes produce more cartilage matrix, causing an increase in the length of the cartilage bone model. This is called interstitial growth. The increase in the thickness of the bone ( appositional growth) comes from new chondroblasts differentiating from the precursors in the perichondrium and becoming incorporated into the cartilage bone model.
As the cartilage model grows, the chondrocytes in the centre of the model hypertrophy. Some of the hypertrophied cells burst and release their contents into the cartilage matrix around them. This changes the pH of the matrix and it is this change in pH that triggers the calcification of the cartilage matrix. As the cartilage begins to calcify, chondrocytes begin to die because nutrients can no longer diffuse through the calcifying matrix. Spaces called lacunae form where the chondrocytes had been, and these eventually merge together forming small cavities within the calcifying matrix.
For bone formation or ossification to begin, a nutrient artery must pierce the perichondrium and the calcifying matrix in the mid region of the cartilage model. This stimulates the osteogenic precursor cells in the perichondrium to become osteoblasts. Initially, these remain just under the perichondrium and secrete a thin shell of compact bone called the periosteal bone collar . Once the perichondrium starts to produce bone rather than cartilage it becomes the periosteum . Osteoblasts are carried into the disintegrating calcified matrix by the nutrient artery and its capillaries. Once these are within the cartilage model they begin to form bone and the primary ossification centre is formed. Spongy bone is formed as osteoblasts deposit bone matrix on the remains of the calcified cartilage. As the ossification centre enlarges toward the ends of the bone, osteoclasts start to break down the new spongy bone to form the medullary cavity in the centre of the bone, where the bone marrow will be located. Primary ossification therefore proceeds inwards from the external surface to the centre of the bone.
Primary ossification forms the diaphysis or shaft of the long bones, composed of an outer core of compact bone, lined with spongy bone surrounding a medullary cavity which is filled with red bone marrow. Secondary ossification centres form the epiphyses at the ends of the long bones and these usually develop around the time of birth. The formation of bone in the secondary ossification centres is much the same as for that in the primary ossification centres. However, there is one difference in that spongy bone remains in the interior