Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Specific knowledge of relevant blood group systems is essential for a thorough understanding of blood bank pretransfusion testing methodology and the potential practical applications of that information to clinical practice. In any given patient care setting, the blood bank and transfusion medicine service are tasked with integrating this information to improve the safety of blood transfusion and related activities.
The first portion of this chapter (Blood Group Systems) introduces the commonly assessed carbohydrate and protein blood group systems relevant to clinical and transfusion service practices, including ABO, Lewis, Ii, P1PK, Rh, MNS, Kell, Duffy, and Kidd blood group systems. Salient laboratory details and/or clinical associations are also provided. The second portion of this chapter (Pretransfusion Testing) introduces basic tenets and methods of blood bank laboratory serologic testing and describes the usual stepwise approach for routine pretransfusion testing, including ABO and Rh type, antibody screen and identification, and crossmatch. Direct antiglobulin testing is also briefly discussed, as are situations involving emergency release of blood products.
The earliest published reports documenting the physical properties of red blood cells (RBCs) and those of coincident agglutinating substances in plasma paved the way for the subsequent discoveries of what are now described as blood group antigens. At the time of this writing, more than 36 distinct blood group systems and collections have been codified based on biochemistry and genetics, most with multiple sub-categorized distinct antigens. While blood banks and transfusion medicine services regularly encounter only a handful of RBC antigens and/or their corresponding antibodies, discoveries continuously expand the field and reorganize an already complex body of knowledge.
Although there is certainly more biochemical nuance, the commonly encountered blood group system antigens relevant to clinical practice can be broadly categorized as predominantly carbohydrate or protein in structure, and are organized as such in this chapter. From there, specific knowledge of approximate incidence ( Table 90.1 ), biosynthetic pathways, genetics, serologic properties, and clinical associations help to contextualize blood bank testing and inform the transfusion medicine service of the most appropriate approaches to a given clinical situation.
| Antigen | Incidence | Antigen | Incidence |
|---|---|---|---|
| D | 85% | Fy a | 66% |
| C | 68% | Fy b | 83% |
| E | 29% | M | 78% |
| c | 80% | N | 72% |
| e | 98% | S | 55% |
| K | 9% | s | 89% |
| k | 99.8% | U | 99.9% |
| Jk a | 77% | Le a | 22% |
| Jk b | 74% | Le b | 72% |
The ABO blood group system was initially discovered in 1900 by Landsteiner and remains the most important blood group system for blood transfusion and transplantation. , ABO antigens are widely expressed on cellular blood components and other human tissues. Consequently, the necessary ABO compatibility between donor and recipient dictates the provision of blood products and organ transplants. For blood transfusion, in particular, ABO compatibility remains the most critical serologic compatibility to assure and constitutes a significant proportion of routine blood bank testing.
The two main ABO antigens are the A and B antigens. The structural basis and biosynthetic precursor of both A and B antigens is the H antigen, an abundant structure on all RBC surfaces that is characterized by a terminal fucose found on certain glycoproteins and glycolipids. H antigen is also expressed in secretory epithelial cells and is present in bodily secretions, although this H antigen is structurally distinct from those found on RBCs due to a difference in precursor molecules (see Lewis section below). Whether the H antigen is present on cell surfaces or in secretions, further modification of the subterminal galactose with the covalent addition of a carbohydrate moiety determines its designation as either an A antigen (addition of N -acetyl-galactosamine [GalNAc]) or a B antigen (addition of galactose) ( Fig. 90.1 ).
