Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The practice of transfusing blood has been around for centuries. The science of transfusion medicine started in the early twentieth century with the discoveries of human blood types and sodium citrate. Human blood typing is the pillar and foundation for safe blood transfusions, and citrate allowed for the storage of blood. The concept of collecting and storing blood (blood banking) was another major development, beginning in the 1930s. In the 1960s and 1970s transfusion practice focused on using whole blood in trauma (and war) for resuscitation and to stop bleeding. In the 1970s, 1980s, and 1990s transfusion was focused on the reduction in transmission of infections from a blood transfusion and moving toward blood component therapy instead of whole blood. In the 1990s and 2000s important topics in transfusion included when not to transfuse and what to transfuse. From the 2010s until now, the focus has been on patient blood management, which includes strategies to mitigate the need for transfusion and refining the precision of the indications for blood transfusions.
Red blood cell (RBC) transfusions given for specific clinical situations can decrease mortality rates. Conversely, severe complications can occur when multiple transfusions are given to a patient. For example, the term lethal triad describes hypothermia, acidosis, and coagulopathy and is an important negative indicator for trauma patient outcome in transfusion medicine. Similarly, the patient who requires high-volume transfusion (>10 units packed red blood cells [PRBCs] in 24 hours) experiences an increased mortality rate: a 10% increase for every 10 units of blood given. Thus if 50 units of blood are given, there is a 50% mortality rate.
Despite the understanding that blood is a scarce resource and comes with possible complications, the practice of transfusing blood is quite commonplace. In 2019 in the United States alone over 10,000,000 units of blood products were transfused. This underscores the importance of transfusion medicine and how the need for blood transfusions has helped this field evolve over the past 60 years.
The American Society of Anesthesiologists (ASA) Task Force on Perioperative Blood Management analyzed the literature and solicited opinions from experts and ASA members that were published as a practice guideline in 2015. These guidelines will be periodically reviewed and updated in the future after review by the ASA Committee on Standards and Practice Parameters.
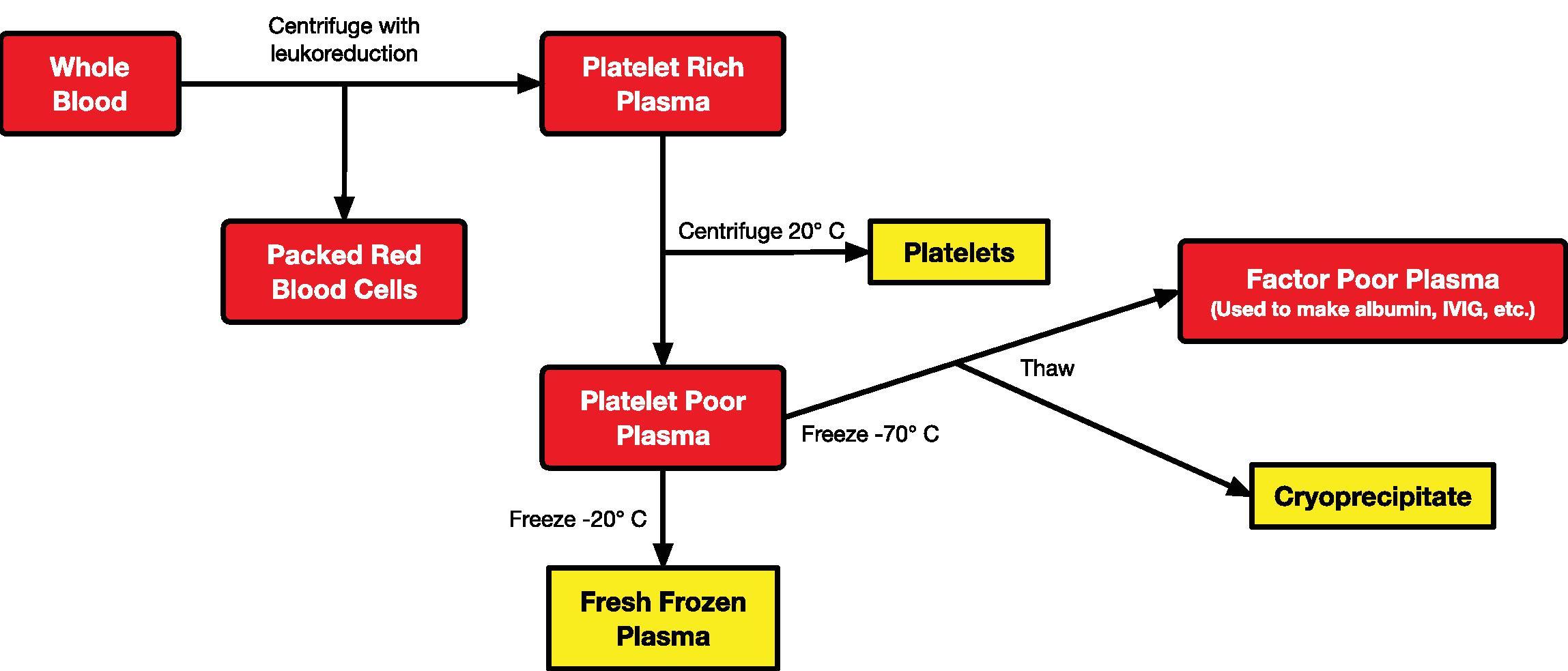
Currently, the majority of the blood supply in the United States is in its component form (PRBCs, fresh frozen plasma [FFP], cryoprecipitate, platelets) because, in this way, it can be used in a more efficacious manner (individualized component therapy). Whole blood, if available, is likely to be autologous in nature. Soon after collection, whole blood is separated into PRBCs and platelet-rich plasma. The platelets are then separated from the plasma, and the plasma is then frozen. Depending on how the plasma is frozen, it can either be thawed for patient use or further processed to cryoprecipitate and, eventually, albumin ( Fig. 25.1 ). All components are stored appropriately until ready for use or discarded if not used within the designated time frame (see later). Of course, this is an oversimplification of the process that is highly regulated and prescribed by the AABB (formerly known as the American Association of Blood Banks).