of the epiphyses. Secondary ossification also starts in the centre and proceeds towards the outer surface of the bone. This secondary ossification process leaves an outer ring of hyaline cartilage around the epiphysis which is important for forming the articular cartilage of the joint surface and the epiphyseal growth plate . The epiphyseal growth plate is located between the epiphysis and the diaphysis, and is responsible for the growth in length of the bones during childhood. The articular cartilage cap at the end of the bone is retained throughout life and helps to reduce the friction between the articulating bones.
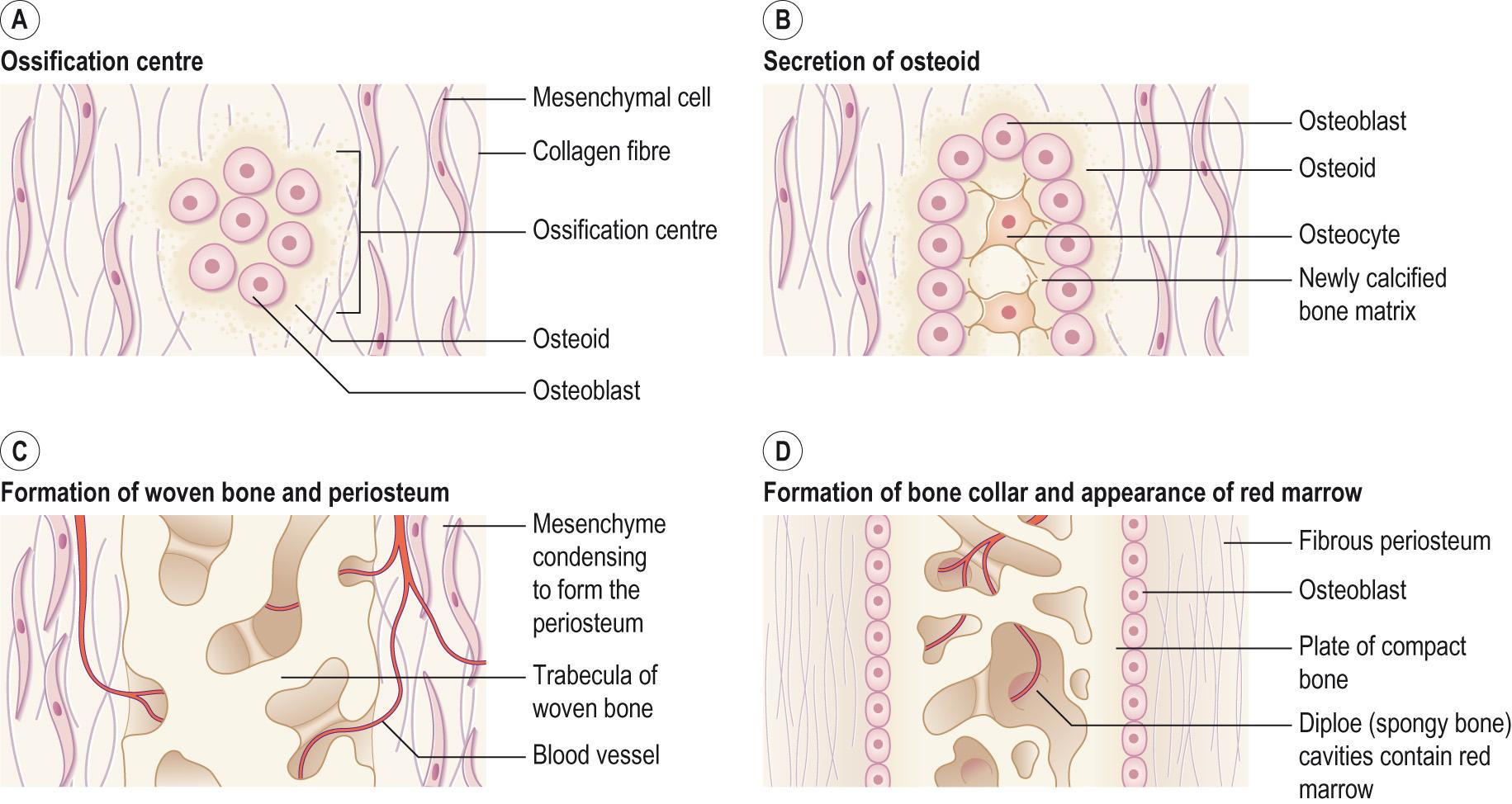
The second process by which bone is formed is intramembranous ossification , where bone forms directly in the condensed mesenchymal cells without first going through a cartilage step ( Fig. 9.6 ) . This type of ossification tends to be found in the flat bones of the skull, the lower jaw, and parts of the clavicle and scapula of the pectoral girdle. At the site of future bone, the mesenchymal cells condense as for endochondral ossification, but they differentiate into osteogenic cells rather than chondroblasts. The osteoblasts cluster together forming a centre of ossification and secrete the organic bone matrix around themselves. Once surrounded, the osteoblasts become osteocytes located in lacunae and they extend fine cytoplasmic processes into canaliculi in all directions. Calcium and other mineral salts are deposited in the matrix within a few days and this hardens and calcifies forming bony spicules . The matrix develops into spongy bone with trabeculae (small pieces of bone) separated by spaces. The connective tissue associated with the blood vessels in the trabeculae differentiates into red bone marrow that fills the spaces. On the outside of the bone the mesenchyme condenses and develops into the periosteum. Finally, the most superficial layers of spongy bone are remodelled into compact bone with spongy bone remaining in the centre.
During childhood the bones grow both in thickness and in length. The bones continue to grow in length until approximately 25 years of age, although they may still increase in thickness after this time.
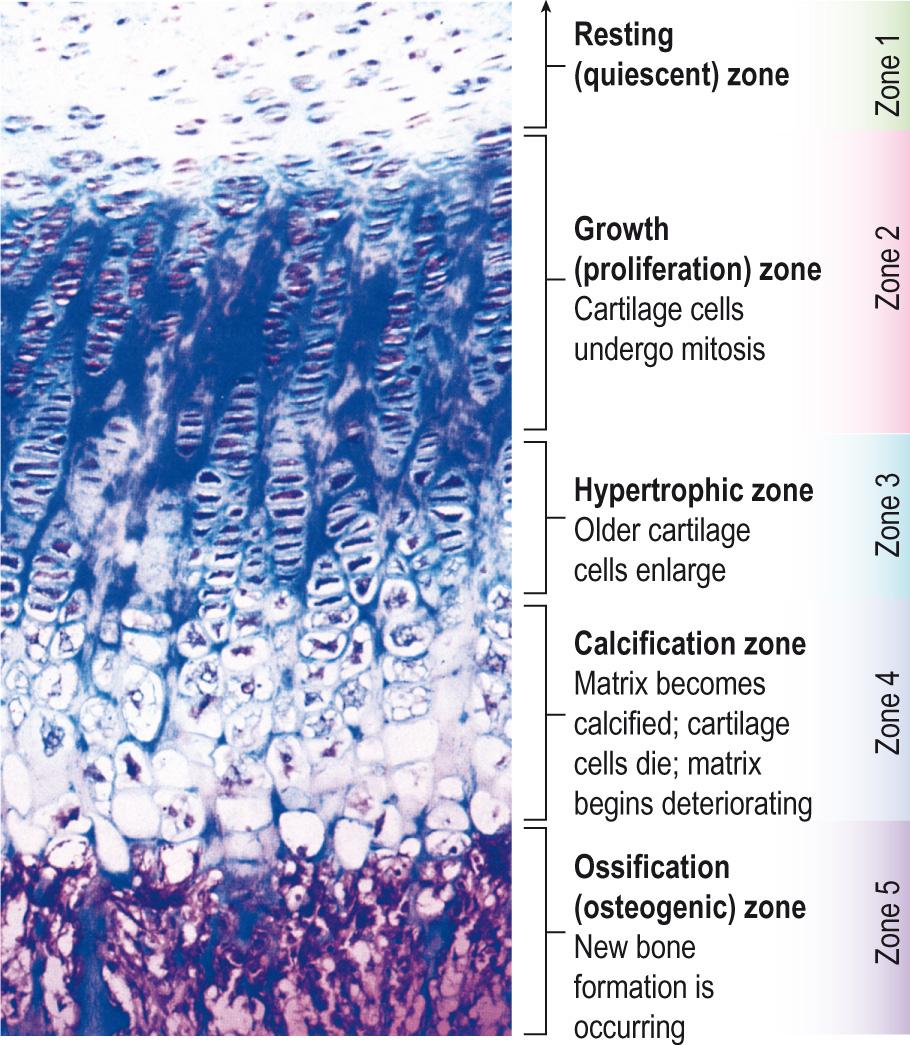
Bone growth in length, especially the long bones, is by the addition of new bone on the diaphyseal side of the epiphyseal growth plates. The epiphyseal growth plate is composed of hyaline cartilage and separates the epiphyses from the diaphysis of the growing bones. It can be divided into four zones ( Fig. 9.7 ) .
