Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pilosebaceous unit and nail apparatus are epidermal derivatives that produce two of the hardest epithelial structures in mammalian biology
The hair follicle is the only continuously cycling organ in mammals, while the nail exhibits uninterrupted growth
Hair and nails serve multiple functions, including protection, sensation, and social communication
Hair shafts and nail plates largely consist of dead, terminally differentiated keratinocytes (trichocytes and onychocytes, respectively) whose durability is based on the presence of abundant keratin intermediate filaments
The hair cycle includes phases of growth (anagen), regression (catagen), resting (telogen), and hair shaft shedding (exogen/teloptosis)
The hair follicle is a site of immune privilege and a major reservoir for epithelial and melanocyte stem cells; immune privilege and stem cells within the nail unit are also becoming recognized
The nail apparatus consists of the fully cornified nail plate, which is produced by the nail matrix, and other specialized epithelial tissues such as the nail bed and nail folds
Skin adnexa in mammals develop through evolutionarily conserved mechanisms. Similar processes lead to modifications of epithelial cells and proteins in order to form functional structures such as hairs, feathers, nails, glands, and teeth. In all cases, adnexa result from interactions between adjacent tissue layers, the ectoderm (epidermis) and underlying mesoderm (dermis) ( Fig. 68.1 ). For example, the hair follicle (HF) first arises as a small epidermal bud and then, via reciprocal signaling involving the underlying dermis, progresses to a complex, multi-cylindrical organ with associated sebaceous and apocrine glands (see Fig. 2.4 ). The developmental relationships among different adnexal structures is evidenced by the hair, nail, sweat gland, and tooth defects observed in ectodermal dysplasias (see Ch. 63 ).
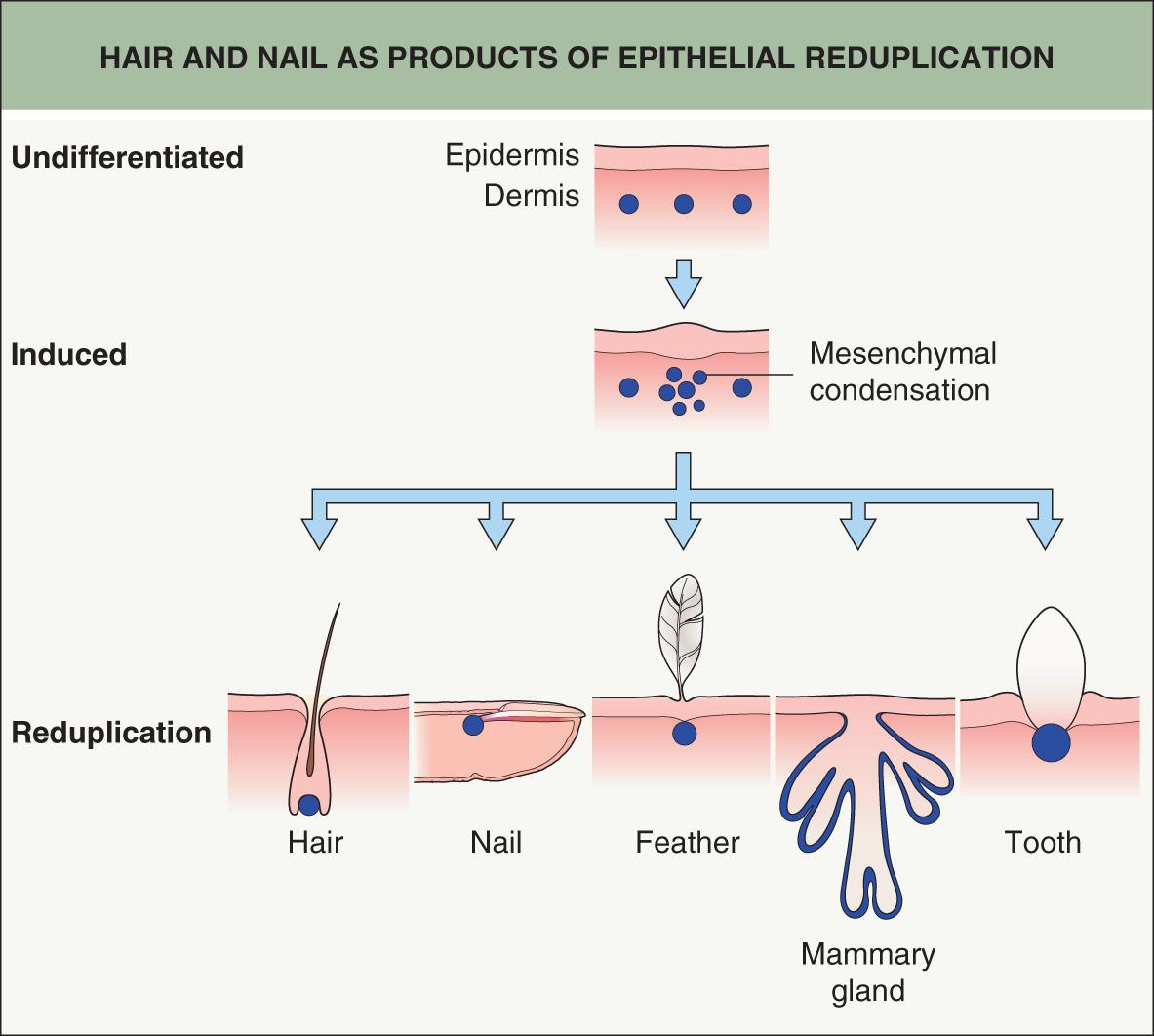
The basic principles of adnexal development were defined by a series of classic tissue recombination experiments performed in the 1970s, which have served as the foundation for research in this field . These experiments demonstrated that the dermal mesenchyme emits the initial “message” for the development of all types of skin adnexa (stage 0). The mesenchyme itself does not change morphologically but rather signals the overlying epithelium to thicken and form a placode (stage 1) ( Fig. 68.2 ). The type of appendage that develops is determined within the epidermis. Canonical β-catenin-dependent WNT ( w ingless-type i nt egration site) signals are candidates for the dermal message, and it is thought that they precede other activators and regulators of adnexal development .