In addition to separating blood into its components for individualized therapy, the blood bank is responsible for determining donor–recipient compatibility. These procedures will be discussed next.
Determining the blood type (A, B, O, Rh) of the recipient and donor is the first step in selecting blood for transfusion therapy. Routine typing of blood is performed to identify the major surface antigen(s) (A, B, Rh), or their absence, on the membranes of erythrocytes ( Table 25.1 ). Naturally occurring antibodies (anti-A, anti-B) are formed whenever erythrocyte membranes lack surface A or B antigens, or both. These antibodies are capable of causing rapid intravascular destruction of erythrocytes whose membranes express the corresponding antigen(s). Therefore in blood type A erythrocytes express the A antigen and have anti-B antibodies; in blood type B erythrocytes express the B antigen and have anti-A antibodies. For blood type AB, both antigens are expressed, whereas in blood type O, no antigens are expressed. Rh factor can also be expressed on the erythrocyte cell surface. However, unlike the A and B naturally occurring antibodies, Rh antibodies are only produced after exposure and are not produced de novo .
| Blood Group | Antigen on Erythrocyte | Plasma Antibodies |
|---|---|---|
| A | A | Anti-B |
| B | B | Anti-A |
| AB | AB | None |
| O | None | Anti-A, Anti-B |
| Rh | Rh | Anti-Rh; acquired after exposure |
In addition to determining a patient's blood type, an antibody screen is performed using the recipient's serum to identify the presence of antibodies against common minor RBC surface antigens. It is a simple agglutination process in which the recipient's serum is placed in wells with known common RBC surface antigens; the presence of agglutination indicates the likely presence of an antibody. The “type and screen” is typically used when the surgical procedure is unlikely to require transfusion of blood (e.g., many laparoscopic procedures) but is one in which there is still a chance that blood could be needed. Blood typing and screening permit more cost-efficient use of stored blood because the blood is available to more than one patient. The chance of a significant hemolytic reaction related to the use of typed and screened blood is approximately 1 in 70,000 units transfused.
A crossmatch occurs when the donor's erythrocytes are incubated with the recipient's plasma. A crossmatch is always done before the transfusion of blood. This entire process usually takes around 45 to 60 minutes. Rapid agglutination (within 5–10 minutes) occurs if the crossmatch is incompatible secondary to a major blood typing (A, B, O, Rh) mismatch or in the presence of recipient antibodies against MN, P, and/or Lewis. In addition to major incompatibility, the crossmatch checks for minor incompatibility by assessing for the presence of antibodies against minor surface antigens (e.g., Kell, Kidd, Fya, Fyb). This minor agglutination reaction can take as long as 45 to 60 minutes to occur. The term type-specific blood refers to the fact that only the ABO-Rh type has been determined. Presently, crossmatching can be done electronically when specific criteria are met and, mainly, several concordant blood types are in the electronic medical record along with a current negative antibody screen. With electronic crossmatching, PRBC allocation of compatible units can be done in 5 to 10 minutes.
In certain clinical situations (e.g., aortic rupture) blood needs to be released from the blood bank on an emergent basis before a patient's blood type can be determined. Every hospital should be able to release blood rapidly via a massive transfusion and/or emergency release protocol. This usually involves a call to the blood bank stating the need to activate the massive transfusion/emergency release protocol that results in the immediate release of a prespecified number of units of uncrossmatched RBCs (type O-negative), prespecified number of units of FFP (type AB-negative), and prespecified number of platelet units.