Zone 1 is the zone of resting cartilage . It is closest to the epiphysis and is made up of small, scattered chondrocytes that have a low rate of proliferation. These cells are relatively quiescent but this is also the germinal layer that supplies the developing cartilage cells. Another major role is to anchor the epiphyseal growth plate to the bone of the epiphysis as the ratio of matrix to cell volume is high, and this high matrix allows the diffusion of nutrients from the blood supply to the epiphysis to maintain the chondrocytes in the deeper layers.
Zone 2 is the zone of proliferating cartilage . The chondrocytes are slightly larger and are stacked like coins. The chondrocytes are dividing and replacing the ones that are dying at the diaphyseal side of the epiphyseal growth plate. These chondrocytes produce the necessary matrix and are responsible for longitudinal growth of the bone via active cell division.
Zone 3 is the zone of hypertrophic cartilage . This zone can be further subdivided into maturation, degeneration and provisional calcification zones. The chondrocytes increase in size, still in their columns, and they accumulate calcium within their mitochondria; this causes them to deteriorate and ultimately leads to their cell death. Upon their death, calcium is released from matrix vesicles, impregnating the matrix with calcium salt. The calcification of the matrix is necessary for invasion of metaphyseal blood vessels, destruction of cartilage cells, and the formation of bone along the walls of the calcified cartilage matrix. No active growth occurs in this layer; columns of cells extending toward the metaphysis are at various stages of maturation. This is the weakest portion of the epiphyseal growth plate and is commonly a site of fracture or alteration (e.g. widening, as in rickets).
Zone 4 is the zone of calcified cartilage . This layer is only a few cells thick and is composed of mainly dead or dying chondrocytes because they have become surrounded by a calcified matrix. The calcified matrix is removed by the action of osteoclasts and is then invaded by osteoblasts. The osteoblasts lay down new bone matrix and therefore result in the diaphyseal border being firmly attached to the epiphyseal growth plate. It is only by the action of the epiphyseal growth plate that the diaphysis can increase in length. Cartilage is replaced by bone at the diaphyseal end of the growth plate and new chondrocytes are added to the epiphyseal growth plate to maintain its size. Thus the thickness of the epiphyseal growth plate is maintained.
Between the ages of 18 and 25 the epiphyseal growth plates begin to close. The main stimulus for growth by the epiphyseal growth plate is human growth hormone (hGH) , which is secreted by the pituitary gland and promotes growth during childhood and adolescence. Growth hormone acts on the liver and other tissues to stimulate production of insulin-like growth factor 1 (IGF-1) , which is responsible for the growth-promoting effects of growth hormone and also reflects the amount produced. The amount of hGH, and therefore IGF-1, declines with age.
As the levels of hGH and IGF-1 begin to decline, the chondrocytes in zone 2 stop dividing and so the thickness of the epiphyseal growth plate gets thinner as bone gradually replaces the cartilage. Eventually only the epiphyseal line remains as a bony feature on the bones, indicating that the bones have stopped growing. The last bone to finish growing is the clavicle. On X-rays of children and young adults, the epiphyseal growth plates are visible as a black region between the bone of the epiphysis and the diaphysis, as cartilage is radiolucent. If a fracture damages the epiphyseal growth plate while it is still open, then the fractured bone may be shorter than normal. This is because the epiphyseal growth plate is an avascular structure and damage to it accelerates the closure of the plate; thus growth of the bone is reduced. If the rate of bone formation is reduced, then the affected bone will be shorter and may cause misalignment of joint surfaces and, in severe cases, shorter stature ( Clinical box 9.6 ).
Achondroplasia is the commonest cause of short stature, with an average adult height of approximately 1.2 m (4 ft) for affected men and women, and it occurs in approximately 1 : 22 000 live births. Achondroplasia is an autosomal dominant disorder, but approximately 80% of cases are de novo mutations. Achondroplasia is due to a mutation in the fibroblast growth factor receptor 3 ( FGFR3 ) gene on chromosome 4, with 99% of them being due to an A to C point mutation leading to an amino acid substitution.
The mutation affects endochondral ossification through inhibition of chondrocyte proliferation in the growth plate cartilage, affecting the growth of the limbs in particular and leading to short bones and reduced height. The diagnosis of achondroplasia is based on a number of very specific features that can be seen in X-rays, such as frontal skull ‘bossing’ and a ‘champagne glass’ pelvis, and ‘trident’ hands, in which the fingers are of similar length.
Children affected with achondroplasia frequently have delayed motor milestones, otitis media and bowing of the knees. Although infants with the condition are at an increased risk of death, most thereafter lead independent and productive lives and are of normal intelligence.
Bone can increase in thickness by appositional growth . The periosteal cells at the bone surface differentiate into osteoblasts and components secrete collagen fibres and other organic and inorganic matter forming the bone matrix. The osteoblasts become surrounded by the matrix and develop into osteocytes. This forms bone ridges along the bone on either side of a periosteal blood vessel. As more bone matrix is produced, the ridges grow and form a groove for the blood vessel, eventually fusing into a tunnel for the vessel. The former periosteum now becomes endosteum lining the tunnel.
Bone deposition continues from the osteoblasts in the endosteum, forming concentric lamellae that proceed towards the centre of the tunnel. Once the tunnel has produced new bone to reach the blood vessel it is a new osteon. As an osteon is forming, osteoblasts under the periosteum deposit a new circumferential lamella, which further increases the thickness of the bone. This process continues as new periosteal blood vessels become enclosed.