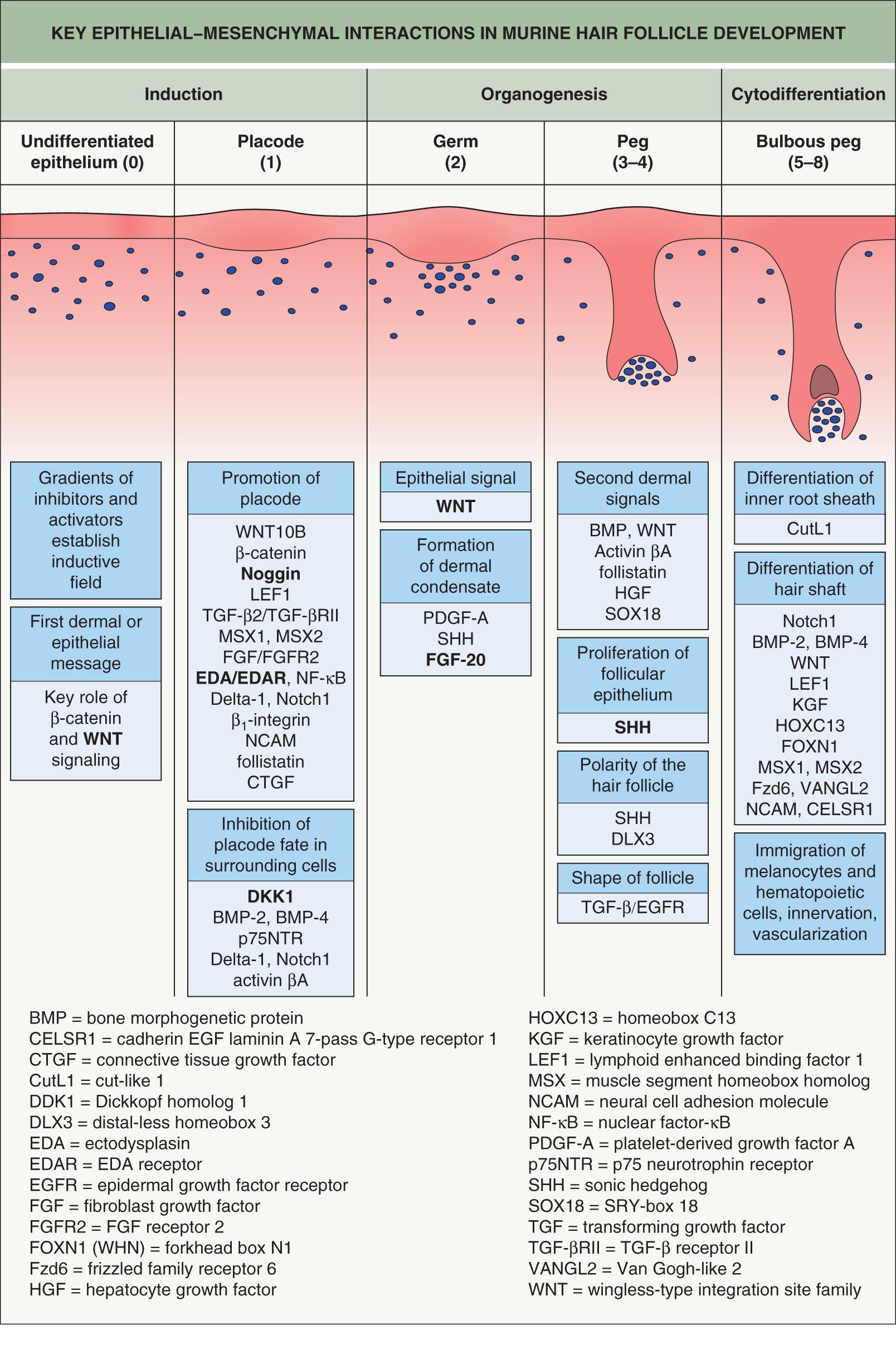
WNT signaling plays key roles in HF development. In mice, lack of WNT signaling through overexpression of the WNT inhibitor Dickkopf homolog 1 (DKK1) prevents HF formation , while overexpression of β-catenin in the epidermis (acting downstream of WNT; see Fig. 55.7 ) promotes conversion to a placode fate . Loss-of-function mutations in WNT10A , which encodes an activator of WNT signaling, cause several forms of ectodermal dysplasia in humans, including odonto-onycho-dermal dysplasia and Schöpf–Schulz–Passarge syndrome (see Ch. 63 ). The spectrum of cutaneous findings that can result from WNT10A mutations includes hypotrichosis, nail dystrophy, palmoplantar keratoderma with eccrine syringofibroadenomatosis, and apocrine hidrocystomas ; in addition, WNT10A polymorphisms have been associated with hair shape, tooth morphology, and corneal thickness . The APCDD1 (adenomatosis polyposis coli down-regulated 1) protein normally inhibits WNT/β-catenin signaling by binding to WNT and low-density lipoprotein receptor-related proteins (LRPs) upstream of β-catenin. APCDD1 mutations that lead to dysregulation of this pathway result in hypotrichosis simplex characterized by hair shaft miniaturization (see Fig. 55.7 ) .