In an emergency situation that requires transfusion before full compatibility testing is completed ( Table 25.2 ) the most desirable approach is to transfuse type-specific, partially crossmatched PRBCs. The donor erythrocytes are mixed with recipient plasma and observed for macroscopic agglutination. If the time required to complete this examination (typically <10 minutes) is not acceptable, the second option is to administer type-specific, noncrossmatched PRBCs, if available, or O-negative PRBCs (universal red blood cell donor, as it has no major surface antigens). O-negative whole blood is not selected because it may contain high titers of anti-A and anti-B hemolytic antibodies in the plasma component. For adult patients, with the exception of women of childbearing age, emergency administration of O-positive PRBCs is considered acceptable practice until the patient's blood type is determined. If the patient's blood type becomes known and available after 2 units of type O-negative PRBCs have been transfused, classic teaching was that subsequent transfusions should probably continue with O-negative PRBCs. However, it is not clear if this practice is necessary, and the generally recommended approach is to switch to type-specific blood when it becomes available.
| Component | ABO/Rh | Screen | Crossmatch |
|---|---|---|---|
| WB/PRBCs | Yes | Yes | Yes |
| FFP | Yes | Yes | No |
| Cryo | No | No | No |
| Platelets | No/Yes a | No/Yes a | No/Yes a |
a On rare occasion, platelets can be typed and crossmatched to improve longevity after transfusion (e.g., for patients with thrombocytopenia who are refractory to random-donor platelets).
The general approach to the administration of FFP during an emergency is similar to that of PRBCs. If type-specific plasma is not available, the plasma of AB-negative blood type donors can be given to all individuals, as it does not contain antibodies against the major surface antigens found on RBCs.
As platelets contain no RBCs and very little plasma, the blood type of the donor and recipient are irrelevant in the majority of emergency transfusions.
Of note, despite its relative unavailability, fresh whole blood is extremely effective in restoring normal coagulation after severe injury. The effectiveness of fresh whole blood depends on how long it has been stored and its temperature. In Vietnam in the late 1960s the military used type-specific blood that was maintained at room temperature and stored for no longer than 24 hours. They found it was extremely effective in preventing and treating trauma-induced coagulopathies. Not surprisingly, more recently, the use of fresh whole blood by surgical teams in Afghanistan was associated with improved survival compared with component therapy without platelets.
PRBCs can be stored in a variety of solutions that contain phosphate, dextrose, and/or adenine at temperatures of 1 to 6°C. Accepted storage time (hemolysis <1% with 75% viability of transfused erythrocytes 24 hours after transfusion) is up to 42 days, depending on the donor and storage medium. Adenine increases erythrocyte survival by allowing the cells to resynthesize the adenosine triphosphate needed to fuel metabolic reactions. Changes that occur in RBCs during storage are affected by the length of storage and the type of preservative used. For many years, fresh PRBCs (<5 days of storage) had been recommended for critically ill patients in an effort to improve the delivery of oxygen (2,3-diphosphoglycerate [2,3-DPG] concentrations are higher in fresh blood), though nowadays, it is more determined by what is available in the hospital blood bank. In the past, administration of younger PRBCs (<14 days of storage) was thought to be associated with better outcomes (i.e., decreased mortality rate and fewer postoperative complications), especially with major surgery. However, a 2016 study concluded that the death rate among a general hospital population was not related to the duration of blood storage. This was further validated by a large European study published a year later and by a Cochrane Database systematic review published in 2018. ,
FFP and cryoprecipitate are stored at −20°C; platelets are kept at room temperature (20°C) and are continuously gently agitated to prevent clumping. Because platelets are kept at room temperature, they have a short shelf life of 5 days. On the other hand, although FFP and cryoprecipitate can be kept frozen for a year, once thawed, they have to be used within 24 hours and 4 hours, respectively.
( Table 25.3 )
| Component | Dose | Volume per Unit | Shelf Life | Storage | Response |
|---|---|---|---|---|---|
| PRBCs | 1 unit | 200–250 mL | 21–42 days | 1–6°C | 1 g/dL increase |
| FFP | 10–15 mL/kg | 200–300 mL | Frozen: 1 year Thaw: 24 hours |
Frozen: <−18°C Thaw: 1–10°C |
30% of normal coagulation factors |
| Cryo | 1 unit/5 kg | 15–20 mL | Frozen: 1 year Thaw: 4 hours |
Frozen: <−18°C Thaw: 1–10°C |
100 mg/dL increase in fibrinogen |
| Platelets | 4–6 pooled WB derivative or 1 apheresis unit | 200–250 mL | 5 days | 20–24°C with gentle agitation | 30–60 × 10 9 /L increase |
In the perioperative period PRBCs (200–250 mL volume with a hematocrit of 70%–80%) are used for the treatment of anemia associated with anticipated and/or actual surgical blood loss. The major goal is to increase the oxygen-carrying capacity of blood with a resultant increase in oxygen delivery to tissues and vital organs. Although PRBCs can increase intravascular fluid volume, nonblood products, such as crystalloids and colloids, can also achieve that endpoint. A single unit of PRBCs will increase adult hemoglobin concentrations approximately 1.0 to 1.5 g/dL. Theoretically, the use of hypotonic solutions with PRBCs may cause hemolysis, and the calcium present in lactated Ringer's solution may lead to clotting in the blood filter and/or transfusion line.