As new bone is being added on the outer surface of the bone, the bone lining the medullary cavity is being destroyed by osteoclasts in the endosteum. Therefore, the medullary cavity gets larger as the bone increases in diameter.
Adequate dietary intake of minerals and vitamins is essential to maintain the growth of bone, as well as sufficient levels of several hormones.
Calcium and phosphorus are needed in considerable quantities during bone growth. Fluoride, magnesium, iron and manganese are required in smaller amounts. Vitamin C is required for the synthesis of the collagen – which is the main bone protein – and is also needed for the differentiation of osteoblasts into osteocytes. The vitamins K and B 12 are required for protein synthesis, and vitamin A stimulates the activity of osteoblasts.
IGFs are the most important hormones during childhood to stimulate growth of the bones and help maintain bone mass in the adult, stimulating osteoblastic differentiation of mesenchymal cells in bone remodelling. IGFs are produced by the bone tissue itself and also by the liver. They act by promoting cell division and synthesis of new bone proteins at the epiphyseal growth plate and periosteum. The production of IGF is stimulated by hGH produced by the anterior pituitary. The thyroid hormones T 3 and T 4 and insulin are also required for normal bone growth.
At puberty, the ovaries and testes secrete sex steroids which stimulate IGF-1. Initially the sex steroids cause a sudden growth spurt and the oestrogens in the female start to cause changes in the female skeleton, such as a wider pelvis. The sex steroids, especially oestrogens, contribute to the shutting down of the epiphyseal growth plate. Females have more circulating oestrogens than males, who have higher androgens; therefore, the lengthwise growth of bones stops earlier in females than in males.
In normal life there is constant bone remodelling, where the resorption of bone by osteoclasts matches the formation of new bone by the osteoblast cells. The process is vital to remove the old bone matrix before it fails and to also redistribute bone matrix to regions of higher stress. The osteocyte processes that fill the canaliculi sense the stress on the bone matrix and signal for osteoclasts to move in and start the remodelling process.
Resorption . Activated osteoclasts move to the old bone that is about to be resorbed. The ruffled border of the osteoclast faces the bone surface; they clamp down onto the bone surface and form a leak-proof seal. They secrete enzymes, such as collagenases and lysosomal enzymes , that attack the organic portion of the bone under the leak-proof seal beneath their ruffled border. They also secrete hydrochloric acid to dissolve the inorganic salts of the bone matrix. Both bone proteins and minerals – mainly calcium salts – enter the osteoclasts and pass through to be excreted into the extracellular space ( Fig. 9.8 ) .
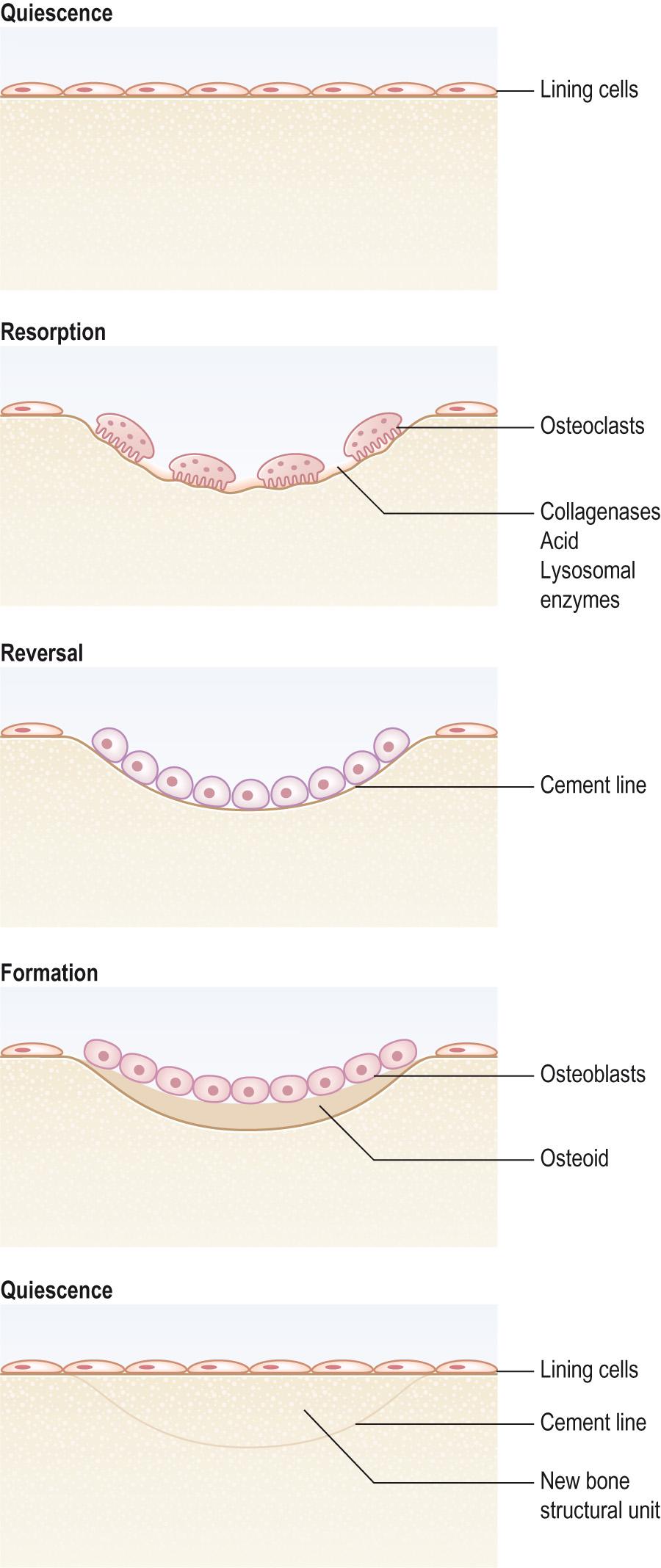
Formation . The next step is that osteoblasts migrate into the hollowed-out space prepared by the osteoclasts. They synthesise type I collagen , osteocalcin (also known as bone Gla protein ), and the other organic components of bone, such as proteoglycans and growth factors, that together form osteoid. The osteoblasts also control the mineralisation of the bone. The osteoid matrix is gradually coated in calcium salts and hardens.