In normal mouse HF development, WNT and β-catenin activation occurs in the placode following the initial dermal signal. This induces transcription of the ectodysplasin A receptor gene ( Edar ), which mediates nuclear factor-κB (NF-κB) signaling . Expression of EDAR within the primary follicle placode also suppresses inhibitory signals from the surrounding epidermis, which refines WNT/β-catenin expression within the placode. A reaction-diffusion mechanism (the Turing model) involving competing gradients of WNT and DKK within the epidermis is believed to modulate HF spacing, establishing regular intervals between follicles .
After determination of placode fate, reciprocal signaling between the mesenchyme and epithelium continue to drive HF morphogenesis through a complex interplay among secreted molecules (see Fig. 68.2 ). Fibroblast growth factor (FGF)-20 secreted by the placode promotes formation of a dermal condensate . A second dermal signal follows, which induces proliferation of the epidermis and invasion of the dermis by a column of epithelial cells that forms the hair germ (stage 2), followed by the hair peg (stage 3–4) and subsequently the bulbous peg (stage 5–8) (see Fig. 68.2 ) . WNT and EDAR signaling control the expression of sonic hedgehog (SHH), which drives the downgrowth of the hair germ . Primary cilia on dermal cells within the condensate coordinate SHH signaling. In mice that lack SHH or have abnormal cilia, the dermal condensate forms but proliferation and downgrowth of the epithelium are arrested . Hemidesmosomal integrins also have a physical role in hair germ development, and mice with deficient β1 or β4 integrins have hair germs that cannot remodel their basement membrane and descend into the dermis .
During normal follicle development, the mesenchymal condensate is progressively engulfed by epidermal cells and becomes enclosed, thereby evolving into the dermal papilla. Although epithelial growth is initially downward, a switch to an upward differentiation program occurs in response to WNT and bone morphogenic protein (BMP) signaling from the dermal papilla. This gives rise to concentric layers of the hair shaft and inner root sheath. Epithelial cell fate depends upon the relative position of a matrix cell with respect to the dermal papilla . An early effector of this signaling within the matrix is the distal-less homeobox 3 (DLX3) transcription factor, which directly regulates expression of hair keratin genes in the hair shaft and inner root sheath . A dominant frameshift mutation in DLX3 results in tricho-dento-osseous syndrome, a form of ectodermal dysplasia characterized by dystrophic hair, enamel hypoplasia, and thickening of skull and jaw bones .
Animal models have increased our understanding of hair biology and facilitated the identification of genes essential to the structure and function of the HF . Mutations in these genes underlie human diseases, including non-syndromic hair disorders, ectodermal dysplasias, and syndromes with extracutaneous manifestations (see Table 69.8 and Ch. 63 ). Recent transcriptional profiling of various progenitor populations have refined markers and signaling signatures of each HF compartment, contributing to our understanding of hair morphogenesis .
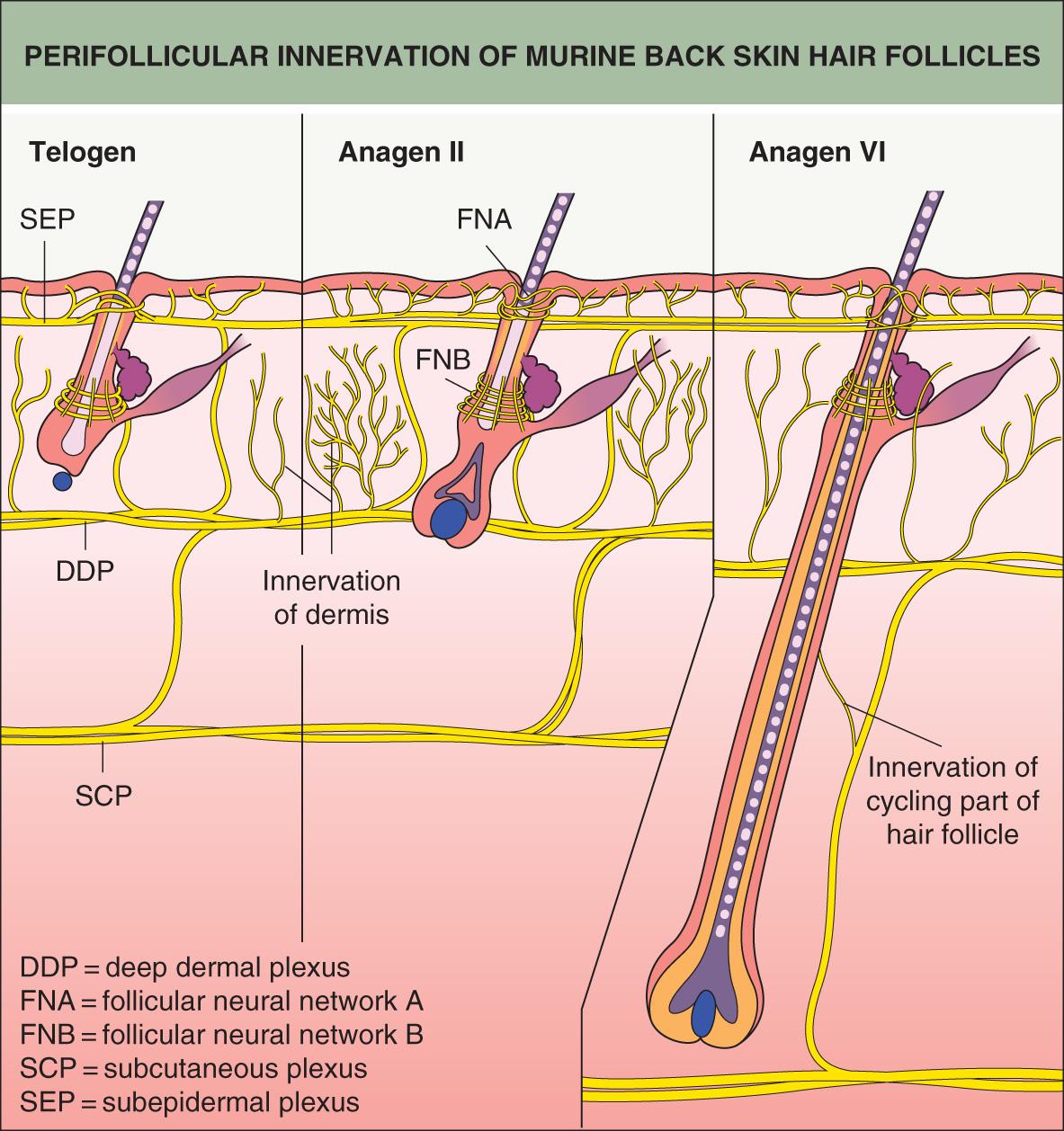
Complex neural networks develop in conjunction with HF morphogenesis. Nerve fibers arrive in the skin first and are located at presumptive follicular sites, running parallel to a dense network of blood vessels ( Fig. 68.3 ). Developing follicles become innervated around stage 5, with nerve fibers encircling the isthmus and bulge regions . Neurotrophins released from the follicular epithelium regulate this innervation, and in turn the nerves may influence the stem cell niche of the HF . Similarly, signaling proteins that promote angiogenesis, such as vascular endothelial growth factor (VEGF), are released from the epithelium and affect development of the vascular network surrounding HFs .
