Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Polyomavirus (PyV) infections were first described in mice in 1952 as a cause of tumors in newborn animals. Since then, PyVs have been found in virtually all vertebrates, including primates, monkeys, cows, rabbits, birds, and fish. BK polyomavirus (BKPyV) was isolated in 1971 from a kidney transplant patient shedding decoy cells in the urine. The isolation of JC polyomavirus (JCPyV) was also reported in 1971 from postmortem tissues of patients with progressive multifocal leukoencephalopathy (PML), but PyV particles had been noted by electron microscopy in PML tissue sections as early as 1965. Since then, more than 10 human polyomaviruses (HPyVs) have been characterized molecularly, and there is serologic evidence that healthy adults are concurrently infected with at least six to seven different HPyVs. Clinical and histopathologic evidence of disease is currently available for six HPyVs ( Table 23.1 ), which almost exclusively affect immunocompromised patients. , This chapter reviews the recent literature on HPyVs in immunocompromised children undergoing cancer/chemotherapy, solid organ transplantation (SOT), or hematopoietic cell transplantation (HCT).
| Common Name | Genus | Taxonomic Name | Organ of Latency | Human Disease |
|---|---|---|---|---|
| BK polyomavirus | Betapolyomavirus | Human polyomavirus 1 | Kidney and urinary tract | Nephropathy, hemorrhagic cystitis |
| JC polyomavirus | Betapolyomavirus | Human polyomavirus 2 | Kidney, brain, blood cells | Progressive multifocal leukoencephalopathy |
| Merkel cell polyomavirus | Alphapolyomavirus | Human polyomavirus 5 | Skin | Merkel cell carcinoma |
| Trichodysplasia spinulosa | Alphapolyomavirus | Human polyomavirus 8 | Skin | Trichodysplasia spinulosa |
| New Jersey polyomavirus | Alphapolyomavirus | Human polyomavirus 13 | Unknown | 1 pancreatic transplant patient with muscle, skin, and eye disease |
| Human polyomavirus 7 | Deltapolyomavirus | Human polyomavirus 7 | Skin | Pruritic hyperproliferative keratinopathy in lung transplant patients |
PyVs share a common morphology of non-enveloped icosahedral virion particles of 40 nm to 45 nm that contain a double-stranded DNA genome of approximately 5000 base pairs wrapped around histones. PyV genomes can be divided into three regions called the non-coding control region ( NCCR ), early viral gene region ( EVGR ), and late viral gene region ( LVGR ). After virion uptake and delivery of the viral genome to the nucleus, the NCCR coordinates, together with host cell factors, the start of the PyV replication cycle by initiating expression of the EVGR-encoded regulatory large T-antigen (LTag) and small-antigen (sTag). LTag and sTag inactivate tumor suppressor proteins pRB and p53 and shift cells into G 1 /S phase to provide necessary building blocks and recruit the host cell DNA polymerase complex for efficient bidirectional replication of the viral DNA genome from the NCCR ori. This is followed by NCCR -driven expression of the LVGR -encoded capsid proteins Vp1, Vp2, Vp3, and the regulatory agnoprotein. The LVGR also encodes viral micro-ribonucleic acids (miRNAs). Vp1 forms the outer shell of the virions consisting of 72 pentamers and assembles spontaneously in virus-like particles used for serologic studies. Vp2 and Vp3 are minor capsid proteins inside the particles adjacent to the viral DNA genome. Enlarged nuclei with prominent intranuclear inclusions consisting of densely packed PyV particles are the hallmark of PyV replication in the late phase of the viral life cycle. Immunohistochemistry for LTag using cross-reactive monoclonal antibody raised originally against the monkey SV40 LTag protein is commonly used for proven BKPyV or JCPyV pathology erroneously called “SV40 positive,” but detection of Vp1 or in situ hybridization has also been used by some centers. Although the principal virology of PyVs is conserved, there are significant differences which permit concurrent infections and mediate differences in host cell tropism, virus biology, and pathology. Thus the outer part of the Vp1 virions is more diverse, and is responsible for primary host cell tropism as judged from selective binding to gangliosides carrying differently branched sugar residues. These Vp1 domains are also the target of neutralizing antibodies. Conversely, the inner parts seem more conserved and mediate interactions between Vp1 pentamers or to Vp2. Binding of immunoglobulin G (IgG) are more frequently cross-reactive between different HPyVs and have less frequently neutralizing activity. The NCCRs of HPyVs differ in length, type, and number of transcription factor binding sites, and are critical for the secondary host cell tropism, which is realized inside the host cell nucleus by the exact timing of EVGR and LVGR expression. PyV micro-RNAs 5p and 3p target the EVGR transcripts and downregulate natural killer cell targets on the cell surface, thereby facilitating immune escape and latency. Taken together, HPyV biology subverts and hijacks the host cell metabolism without offering classic antiviral targets of high selectivity.