Quiescence . As the osteoblasts become embedded in the mineralising matrix, they slow their production of matrix protein and become osteocytes. The bone is now in a resting phase of the remodelling cycle.
Resorption and new bone formation are normally well balanced, but where resorption is increased, a basophilic lesion occurs (the cement line ) where remodelling is obvious histologically as new bone fills a resorbed cavity.
This remodelling cycle is the same for both compact cortical bone and the trabecular network of the internal spongy bone. Uncoupling bone resorption from bone formation leads to the bone conditions with a loss or increase in the body's bone mass, as seen in osteoporosis, osteomalacia or Paget disease ( Clinical box 9.7 ).
In Paget disease there is accelerated bone turnover, which is indicated by an increased osteoclast-mediated bone resorption. The osteoclasts seen in Paget disease patients are numerous and large, with up to 100 nuclei in them. The alkaline phosphatase levels are very high as well, indicating increased osteoblast activity. The increase in bone turnover leads to thicker but softer bones and is frequently associated with fractures.
Paradoxically there is also bone thickening , but the new bone is new woven bone as there is no remodelling to stronger compact and trabecular bone. This thickening of the bone may trap nerves, resulting in severe bone pain. Untreated, the disease progresses and the legs may bow, the spine develops a curvature and the skull may increase in size. If there is severe skull enlargement there can be problems with the patient's vision and hearing as the nerves become trapped. Treatment includes the administration of drugs that reduce bone turnover, such as bisphosphonates and analgesics for pain.
Paget disease increases in prevalence with age and is more common in those with northern European ancestry. The cause is unknown, although both genetic and environmental factors are thought to play a part.
The whole process of bone remodelling takes between 160 and 200 days from when the osteoclasts begin to remove the old bone and when the osteoblasts have become embedded in the mineralised bone matrix. The final stage of bone healing after a fracture occurs by the same bone remodelling process (see later).
The main calcium store for the body is the bone, with 99% of the total body calcium stored in the skeleton; serum calcium represents less than 1% of the body's total calcium. The serum calcium level is extremely important for many vital bodily functions, such as blood clotting, nerve cell activity and many other cellular activities. The concentration of calcium ions in the blood plasma must be maintained within a narrow range (9–10.5 mg/dL, 2.2–2.6 mmol/L), as even small changes outside this window can be fatal, from heart or respiratory arrest due to calcium concentrations that are too high or too low, respectively. The level of calcium in the blood must therefore be very tightly regulated by controlling the rate of calcium resorption from bone into the blood, and calcium deposition into the bones. There are two major hormones and one minor hormone that control calcium homeostasis.
Parathyroid hormone (PTH) is an 84-amino acid peptide that is secreted by chief cells within the four parathyroid glands, located on the back of the thyroid gland in the neck. PTH is the most important regulatory hormone of calcium concentration in the bone and blood and is linked to several negative feedback systems that adjust blood calcium ion concentration. A fall in blood calcium ions is detected by the PTH receptors, and PTH synthesis is increased and released into the blood. PTH affects the activity of osteoclasts indirectly through its binding to the PTH receptor on osteoblasts; the osteoblasts then produce RANKL, which stimulates osteoclast progenitor cells to proliferate and differentiate, which increase the resorption of bone, thus resulting in the release of calcium from the bone into the blood. PTH also acts on the kidneys to increase calcium reabsorption, phosphate excretion and synthesis of 1,25-dihydroxyvitamin D. Normally 95% of the calcium filtered by the kidney is reabsorbed. PTH actually decreases calcium reabsorption from the proximal tubule but increases the reabsorption from the distal nephron. There is also decreased phosphate reabsorption from the proximal tubule, which results in its increased excretion.
1,25-Dihydroxyvitamin D (dihydroxycholecalciferol) is the second factor that controls serum calcium levels. Vitamin D is derived from two sources: the diet (D 3 ) or by synthesis in the skin (D 2 ). This is then converted to 25-dihydroxyvitamin D in the liver and then to the active 1,25-dihydroxyvitamin D in the kidneys. 1,25-Dihydroxyvitamin D is a very potent stimulator of intestinal calcium and phosphate absorption and is also a stimulator of bone resorption, although only at high concentrations. At normal physiological levels, 1,25-dihydroxyvitamin D is necessary for proper bone mineralisation, and the lack of vitamin D either due to dietary deficiencies or lack of sunlight exposure leads to osteomalacia ( Clinical box 9.8 ).