Individual mammals can produce different types of hairs that share basic architectural principles. In the dorsal skin of mice, HF development occurs in three successive waves: (1) large, straight, primary guard or tylotrich hairs, which comprise 1–3% of total hairs and have two sebaceous glands, form on embryonic day 14.5; (2) secondary awl (straight, ~30% of the coat) and auchene (one bend, ~0.1% of the coat) hairs form on embryonic day 16.5; and (3) tertiary zigzag hairs, which have several bends and account for ~70% of the coat, form on embryonic day 18.5. The mouse coat therefore contains four distinct hair types, in addition to specialized hairs such as vibrissae (whiskers) and tail hairs . EDAR is necessary for placode development in primary HFs but not for induction of secondary HFs, which utilize signaling pathways that involve Noggin and SRY-box 18 (SOX18) expression within the dermal papillae .
In humans, hypohidrotic ectodermal dysplasia (HED) with abnormal morphogenesis of scalp hair, sweat glands, and teeth results from mutations in the genes encoding components of the EDAR/NF-κB signaling pathway, including EDAR, its ligand ectodysplasin A, and the downstream effector EDAR-associated death domain . In addition, a non-synonymous single nucleotide polymorphism (SNP) within EDAR is associated with increased hair thickness in Asian populations . Deep deformations in the hair shaft were identified in Swedish patients with an autosomal dominant form of HED due to an EDAR mutation . These clinical observations underscore the importance of the EDAR/NF-κB signaling pathway in human appendage development. Interestingly, mutations in human SOX18 cause lymphedema and telangiectasias as well as hypotrichosis .
The hairs of the mouse dorsal coat have anterior–posterior polarity along the body axis, which contributes to formation of a sleek coat. SHH and neural cell adhesion molecule (NCAM) within the HF have an asymmetric distribution pattern, which is established by a conserved planar cell polarity (PCP) pathway during hair germ development . PCP proteins Van Gogh-like 2 (VANGL2) and cadherin epidermal growth factor laminin A seven-pass G-type receptor 1 (CELSR1) respond to directional cues and are necessary for the correct polarization of follicles within the skin . Mutations in Vangl2 or the frizzled family receptor 6 ( Fzd6 ) affect the early global alignment of HFs, resulting in germs that first grow vertically. Over time the follicles reorient themselves in competing patterns, producing characteristic whorls on the back of the mouse . In Fzd6 −/− mice, an additional mutation in Astrotactin2 ( Astn2 ), which has roles in neuronal migration, leads to a 180-degree reorientation of the dorsal coat to a posterior–anterior polarity . Hair whorls are frequently observed in mammals, such as on the occipital scalp in humans, between the eyes in horses and cattle, and on the upper chest in dogs .
Under physiologic conditions, human HF morphogenesis occurs only once. Lanugo, vellus, and terminal hairs ( Table 68.1 ) have the same basic architecture. The first “coat” is composed of fine, long, variably pigmented lanugo hair, which is shed in an anterior-to-posterior wave during the third trimester. A second coat of fine, shorter, unpigmented lanugo hair then grows in all areas except the scalp and is shed 3–4 months after birth; pigmented scalp hairs, which have variable lengths and diameters, are also shed postnatally in an anterior-to-posterior wave. Thereafter, the hair grows in an asynchronous “mosaic” pattern rather than waves. In prepubertal children, hair is terminal on the scalp, eyelashes and eyebrows, while it is vellus on the face, trunk, and extremities. During puberty, vellus hairs in some sites become terminal hairs under the influence of androgens. Paradoxically, terminal hairs on the scalp exposed to the same androgens revert to miniaturized vellus-like hairs in androgenetic alopecia.