The exact mode of natural BKPyV and JCPyV transmission is undefined but most likely involves contact with mucosal surfaces in the oral/pharyngeal, gastrointestinal, or respiratory tract. Seroprevalence studies indicate that primary infection with BKPyV occurs in toddlers, reaching IgG positivity rates of greater than 90% between the ages of 2 and 4 years. There are no known symptoms associated with primary BKPyV infection and a nonspecific, constitutional illness cannot be excluded. Primary JCPyV infection appears to occur significantly later as only 35% of adolescents have JCPyV-specific IgG antibodies compared with 60% of blood donors. In a study of 18 patients undergoing thymectomy in children owing to congenital heart surgery, the IgG seroprevalence of BKPyV and JCPyV was 70% and 25%, respectively. However, some differences in seroprevalence rates across different studies reflect patient age and waning antibody responses over time, but particular attention should be paid to the different antigens used (e.g., Vp1 monomers or fusion proteins, Vp1-pentamers or Vp1-virus-like particles).
HPyVs have been detected in the urine of 30% of healthy children and adults as well as 40% of stool samples from hospitalized children. Detection of BKPyV and JCPyV in human sewage systems supports the possibility of secondary indirect environmental exposures other than the direct transmission route (e.g., from child to child). Indeed, PyV particles are fairly resistant to environmental inactivation and can withstand heating to 60°C for 30 minutes and many disinfectants. Other routes of transmission are less well defined and even controversial (e.g., via transfusion, transplacental, seminal fluids, or organ transplantation), with the notable exception of kidney transplantation, where transmission has been shown to occur from donor to recipient.
After primary infection, BKPyV and JCPyV reach the renourinary tract presumably by DNAemia, where they preferentially infect the renal tubular and bladder epithelial cells in the case of BKPyV and the urothelial cells of the renal pelvis and the bladder in the case of JCPyV. , Moreover, detection of JCPyV DNA has been reported in tonsils, bone marrow, and the central nervous system. Taken together, large parts of the general human population have been infected with HPyVs, including BKPyV and JCPyV, but neither primary infection, persistence, nor shedding has been linked to significant pathology or disease in immunocompetent persons. Conversely, as outlined in Table 23.2 , immunocompromise appears to be a conditio sine qua non for HPyV disease, but the differences in incidence rates in the respective clinical settings strongly suggest that specific risk signatures beyond mere immunodeficiency appear to play a role. Thus BKPyV-associated nephropathy is most frequently diagnosed in adult and pediatric kidney transplant recipients at rates of 1% to 15%, but only rarely in non-kidney SOT or allogeneic HCT, despite similar or higher intensity of immunosuppression as evidenced by other opportunistic infectious diseases caused by Pneumocystis jirovecii or cytomegalovirus (CMV) replication. Conversely, hemorrhagic cystitis is most frequently seen after allogeneic HCT, but only rarely after kidney transplantation, wherein local toxic damage of the bladder urothelia from conditioning and impaired immune control are followed by increased inflammatory responses postengraftment. JCPyV-mediated PML has reached the highest rates in human immunodeficiency virus/AIDS before the availability of combination antiretroviral therapy or in refractory relapsing multiple sclerosis treated with natalizumab, but less data are available for treatment with dimethyl fumarate or fingolimod, or in SOT or HCT. Accordingly, risk-adapted consultation, screening, and intervention are currently recommended for these respective patients.
| Pediatric Population | Clinical Manifestation (Reported Pediatric Rates) |
|---|---|
| BKPyV | |
| Kidney transplant | Nephropathy (5%-15%) Hemorrhagic cystitis (rare) |
| Hematopoietic cell transplant | Hemorrhagic cystitis (8%-25%) Nephropathy (rare) |
| Liver Transplant | Nephropathy (rare) |
| Heart transplant | Nephropathy (rare) |
| Lung transplant | Hemorrhagic cystitis (rare) |
| Malignancy | Hemorrhagic cystitis (rare) |
| JCPyV | |
| HIV/AIDS, refractory multiple sclerosis | Progressive multifocal leukoencephalopathy |
| Kidney transplant | Nephropathy (rare) Progressive multifocal leukoencephalopathy (very rare) |
| Malignancy | Progressive multifocal leukoencephalopathy |
| Less common clinical manifestations: gastrointestinal, pulmonary, ophthalmologic, hepatic, neurologic, cancer | |
a Nephropathy and hemorrhagic cystitis associated with BKPyV are the predominant clinical manifestations affecting kidney transplant and hematopoietic cell transplant recipients, respectively. Progressive multifocal leukoencephalopathy associated with JCPyV is the predominant clinical manifestation affecting patients with human immunodeficiency virus/AIDS and refractory multiple sclerosis.