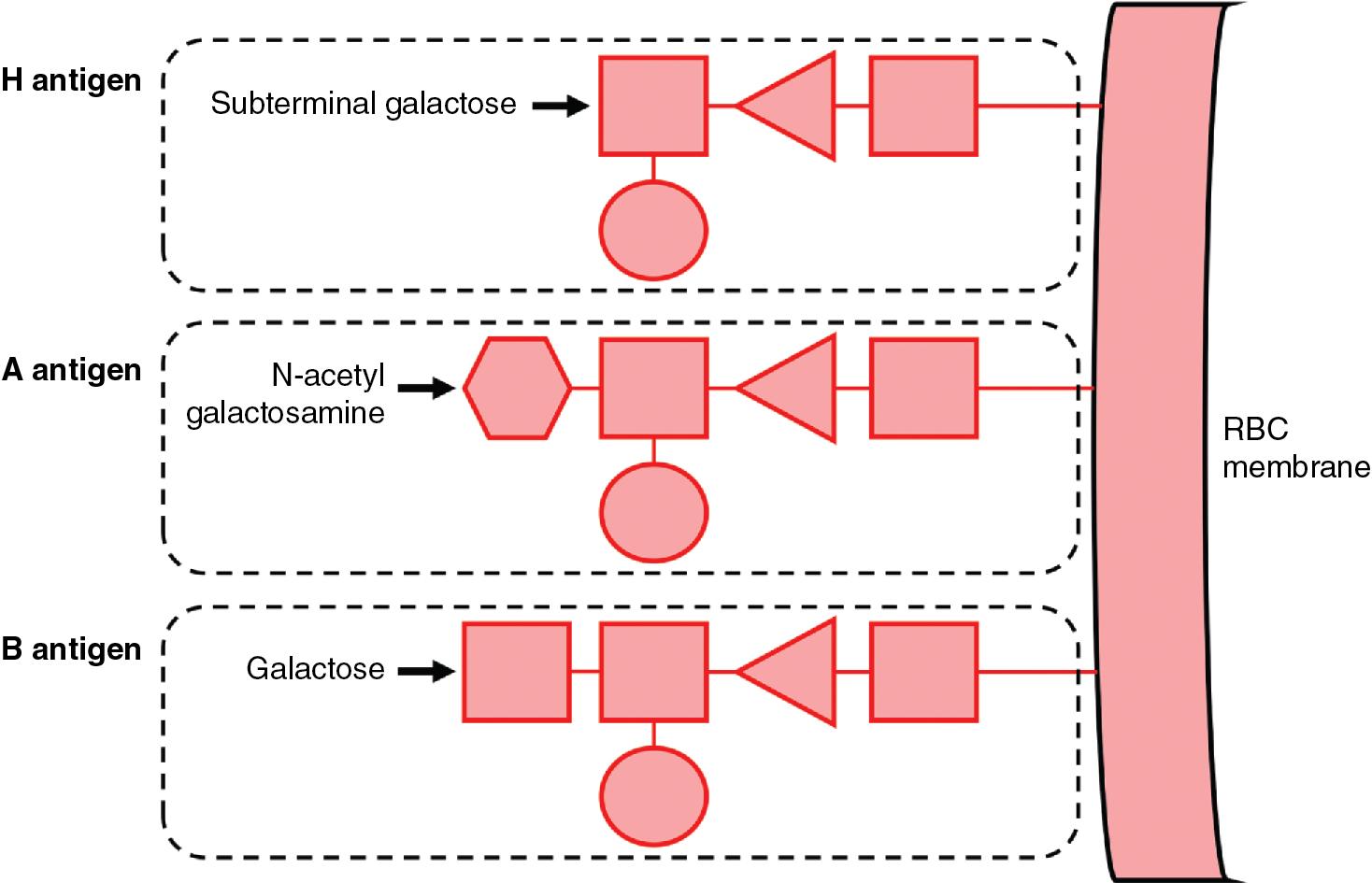
The ABO gene locus resides on chromosome 9q34 and encodes a glycosyltransferase enzyme that modifies the H antigen. The A and B alleles of this gene locus encode enzymes responsible for adding GalNAc or galactose, respectively, to form A or B antigens. These antigens are expressed co-dominantly on RBC surfaces, and the presence or absence of A and B antigens determines an individual’s ABO phenotype: blood groups A, B, AB, or O ( Table 90.2 ). Group AB individuals express both A and B antigens on RBCs, whereas group O individuals express neither. The O allele encodes a nonfunctional ABO gene product that lacks the enzymatic activity needed to modify the H antigen. Consequently, group O RBCs express unmodified H antigens exclusively, although groups A, B, and AB RBCs will still express some amount of these unmodified H antigens.
| ABO Group | Incidence a | Possible Alleles | Antigens | Isohemagglutinin Antibodies | Antibody Isotypes |
|---|---|---|---|---|---|
| O | 45% | O/O | H | Anti-A Anti-B Anti-A,B |
Mostly IgG (less IgM) |
| A | 40% | A/A A/O |
H A |
Anti-B | Mostly IgM |
| B | 11% | B/B B/O |
H B |
Anti-A | Mostly IgM |
| AB | 4% | A/B | H A B |
None | N/A |
a Approximate incidence percentage based on Caucasian blood donors.