| TRICHOLOGY TERMS | |
|---|---|
| Hair follicle (HF) cycle | Autonomous, rhythmic transformation of fully developed HFs through phases of growth, regression, and resting; controlled by the follicle itself by an as yet enigmatic “hair cycle clock”, with modulation by numerous systemic/extrafollicular factors |
| Anagen | Growth stage of the HF cycle |
| Catagen | Involution of the lower two-thirds of the HF by massive keratinocyte apoptosis |
| Telogen | Resting phase of the HF cycle |
| Exogen | Phase of active hair shaft shedding (during anagen in rodents; most likely during the telogen to anagen transition in humans) |
| Kenogen | Telogen follicle with no club hair present |
| Hair bulb | Lowermost portion of the HF |
| Hair matrix | Rapidly proliferating keratinocytes that terminally differentiate to produce the hair shaft |
| Club hair/fiber | Fully keratinized proximal tip of hair shaft, formed during late catagen and telogen; brush-like appearance; characteristic of telogen follicles |
| Vellus hair (follicle) | Very short, non-pigmented, and usually non-medullated; absence of arrector pili muscle; tiny vellus follicles can have large sebaceous glands (face); undergo full hair cycle, yet much shorter than terminal hair |
| Terminal hair | Large, usually pigmented and medullated hair |
| Lanugo hair | Fine hair on the fetal body; shed in utero or during the first weeks of life |
| Vibrissae | Special sensory HFs found on the upper lips/snout region in non-human mammals, especially rodents; largest and most densely innervated HFs with special sinusoid blood supply; first HFs to develop |
| Tylotrich HF | Large sensory HFs interspersed within truncal skin of non-human mammals, most notably rodents; extra large, long hair shaft, typically associated with double sebaceous gland and an innervated epidermal Merkel cell complex (Pinkus' Haarscheibe); second type of HF to develop |
| Non-tylotrich pelage hair | Majority of all HFs in non-human mammals; last to develop |
| Effluvium | Process of excessive shedding of hair shafts |
| Alopecia | Result of abnormal hair loss |
| Hirsutism | Excessive vellus-to-terminal hair conversion in androgen-dependent areas in women |
| Miniaturization | Terminal-to-vellus hair conversion, e.g. on the balding scalp in androgenetic alopecia; these miniaturized follicles still have an arrector pili muscle, unlike true vellus hairs |
| Infundibulum of follicle | Region extending from the junction with the interfollicular epidermis to the opening of the sebaceous gland; displays cornification similar to that of the interfollicular epidermis |
| Isthmus of follicle | Region located between the opening of the sebaceous gland and the site of insertion of the arrector pili muscle; displays trichilemmal keratinization |
| Arrector pili muscle | Inserts at the level of the bulge; pulls up hair (“goose bumps”) |
| Bulge | Segment of the outer root sheath located at the level of arrector pili muscle insertion; major seat of epithelial stem cells of the HF |
| Secondary hair germ | Additional seat of epithelial stem cells and site of melanocyte stem cells; located between club hair and dermal papilla in telogen HF |
| Dermal sheath | Special mesenchymal follicular sheath that is tightly attached to the HF basement membrane and is continuous with the follicular dermal papilla |
| Follicular dermal papilla (DP) | Onion-shaped, closely packed, specialized fibroblast population with inductive and morphogenic properties; hair cycle-dependent fibroblast trafficking occurs between the dermal sheath and DP; volume of DP determines size of hair bulb and, thus, hair shaft diameter |
| Inner root sheath (IRS) | Packages and guides the hair shaft; cornifies |
| Outer root sheath (ORS) | Merges distally into the epidermis and proximally into the hair bulb; provides slippage plane, nutrition, regulatory molecules, and stem cells |
| Follicle pigmentary unit | Melanin-producing HF melanocytes located above and around the upper one-third of the DP; transfer eu- or pheomelanosomes to differentiating HF keratinocytes in the precortical matrix; goes largely into apoptosis during each catagen phase, regenerated from melanocyte stem cells in hair germ (and possibly from non-melanogenic ORS melanocytes) during anagen |
Congenital generalized hypertrichosis (Ambras syndrome) is characterized by a disruption in the patterning of hairs, with terminal hairs growing at sites that normally have vellus follicles . A position effect that down-regulates the expression of the trichorhinophalangeal syndrome I gene ( TRPS1 ) was found to underlie this hypertrichosis phenotype in both humans and mice . In addition, X-linked congenital generalized hypertrichosis in a Mexican family was associated with a position effect that decreased the expression of FGF13 .
In most mammals, the hair pattern differs depending on the body site. While the ectoderm involved in skin and hair development is non-neural and relatively homogeneous, the mesenchyme has various origins which may explain this regionalization. The dorsal dermis is derived from the somitic dermomyotome, the ventral dermis has a somatopleural origin, and the craniofacial dermis arises from the neural crest . The dermal progenitors at each of these sites are specified by different signaling pathways. Mice display regional differences in skin thickness as well as hair color and type. The postnatal identity of their skin is acquired by embryonic day 12.5, with the T-box 15 (TBX15) transcription factor appearing within dorsal skin to demarcate the dorsal–ventral boundary .
In humans, the effects of androgens on HFs depend upon the body site. Follicles in the frontal scalp are normally androgen-dependent, whereas occipital scalp follicles are hormonally insensitive and therefore less susceptible to androgenetic alopecia. When HFs from the occipital scalp are transplanted to the frontal scalp, they typically retain the pigmentary characteristics, androgen sensitivity, and growth rate of the donor site . However, in other instances, transplanted hairs take on characteristics of the recipient site, such as scalp hairs moved into the eyebrow region. It remains to be established exactly which characteristics are more influenced by the recipient site versus the donor site following transplantation.