The key steps of BKPyV reactivation to nephropathy have been described in detail, but they were mostly derived from adult patients after kidney transplantation. These include the following:
Low-level viruria in approximately 5% to 10% of patients with residual urine production before kidney transplantation
High-level replication with urine BKPyV loads greater than 10 million copies/mL and decoy cell shedding in 20% to 50% of patients after transplantation
Detection of BKPyV DNA in plasma in 10% to 40% of patients after transplantation
Histologically proven BKPyV-associated nephropathy with little inflammation and baseline allograft function (PyVAN-A)
Increasing allograft damage due to BKPyV replication and inflammation decreasing kidney allograft function (PyVAN-B1, -B2, -B3); and
Irreversible fibrosis and tubular atrophy causing decline in allograft function (PyVAN-C).
Accordingly, screening for high-level viruria and/or DNAemia using nucleic acid testing (NAT) is recommended to identify patients with persistent DNAemia who would benefit from preemptive reduction in immunosuppression.
Most of the literature describing BKPyV infection replication and disease in children has been published in the past decade. In a prospective clinical and laboratory study from 2002 to 2005 in Italy, Ginevri and colleagues followed up with 62 children who received basiliximab and standard maintenance triple therapy with a calcineurin inhibitor, mycophenolate mofetil, and corticosteroids. Only 3 of the 62 patients received induction that included antithymocyte globulin. Blood and urine samples were collected at months 1, 3, 6, 9, 12, 18, 24, 36, and 48 after transplant for NAT testing (458 samples, average of 7 samples per patient after transplant). The cumulative risk of viruria was 64% (95% confidence interval 53% to 78%) and that of DNAemia was 22% (95% confidence interval 13% to 35%), and was first detected at a median of 3 months (range 1 to 24 months for viruria and 1 to 18 months for DNAemia) after transplant. Using a protocol to reduce immunosuppression, no cases of BKPyV-associated nephropathy occurred in this series.
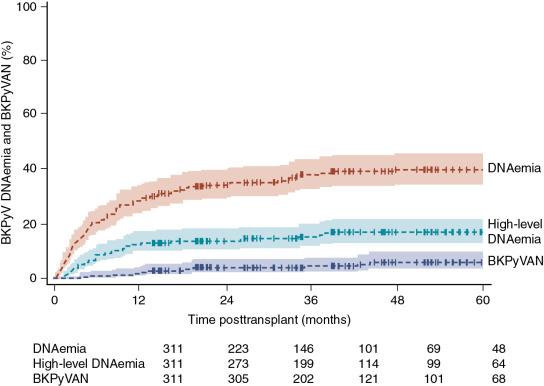
Risk factors for BKPyV replication after kidney transplant have included the depth and type of immunosuppression, including the use of antithymocyte globulin for induction or rejection treatment and the level of exposure to tacrolimus and mycophenolate mofetil. Other reported risk factors include human leukocyte antigen (HLA) mismatches, deceased donation, older age, male gender, donor antibody high/recipient antibody low, and ureteral stents. In their analysis of 313 children receiving a kidney transplant in Europe, Höcker and colleagues reported the cumulative incidence of DNAemia and biopsy-proven nephropathy after kidney transplant ( Fig. 23.1 ). In a multivariate model, they found that a higher degree of immunosuppression (odds ratio [OR] 1.3, P < .01), tacrolimus use (vs. cyclosporine) (OR 3.6 P < .01), younger recipient age (OR 1.1 per year, P < .001), and obstructive uropathy (OR 12.4, P < .01) were associated with higher risk of BKPyV infection.
The serostatus of the donor and recipient may also contribute to posttransplant BKPyV risk. Ali and colleagues conducted a retrospective analysis of pediatric kidney transplant patients at their center in Canada from 1986 to 2007. All patients had follow-up for at least 1 year after transplant. Using an enzyme-linked immunosorbent assay to detect IgG against the BKPyV Vp1 virus-like particle, the authors tested stored blood samples from donors and recipients. An antibody titer of 1:2560 or less was categorized as a low titer and a titer of 1:10,240 or more was defined as a high titer. Of the 94 included transplant recipients, 34% had low anti-BKPyV IgG serostatus and 66% had high serostatus before transplant. Consistent with published reports for healthy children, pretransplant antibody titers were higher with increasing age, especially after the age of 6 years. Blood was also available to test 40 donors, 73% of whom had antibody titers in the high anti-BKPyV IgG serostatus group. Recipients with a low serostatus had a significantly higher risk of BKPyV DNAemia in the first year after transplant. The highest risk of BKPyV DNAemia was found in children with high donor serostatus and low recipient serostatus. Although BKPyV-specific IgG mediate part of the antiviral immune response, a higher antibody status may be a surrogate of recent exposure or reactivation, and hence reflect a higher tissue BKPyV load in the allograft that cannot be countered by sufficient immune effector functions in the recipient with low BKPyV-specific immunity evidenced by low antibody titers. Although the numbers were small, BKPyV DNAemia occurred in 4 of 7 (57%) transplants from a high donor serostatus to a low serostatus recipient, in none of the 3 transplants in which both the donor and the recipient had low serostatus, and in only 1 of 26 (4%) transplants in which the recipient had high serostatus before transplant. Unlike CMV and Epstein-Barr virus (EBV), it is not standard to test for donor and recipient antibodies against BKPyV before kidney transplant in children or adults. This may change, however, as more research becomes available on the functional and surrogate role of antibody titers and specificities in donor-recipient pairs, and may help to better determine the posttransplant risk of uncontrolled BKPyV replication and disease.