Osteomalacia is a softening of the bones. The childhood variety of this condition is rickets . There is no loss in the mass of the bones, but the ratio of matrix to mineral changes so that there is more bone matrix and less bone mineral than in the normal bones. It can produce similar symptoms to osteoporosis but they have different causes. Osteomalacia is due to the inadequate mineralisation of newly formed bone matrix. In contrast, in osteoporosis, mineralisation is normal. Rickets is only seen before the epiphyseal growth plate has closed, and causes a bowing of the legs as the bones are softer and bend. This may be due to insufficient calcium absorption because of a lack of dietary calcium, or a lack of activated vitamin D, or phosphate deficiency from increased renal loss. In children, the bones are soft anyway because they are growing, so the deformities produced by osteomalacia may be severe, with curvature of the spine in addition to the bowed legs. In children, the epiphyseal growth plate is widened and may be cup or trumpet shaped, and the line of ossification is less distinct. In adults, because the bones have stopped growing, the deformities are less severe, and the symptoms are more like osteoporosis. On an X-ray of an adult with osteomalacia, there may be pseudofractures visible on regions of bones that have muscle attachments. These radiolucent lines on the bones are called ‘ Looser zones ’ (Looser lines).
The principal role of 1,25-dihydroxyvitamin D is the increased mineralisation of the bone matrix. A part of this is due to the raised plasma Ca 2+ levels from the action of 1,25-dihydroxyvitamin D on the gut. 1,25-Dihydroxyvitamin D can also stimulate the proliferation and activity of the osteoblasts, which are producing the new bone matrix.
Calcitonin (thyrocalcitonin) also contributes to the control of blood plasma calcium. It is released from the parafollicular cells of the thyroid gland in response to rising serum Ca 2+ concentration (e.g. after a meal) and directly inhibits osteoclast activity, inhibits Ca 2+ absorption by the intestines and inhibits renal tubular Ca 2+ reabsorption, thus reducing plasma Ca 2+ (and phosphate) concentrations. Therefore, calcitonin leads to less calcium resorption from the bones and more calcium is incorporated into the bone tissue. Under normal physiological conditions calcitonin has only minor effects, which, as outlined previously, are the opposite of PTH.
Oestrogen increases the activity of osteoblasts and decreases the activity of the osteoclasts that resorb bone. It therefore has a protective effect in women. Loss of oestrogen at menopause removes this protective effect, and there is a rapid loss of bone mass in the 5 years around the time of menopause ( Clinical box 9.9 ).
Osteoporosis literally means porous bones, and there is a generalised loss of bone mass, making the bones more insubstantial and brittle. In both men and women, bones reach their maximum density in early adulthood. By the age of 20 years, 90%–95% of the peak bone mass has been attained and, from approximately 40 years of age, there is a gradual loss of bone mass. However, in women, the loss of oestrogen at menopause means that the protection the hormone provides in preventing osteoclast activity is now removed, and bone loss is increased. As women's skeletons tend to be lighter to begin with, a relatively small loss of bone mass can have severe implications on the health of their bones. There is a loss in the bone mass but the ratio of bone matrix to mineral is unchanged.
The condition is usually diagnosed from the X-ray appearance of the bones where they appear more radiolucent and less dense than normal. However, normal X-rays are relatively insensitive to the loss of bone mass and as much as 50% has to be lost for it to be clearly seen on an X-ray. Instead DEXA scans are used to measure bone mineral density (BMD) in which a density T-score of less than 2.5 standard deviations away from normal is considered to indicate osteoporosis. In most cases of osteoporosis, the loss of bone is not evenly distributed; trabecular (spongy) bone has a higher rate of bone remodelling than cortical (compact) bone. Those that contain a large trabecular bone framework are particularly at risk, with a reduction of between 2% and 3% per year, but this can increase in women to 9% around the time of the menopause, in comparison with cortical bone, for which the loss averages 1%–2% per year. Bones with a high proportion of trabeculae bone, such as the neck of the femur and vertebral bodies, are particularly at risk.
The loss of bone mass has no clinical effect unless a fracture occurs. Fractures of the vertebral bodies create a loss in the anterior height of the vertebrae, leading to kyphosis (excessive posterior curvature) of the back. Fracture of the neck of the femur increases with age until by the age of 75, it is the most common type of fracture, due to the osteoporosis seen in the elderly.
Genetic, hormonal, nutritional and activity factors all play a role in the development of osteoporosis. Excessive glucocorticoid activity, either naturally from an adrenal tumour or from artificial sources, such as the prolonged use of corticosteroids, also lead to the loss of bone mass and osteoporosis. Excessive alcohol and smoking can also lead to the loss of bone mass and brittle bone.
To prevent osteoporosis, children and especially young girls should be encouraged to maintain an adequate dietary intake of calcium and to exercise, both of which will build up the strength of the bones. Throughout life, adequate dietary calcium and exercise should be continued, not to prevent bone loss but to minimise the rate of loss. The use of hormone replacement therapy (HRT) has been shown to slow the development of osteoporosis in postmenopausal women.
Apart from HRT, another treatment available for osteoporosis are bisphosphonates . These are pyrophosphate analogues that bind to the hydroxyapatite crystals of the bone matrix and so inhibit bone breakdown. They also inhibit osteoclast attachment to the bone matrix, as well as stimulate osteoblasts to inhibit osteoclast formation.