Although HF formation in general occurs prior to birth, adult mammalian skin retains the capacity for HF morphogenesis. In adult mouse and rabbit skin, WNT-dependent de novo HF formation occurs after wounding ; this is mediated by FGF-9 secreted by infiltrating γδ-T cells and wound fibroblasts . It is therefore reasonable to consider possible therapeutic interventions that would initiate de novo HF formation on the human scalp. Trans-species end-bulb formation and hair fiber growth has been demonstrated after implantation of intact human dermal papillae onto transected mouse whisker follicles . In humans, allogeneic transplantation of tissue from the dermal sheath that surrounds the dermal papilla can induce new follicle formation in the recipient, with immune privilege providing protection from rejection . In addition, cultured tooth mesenchymal papilla cells from humans and rats have been shown to induce hair fiber formation in an amputated follicle, highlighting the conservation of inductive signals among different adnexa .
Dermal papilla cells in rodents show much more inductive potency than their human counterparts. Rodent HF papilla cells can reprogram differentiated glabrous epidermis to produce a de novo HF, and they are also capable of initiating trans-differentiation of amnion or even adult corneal epithelium into epidermis complete with HFs . Human dermal papilla cells lose their inductive properties quickly in a standard monolayer culture, which hampers the utilization of these cells for hair regeneration. However, dermal papilla cells grown in a three-dimensional spheroid culture have been shown to retain their ability to induce hair growth in glabrous skin, suggesting that cellular interactions are critical to maintenance of dermal papilla identity .
Once the mature HF is generated during embryogenesis and early postnatal life, it begins to cycle and continues to do so throughout its lifetime. The phases of the hair cycle include active growth (anagen), regression (catagen), and rest (telogen) ( Fig. 68.4A ). As noted above, mouse follicles cycle in a synchronous wave along the anterior to posterior axis, while human follicles cycle in a mosaic pattern. HF cycling is an autonomous phenomenon that continues even when HF units are isolated from the skin and grown in culture . The portion of the follicle below the bulge undergoes distinct morphologic changes as it grows and regresses; in contrast, the upper region of the follicle including the sebaceous gland, isthmus, infundibulum, and hair canal remains permanent.
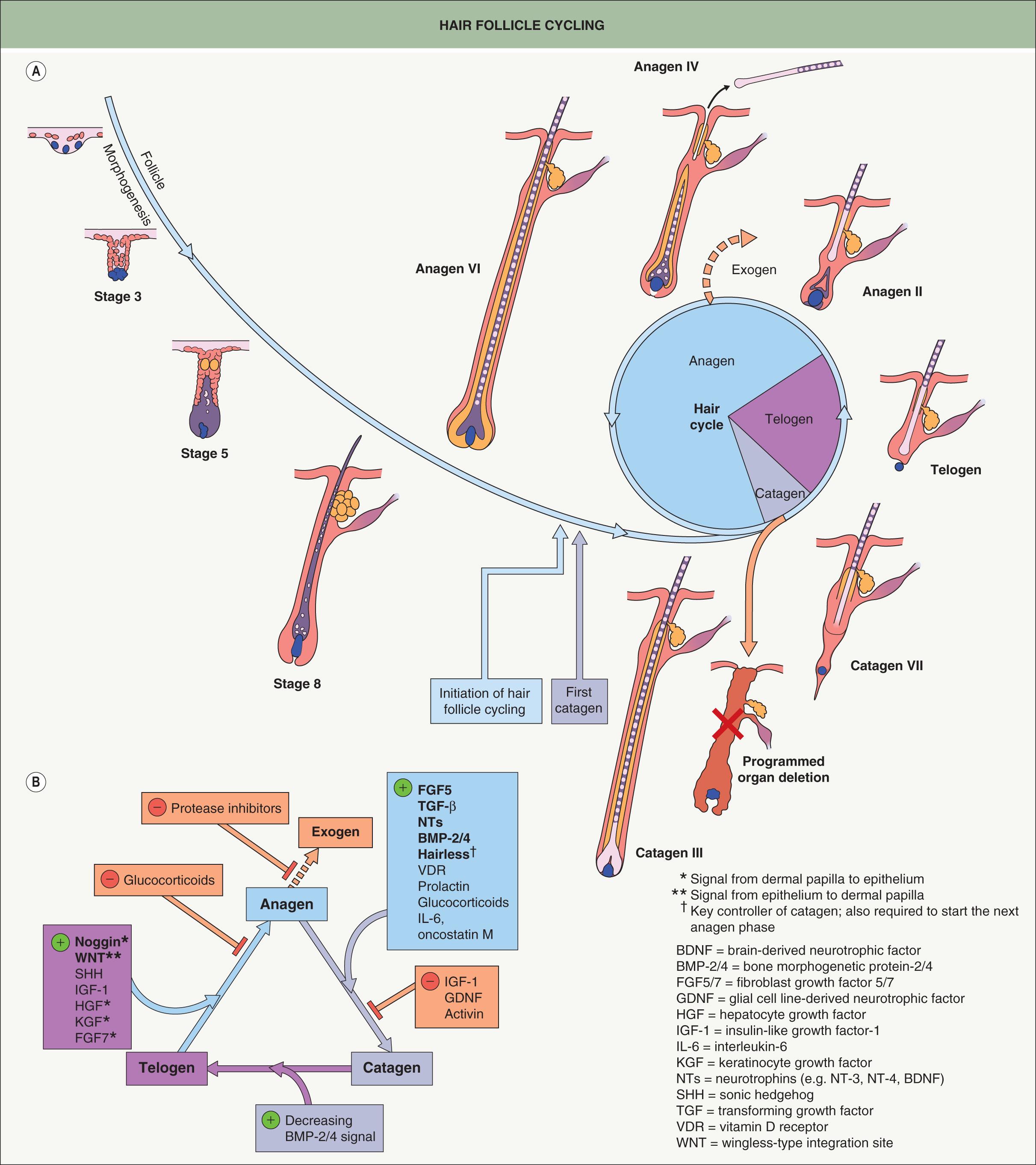
The process of cycling makes the HF a dynamic structure, enabling animals to change their coats depending on the season for camouflage and insulation ( Table 68.2 ) and for the lengths of hairs to vary depending on the body site. Coat changes involve two processes: (1) exogen – signals and structural changes culminating in hair shedding (teloptosis); and (2) initiation of new hair fiber growth . “Kenogen” refers to a telogen follicle that has lost its club fiber but has not yet transitioned to anagen, representing the “true” resting phase of the hair cycle where metabolic activity is at a minimum.