In addition to ABO antigens present on RBCs, the ABO phenotype is characterized by the presence or absence of naturally occurring anti-A and anti-B antibodies, also called isohemagglutinins. An individual will naturally form antibodies directed against the ABO antigens they lack, which are thought to develop following exposure to ubiquitous bacterial or environmental antigens. These antibodies develop in otherwise healthy infants at approximately six months of age, concurrent with other humoral immunity, and are typically of both IgM and IgG isotypes. In addition to anti-A and anti-B antibodies, group O individuals may also develop a distinct anti-A,B antibody with specificity against both A and B antigens and is largely of IgG isotype. In general, isohemagglutinins are reactive at both room and physiologic temperatures and can efficiently initiate terminal complement fixation on RBCs, leading to acute intravascular hemolysis. These isohemagglutinins are designated as “clinically significant” antibodies due to their ability to induce hemolytic transfusion reactions or hemolytic disease of the fetus and newborn (HDFN). Therefore when selecting transfusion support products for a patient with isohemagglutinins, compatible RBC products that lack the corresponding ABO antigens should be provided ( Table 90.3 ).
| Recipient ABO Group | Compatible RBCs | Compatible Plasma |
|---|---|---|
| O | O | O A B AB |
| A | O A |
A AB |
| B | O B |
B AB |
| AB | O A B AB |
AB |
Subgroups exist for both A and B antigens that have quantitative and qualitative implications for their expression on RBCs and in secretions. For instance, the most prevalent group A subgroups are A 1 (approximately 80% of all group A individuals, considered to be the “conventional” A antigen) and A 2 (approximately 20%). The A 2 subgroup, commonly encountered in clinical practice, results from slower enzymatic modification of H antigens into A antigens and has subtle differences in resultant branching structures. This decreased enzymatic activity leads to a phenotype with fewer A antigens expressed per RBC surface. Interestingly, A 2 phenotype individuals may develop an IgM anti-A 1 alloantibody reactive with A 1 phenotype RBCs but not their own. This antibody may cause reactive crossmatches with A 1 RBC products but is generally considered clinically insignificant and unlikely to cause hemolysis. Up to 8% of group A 2 and 35% of group A 2 B individuals may form an anti-A 1 alloantibody.
The rare Bombay phenotype, initially described in Mumbai, India (formerly called Bombay), is a null phenotype of the H antigen. Homozygosity for nonfunctional alleles of the fucosyltransferase-1 ( FUT1 , H) and FUT2 (Secretor) genes lead to a complete absence of H antigens, and consequently A or B antigens, on RBC surfaces and in secretions. As a result, Bombay phenotype individuals naturally develop clinically significant anti-A, anti-B, and anti-H isohemagglutinins. In contrast, rare para-Bombay phenotype individuals carry at least one functional FUT2 allele while being homozygous for null FUT1 alleles. The para-Bombay phenotype leads to detectable H, A, or B antigen expression in secretions, and a lesser extent on RBC surfaces due solely to passive adsorption of secretory antigens rather than actual expression. Similar to Bombay individuals, para-Bombay individuals also naturally develop anti-H isohemagglutinins, albeit of lesser hemolytic potential, as well as anti-A or anti-B antibodies depending on their ABO gene expression.
Mourant described the Lewis blood group system in 1946 through the discovery of naturally occurring anti-Le a (Lewis A) antibodies. The two main Lewis antigens are the Le a and Le b (Lewis B) antigens. In contrast to most other clinically relevant RBC antigens, Lewis antigen-bearing glycolipids are not synthesized by RBCs, nor are their structures directly incorporated into RBC membranes. Rather, these glycolipids are free-floating in plasma and become “expressed” on RBCs once they passively adsorb onto RBC surfaces.
Lewis antigen biosynthesis in plasma occurs from interactions of a soluble precursor molecule with two fucosyltransferase enzymes: Secretor ( FUT2 gene) and Lewis ( FUT3 gene) ( Fig. 90.2 ). , , The direct action of the Lewis enzyme on the precursor results in the formation of the Le a antigen. Alternatively, the Secretor enzyme may act directly on the precursor to form the soluble form of the H antigen, which the Lewis enzyme can then act upon to form Le b antigen. Note that both the Secretor and Lewis enzymes are necessary to form Le b antigen, while only the Lewis enzyme is necessary to form Le a .