As bone is continually being formed and broken down it has the ability to alter its strength in response to mechanical stress . More new bone is deposited along the lines of stress, and calcitonin production is increased to inhibit bone resorption. With no mechanical stress, bone does not undergo normal remodelling and bone resorption outweighs bone formation. The main mechanical stress that bone encounters is from the contraction of skeletal muscles and the pull of gravity. If a person is bedridden or has a fractured bone that is placed in a cast, the strength of the unstressed bones reduces. Astronauts who live in a low-gravity environment for even a short time lose bone mass dramatically – as much as 1% a week. On the other hand, the bones of athletes, which are continually under repetitive stress, becomes thicker. Any weight-bearing activity helps to maintain bone mass and this is especially vital just prior to the closure of the epiphyseal growth plate, as it helps to build up bone mass before the inevitable loss with age. Even in the elderly, weight-bearing activities can help to slow the loss of bone mass.
A calcium intake of between 800 and 1500 mg per day is considered to be adequate for most adults; children and teenagers require higher levels of calcium in their diet as their bones are actively growing. However, the amount of calcium required to be taken in from dietary sources must be considered alongside those of the other dietary components that affect absorption of calcium from the gut, and also those factors that influence calcium losses.
Calcium is lost from the body in urine, gut secretions and sweat. To avoid a net loss of calcium from the bones, the calcium absorbed from food in the gut must balance the losses. Otherwise, the body will take calcium from bone to maintain the required level of calcium in the blood. The body contains approximately 1 kg of calcium in the bones. Even a small excess in the loss of calcium compared with absorption of just 30 mg per day will, after 1 year, result in a 1% loss of calcium from the bones.
The typical diet of North Americans and Europeans contains four dietary components with equal importance that also lead to a net calcium loss: high sodium, high protein, low potassium and low bicarbonate intakes.
Increasing sodium intake from 1000 to 4000 mg/day causes an additional calcium loss of 52 mg/day.
Increasing protein intake from 40 to 100 g/day will cause an additional calcium loss of 66 mg/day.
Decreasing potassium intake from 8000 to 2000 mg/day will increase calcium losses by 31 mg/day.
Decreasing bicarbonate intake from 100 to 20 mmol/day will increase calcium losses by 32 mg/day.
In children, adolescents and younger adults, calcium absorption is more efficient and adapts better to increased losses with better production of 1,25-dihydroxyvitamin D. Older individuals of both sexes show a decline in calcium absorption of approximately 30%–40% less at 80 years than at 30 years of age, and so they are affected more by these dietary factors.
The best foods for increasing calcium absorption without effecting calcium loss are the green leafy vegetables, such as kale and spring greens. There is no evidence that high dairy food consumption increases calcium levels, and these foods are also usually associated with high saturated fat intake; in addition, the high calcium content of many of these foods is often offset by their high sodium and protein content. Foods such as meat, fish and eggs, which are low in calcium, should be eaten in moderation as they drive high calcium losses, whilst some low-calcium foods, such as bananas and oranges, stimulate calcium absorption, providing a boost.
The Asian diet is also not without problems for calcium absorption. Phytic acid is found in high quantities in chapatti flour and combines with calcium in the gut to make it unabsorbable. The pH of the blood also plays a significant factor in osteoblast and osteoclast activity. As the blood pH drops, the balance is shifted in favour of osteoclasts and therefore bone density declines. The pH of the blood decreases with age, as kidney efficiency declines, and it is also more sensitive to the balance between acid and bicarbonate from the diet. Consuming alkaline foods, which are typically high in potassium relative to protein, will increase the pH of the blood, thereby shifting the balance in favour of the osteoblast activity. However, a low protein diet causes a decline in the production of growth hormones and even of bone proteins, such as collagen. It is therefore very important to maintain adequate protein intake while eating plenty of alkaline foods, such as fruits and vegetables, to balance the acid from the protein. It should be noted that proteins that come from vegetable sources (other than grains and some nuts) are usually alkaline, whereas proteins from animal sources are usually acidic.
A fracture is any break in the bone; the process of bone healing is an ordered progression of steps. Bone is about the only tissue in the body that, when it heals, is stronger than before the fracture. Usually scar tissue is weaker than the original, but with bone the healing process leads to new bone tissue which is as strong if not stronger than the old bone that broke.
There are three phases in the healing process: reactive, reparative and remodelling.
Reactive phase . The first step in the repair process is the formation of a fracture haematoma . There are numerous blood vessels throughout the bone. As a result of the fracture, the blood vessels that cross the fracture line are damaged and blood leaks out into the fracture site. A blood clot forms 6–8 hours after the fracture. The blood supply to the bone cells that lie on either side of the fracture is disrupted, and they begin to die. The dead cells induce macrophages and osteoclasts to start removing the dead bone debris and cells from the fracture site. They also cause a localised swelling and inflammation. The haematoma serves as a focus for the healing process.
The next step in the healing process is to re-establish the blood supply to the area, so that new cells can begin to heal the fracture. Blood capillaries grow into the haematoma. This stage may last several weeks, but it is during this process that the presence of nicotine in the system can inhibit this capillary ingrowth, and for smokers there is an increased length of fracture healing compared with non-smokers.
Reparative phase . The next step is the formation of a fibrocartilaginous callus ; the new blood vessels that grow into the haematoma begin to organise it into a granulation tissue, called initially a procallus . Fibroblasts from the periosteum – and osteogenic progenitor cells from the periosteum, endosteum and the red bone marrow – start to invade the procallus. Collagen is produced by the fibroblasts, which become chondroblasts, and these connect the two ends of the fracture together. The osteogenic progenitor cells enter the bordering regions of healthy bone on the edges of the dead bone, ready to start making new bone matrix. As more chondroblasts form, they begin to make fibrocartilage, which replaces the procallus. This stage lasts approximately 3 weeks. The procallus and the fibrocartilaginous callus that replaces it are very soft in the first 4–6 weeks of fracture healing, so there is need for adequate support and bracing until the callus begins to ossify.