| FUNCTIONS OF HAIR AND NAILS |
| Functional properties of hair shafts |
|
| Functional properties of pilosebaceous units |
|
| Functional properties of nails |
|
Anagen is the period of active hair fiber production, and its duration varies depending upon factors such as species and body site ( Table 68.3 ). Anagen is divided into six substages, anagen I–VI; it lasts 1–3 weeks in mice and up to several years on the human scalp , explaining the longer lengths of human scalp hair. The start of anagen (anagen I), as observed in mouse skin, is associated with an increased size of the dermal papilla, due mainly to an influx of cells from the surrounding dermal sheath. Dermal sheath cells at the base of the follicle also contribute to dermal papilla regeneration after removal of the lower follicle . The influx of cells at the start of anagen is accompanied by an increase in ECM material. This doubles the size of the dermal papilla , the width of which is directly proportional to the thickness of the hair shaft produced by the follicle. The degree of axial symmetry within the hair bulb determines the curvature of the final hair structure .
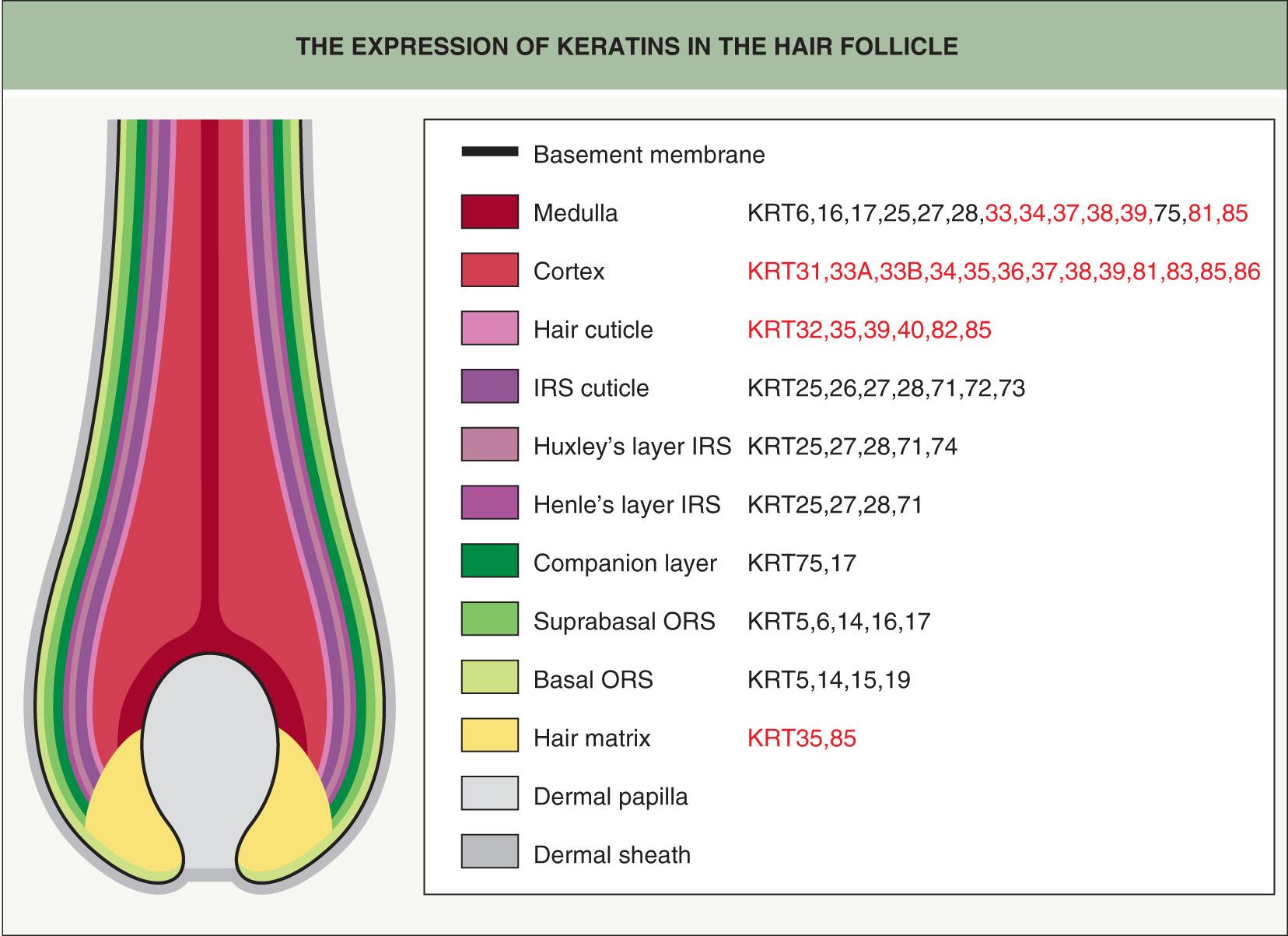
The dermal papilla provides signals to the surrounding matrix cells, whose position with respect to the dermal papilla specifies their fate, e.g. central cells become the medulla . As the matrix cells begin to differentiate, they form precursor cells of the different follicle lineages. Migrating upwards, these cells undergo specialized programs of differentiation, expressing defined sets of keratins to form seven concentric epithelial lineages of the follicle, from the medulla to the companion layer ( Figs 68.5 & 68.6 ). In contrast, the outer root sheath (ORS), which is the outermost layer of the epidermal portion of the follicle, does not originate from upward growth of matrix cells, but rather directly from the bulge (see below) . The growing HF ends up nestled in the subcutaneous fat by anagen VI. In the mouse, dermal adipocyte precursors secrete platelet-derived growth factor-A (PDGF-A), which acts on the dermal papilla to support anagen .