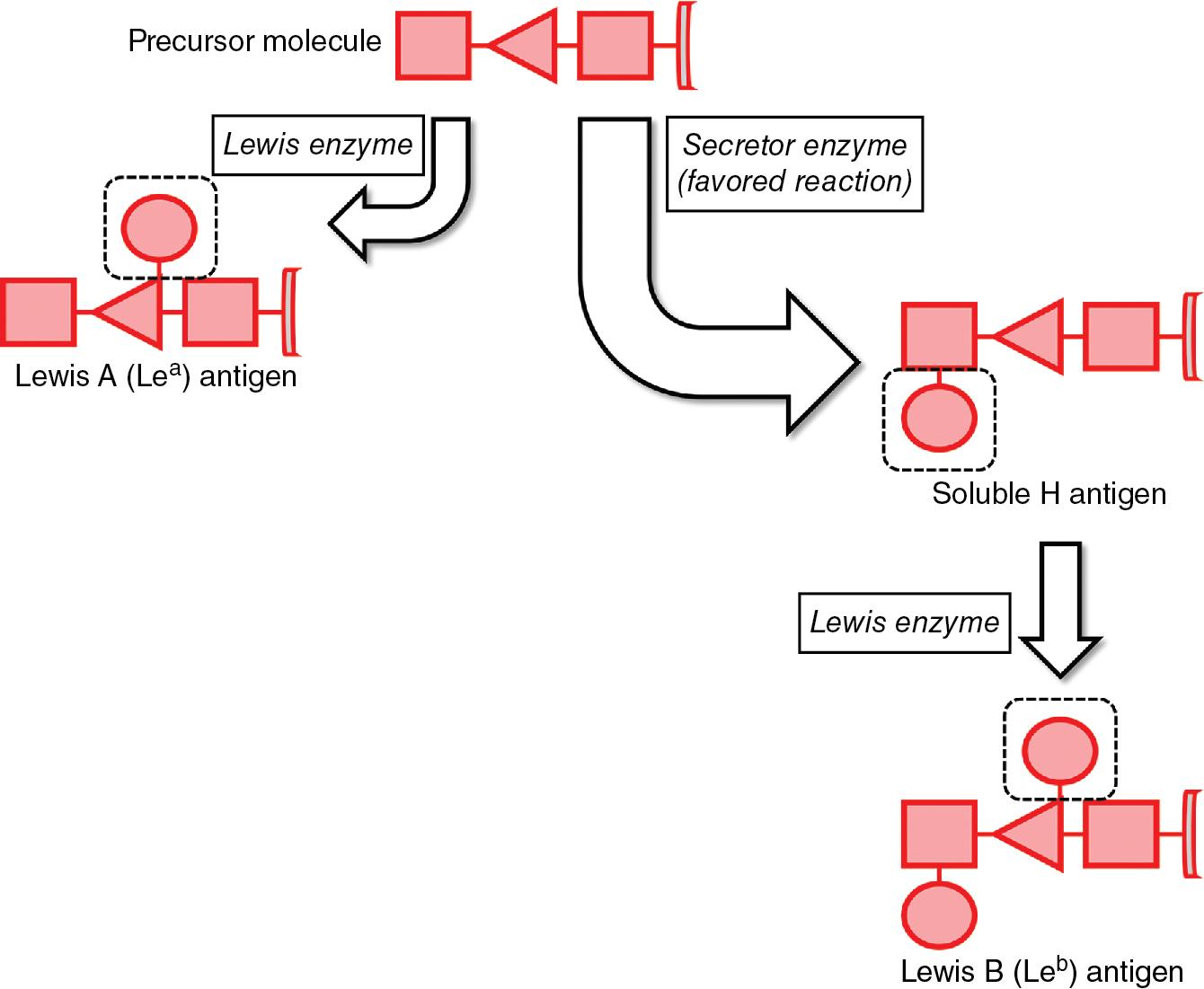
In individuals who express both the Secretor and Lewis enzymes, the Secretor enzymatic action is favored such that the majority of precursors are converted to soluble H antigens rather than Le a antigens. , , Consequently, there is preferential biosynthesis of Le b antigens. This quantity disparity may give the impression of RBCs that are Le(a−b+) on serologic testing when, in reality, Le a antigen is present but expressed to a lesser amount than Le b antigen.
Other Lewis phenotypes are consistent with the presence or absence of Secretor or Lewis enzyme expression ( Table 90.4 ). The rare Le(a+b+) phenotype may be transiently seen in neonates and infants due to relatively weak Secretor enzyme activity which strengthens with age, as well as in individuals with inherited weak Secretor enzyme activity. Additionally, the Le(a−b−) phenotype may also be transiently observed during pregnancy due to the dilution of circulating Lewis antigens in an expanded plasma volume and their adsorption onto certain lipoproteins that increase during pregnancy. ,
Both anti-Le a and anti-Le b antibodies are, in general, naturally occurring IgM isotype antibodies that are reactive at non-physiologic room temperature. They are generally considered “clinically insignificant” (not implicated in hemolytic transfusion reactions or HDFN), although rare reports exist of hemolysis attributed to anti-Le a antibodies. Of note, both anti-Le a and anti-Le b antibodies may be detected alongside the transient Le(a−b−) phenotype observed during pregnancy. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here