This notion may be extended to differences in BKPyV subtype antibody titers. BKPyV can be divided into four major subtypes (I, II, III, and IV) and each subtype can be further divided in subgroups yielding a total of 12 different categories of BKPyV strains. BKPyV subtype I is found in patients worldwide, whereas subtype IV is found in patients mostly from East Asia and Europe. Subtypes II and III are rarely reported. Subtypes II, III, and IV and subgroups Ib1 and Ib2 are also known to be distinct serotypes. Thus a subtype mismatch arising from transplanting of a donor graft harboring BKPyV subtype IV into a recipient with antibody titers to Ib may be followed by preferential replication of the donor BKPyV subtype. Momynaliev and colleagues examined BKPyV subtypes among 6 pediatric kidney transplant recipients and 10 adult controls at their center in Russia from 2008 to 2009. They found that 66% of the identified BKPyV isolates were Ib2 and 24% were IVc2. More research is needed to determine the role of BKPyV genotypes and serotypes in children with respect to posttransplant high-level viruria, DNAemia, and disease, and the risk-benefit of BKPyV mitigation and graft survival through informed screening and organ allocation.
Few studies have examined the risk of BKPyV replication in children after nonrenal SOT with most of the literature limited to cross-sectional analyses of patients who were several years after transplant, when the risk of BKPyV replication events is presumably lower. In a study of 59 pediatric liver transplant recipients in Israel by Amir and colleagues, blood and urine samples were tested at a single time point at least 1 month after transplant and retested if they were positive. At a median of 5 years after liver transplant, 9 of 59 (15.3%) had viruria, and 1 of 59 patients (1.7%) had low-level DNAemia. In all 9 of the patients with viruria, BKPyV replication was transient and there was no longer evidence of DNAemia or viruria by their next clinic visit 5 months later. In Germany, Brinkert and colleagues tested 100 pediatric liver transplant recipients for BKPyV and JCPyV in urine, and plasma was tested only if the initial urine result was positive above 100,000 copies/mL. Of the 100 included patients in this cross-sectional analysis, 15 (15%) had isolated BKPyV viruria at a median of 6 years after transplant, but no DNAemia was identified.
In contrast to liver transplantation, recipients of thoracic transplants (heart and lung) typically receive higher doses of immunosuppression to prevent rejection, placing them at greater risk for infectious disease events. Ducharme-Smith and colleagues conducted a cross-sectional analysis of pediatric heart transplant recipients in the United States by collecting urine samples at regular clinic appointments. Since 2006, urine testing was performed only in patients with a history of chronic kidney disease and all patients were screened starting in 2012. Blood testing for BKPyV DNA was performed only if urine testing was positive. Of the 83 patients screened for viruria at a median of 3.3 years after transplant, 28 (34%) had viruria. Of these 28 patients, 7 had test results for DNAemia (representing 8% DNAemia among the total study population). In multivariate analysis, patients in whom BKPyV viruria developed were significantly more likely to have evidence of EBV detection in the blood. In a follow-up study, the authors prospectively collected urine and blood samples from 10 consecutive pediatric heart transplant recipients from 2013 to 2015. Samples were collected before transplant and at 1 week and months 3, 6, 9, 12, and 15 after transplant. Quantitative blood and urine NAT was only performed if the result of the initial qualitative urine NAT was positive. The 10 subjects had follow up for 15 months after transplant, during which time viruria developed in 2 of 10 and 1 of 10 (10%) had DNAemia.
Several reviews have summarized the literature describing cases in adults and children with BKPyV replication after heart or lung transplant. , In adults after heart transplant, studies have reported a viruria risk of 19% and a DNAemia risk of 5%. After lung transplant, 33% of recipients have been found to have viruria, with less data available on the risk of DNAemia. No studies have systematically examined the risk of BKPyV replication after pediatric lung transplant or in children with cancer. As summarized in later text, there are mainly case reports of BKPyV nephropathy in children after nonrenal SOT, especially in those receiving heart or lung transplants. However, the exact risk of nephropathy in the native kidneys of children with SOT remains unknown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here