The next step in the healing process is the formation of the bony callus . The osteogenic progenitor cells that invaded the procallus and migrated to the borders of the dead/healthy bone region form into osteoblasts and begin to secrete bone matrix. They form spongy bone trabeculae which at this stage are not arranged in an ordered way and so the new bone is called woven bone . The trabeculae join the living bone tissue on either side of the fracture. Depending on the size of the bone and the severity of the injury, it may take up to 3 months for the whole of the fibrocartilaginous callus to be transformed into bone of adequate strength.
Remodelling . The final step in the healing process is bone remodelling. The woven bone lattice is rearranged into the normal cortical and spongy bone arrangement. The woven bone is removed gradually by the action of the osteoclasts and replaced by other osteoblasts. Sometimes the repair is so good that the fracture line is undetectable even on an X-ray.
Joints occur between two or more bones and can be classified according to the type of connecting material into four types:
Bony joints
Fibrous joints
Cartilaginous joints
Synovial joints.
They can also be classified according to the amount of movement that is possible at the joint:
Synostoses and synarthroses – fixed, unmovable joints
Amphiarthroses – where some movement is possible
Diarthroses – which are freely movable.
Bony joints are formed where a previous gap between two bones ossifies, effectively forming a single bone – a synostosis. For example, the right and left frontal and mandibular bones in infants fuse, and in the elderly, some of the cranial bones may fuse. The first rib and the sternum can also fuse with age, as do the epiphyses and diaphysis of the long bones.
Fibrous joints are connected via fibrous tissue, with no cavity between them. These are synarthroses , as there is usually very little movement at the joint because the bones are held together very closely via fibrous tissue crossing the joint. There are four types of fibrous joint:
Suture
Syndesmosis
Gomphosis
Schindylesis
A type of fibrous joint called a suture is found between the bones of the skull where dense connective tissue is found between the closely interlocked bones. They may be serrate , as seen in the parietal bones; bevelled , as seen associated with the temporal, sphenoid and occipital bones; or butt , as seen in the roof of the mouth where the palantine and maxilla bones meet.
At birth, the bones of the skull vault are separated via broad fibrous bands of tissue called fontanelles . These allow an easier passage of the baby's head through the birth canal (pelvic outlet) during labour by letting the skull mould, with the parietal bones over-riding each other. This makes the baby's head temporarily smaller and a few days after birth the head returns to its normal shape. The fontanelles are also vital after birth as they allow the brain to grow.
The anterior fontanelle is the largest and generally closes by 18–24 months after birth. While it is open it can be used to help in the diagnosis of raised intracranial pressure. As the fontanelle is composed of fibrous tissue rather than bone it can be gently palpated; a fontanelle that is dome-shaped and does not depress when gently pressed indicates raised intracranial pressure.
The fontanelles remain open until at least 18 months after birth; however, the sutures are still separated by fibrous tissue. The sutures can still be seen on the adult skull, as most never fully close ( Clinical box 9.10 ).
Clinically there are a number of conditions in which the fontanelles and the sutures close prematurely; these are all classified as craniosynostosis syndromes . The sutures may close before birth, making labour difficult and life threatening for both the baby and the mother; such babies are often delivered by caesarean section. Children born with one of these conditions may have to undergo extensive and repeated surgery to open the sutures to allow the brain to develop and grow; without this, the intracranial pressure rises, which can cause brain damage. The sutures and fontanelles also allow the face to grow after birth, and if they close prematurely then the skull is attached to the base of the skull earlier than normal and the face tends to appear flat with bulging eyes as the orbit fails to grow to accommodate the eyes, and the mandible protrudes giving malocclusion of the teeth. The genetic basis of many of the craniosynostosis syndromes has been linked to mutations in the fibroblast growth factor receptor genes or in the TWIST genes. These mutations result in an activated receptor even in the absence of the ligand, which seems to cause premature ossification of the skull bones. In a few conditions, other regions of the skeleton are also affected, mainly the hands and feet, with fused digits ( syndactyly ).
Another type of fibrous joint is a syndesmosis . These are formed by either a bundle or sheet of fibrous tissue between the bones. The bundles are ligaments , whereas the sheets are called interosseous membranes . Syndesmoses are found in the lower legs between the distal tibia and fibula, forming the distal tibiofibular joints . These joints have a short ligament between the bones so they are very rigid. Another syndesmosis is found in each forearm between the radius and ulna, forming the radioulnar interosseous joints . These bones are connected by an interosseous membrane so these joints do permit a small amount of movement between the bones, especially in the radius and ulna, allowing pronation and supination, and so functionally are amphiarthroses .
Each tooth is connected to the bone of the upper or lower jaw via a fibrous joint called a gomphosis . The dense fibrous connective tissue between the tooth and its socket in the alveolar bone is called the periodontal ligament . In most healthy cases, there should be no or only very limited movement between the elements of the joint; only in dental gum disease does the gomphosis become weakened and the tooth loosen in its socket.
The last type of fibrous joint is the schindylesis ; this is an articulation where a thin plate of bone is received into a cleft or fissure formed by the separation of two laminae in another bone. An example of a schindylesis is the articulation of the rostrum of the sphenoid and perpendicular plate of the ethmoid with the vomer (all found in the upper facial part of the skull), or in the reception of the latter in the fissure between the maxillae and between the palatine bones.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here