The decision to administer PRBCs should be based on measured blood loss and inadequate oxygen-carrying capacity. Acute blood loss in the range of 1500 to 2000 mL (approximately 30% of an adult patient's blood volume) may exceed the ability of crystalloids to replace blood volume without jeopardizing the oxygen-carrying capacity of the blood. Hypotension and tachycardia are likely, but these compensatory responses may be blunted by anesthesia or other drugs (e.g., β-adrenergic blocking drugs). Compensatory vasoconstriction may conceal the signs of acute blood loss until at least 10% of the blood volume is lost, and healthy patients may lose up to 20% of their blood volume before signs of hypovolemia occur. To ensure an adequate oxygen content in blood, PRBCs should be administered when blood loss is sufficiently large. Administration of whole blood, when available, decreases the incidence of hypofibrinogenemia and perhaps coagulopathies associated with the administration of multiple units of PRBCs. In the Vietnam conflict fresh whole blood (typed and crossmatched, but not cooled) was quite effective, especially with massive transfusion-associated coagulopathies. Forty years later in Iraq, military physicians administered fresh whole blood from prescreened “walking donors,” which also can treat or prevent thrombocytopenia. In fact, warm fresh whole blood may be more efficacious than stored component therapy when treating critically ill patients requiring massive blood transfusions. Also, whole blood may be preferable to PRBCs when replacing blood losses that exceed 30% of the blood volume. Practically speaking, specific ratios of PRBC transfusions with FFP and platelets have been used in place of whole blood. For example, a ratio of 1.5 units PRBC with 1 unit of FFP (i.e., for every 1.5 units of PRBCs administered, 1 unit of FFP should be given). In addition, 1 unit of apheresis platelets (or 6 pooled random donor units) for 6 units of RBCs has been recommended in patients with large blood losses and trauma. Interestingly, despite its wide acceptance in massive resuscitation in trauma patients, these ratios have not been conclusively proven to improve mortality. ,
With acute blood loss, interstitial fluid and extravascular protein are transferred to the intravascular space, which tends to maintain plasma volume. For this reason, when crystalloid solutions are used to replace blood loss, they should be given in amounts equal to about three times the amount of blood loss, not only to replenish intravascular fluid volume but also to replenish the fluid lost from interstitial spaces. Albumin is a solution that is useful for acute expansion of the intravascular fluid volume. In contrast to crystalloid solutions, albumin is more likely to remain in the intravascular space for a longer period (about 12 hours). These solutions avoid complications associated with blood-containing products but do not improve the oxygen-carrying capacity of the blood and in large volumes (>20 mL/kg) may cause coagulation defects from ongoing dilution (also see Chapter 24 ).
FFP is the fluid portion obtained from a single unit of whole blood that is frozen within 6 hours of collection. All coagulation factors, except platelets, are present in FFP, which explains the use of this component for the treatment of hemorrhage from presumed coagulation factor deficiencies. FFP transfusions during surgery are probably not necessary unless the prothrombin time/international normalized ratio (PT/INR) or activated partial thromboplastin time (aPTT), or both, are at least 1.5 times longer than normal. More recently, FFP has been given in specific ratios with PRBCs in trauma patients regardless of laboratory values. Other indications for FFP are an urgent reversal of warfarin and management of heparin resistance (i.e., FFP contains antithrombin III).
Cryoprecipitate is the fraction of plasma that precipitates when FFP is thawed from −70°C. It contains factor VIII, fibrinogen, fibronectin, von Willebrand factor, and factor XIII. This component is useful for treating hemophilia A (it contains high concentrations of factor VIII in a small volume) or von Willebrand factor deficiency that is unresponsive to desmopressin. Cryoprecipitate can also be used to treat hypofibrinogenemia because it contains more fibrinogen than FFP.
Administration of platelets allows specific treatment of thrombocytopenia without the infusion of unnecessary blood components. Platelets are derived from volunteer donors and can be pooled from platelet concentrates derived from whole blood donation (random-donor platelets) or from a single donor (apheresis platelets). During surgery, platelet transfusions are probably not required unless the platelet count is less than 50,000 to 100,000 cells/mm 3 (usually dictated by the type and location of surgery) as determined by laboratory analysis or in predetermined ratios with PRBC and FFP in trauma patients, as described previously.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here