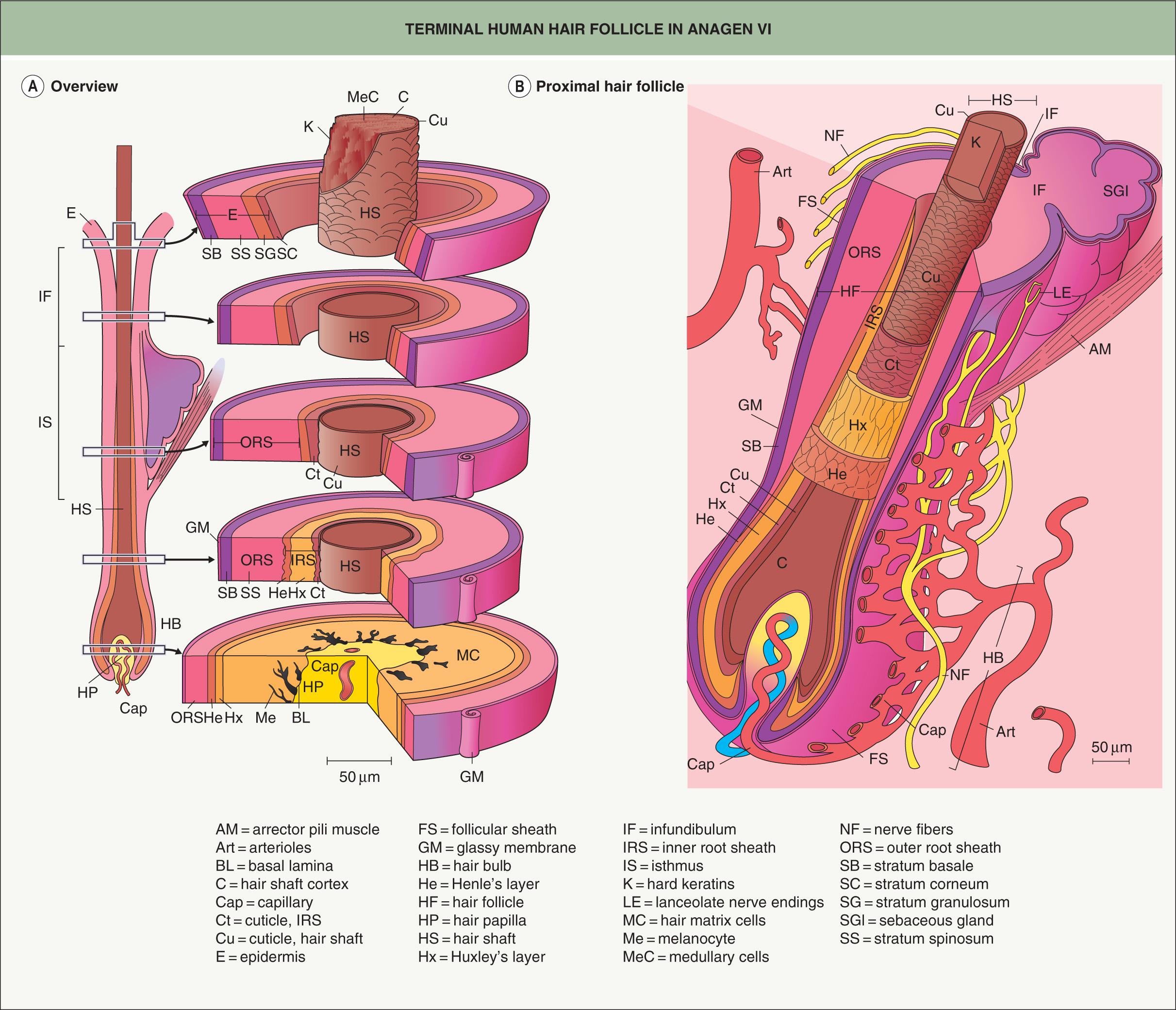
In rodents and rabbits, plucking telogen hairs can accelerate onset of the next anagen phase, and this has been utilized to study the mammalian hair cycle in a controlled manner. The trauma of plucking causes release of chemokine C-C motif ligand 2 (CCL2) by the follicle, leading to recruitment of macrophages that secrete tumor necrosis factor (TNF), which promotes local follicles to enter anagen . Other microenvironmental cues and “quorum-sensing” among HFs also likely play a role in hair cycle coordination.
At the end of anagen, the follicle enters the regressive catagen phase, which is divided into eight substages (catagen I–VIII) . Catagen lasts ~2 weeks in humans, regardless of the site and follicle type . The initiation of catagen is characterized by a nearly 50% reduction in the volume of the dermal papilla due to loss of ECM and migration of cells into the surrounding dermal sheath, which acts as a repository that replenishes the dermal papilla in the subsequent anagen . Melanocytes in the hair bulb also down-regulate melanogenesis. Later, there is cessation of mitotic activity within the matrix cells of the bulb, and the remaining hair precursor cells at the base of the shaft differentiate to form a “club fiber”.
The club fiber migrates up the follicle, and a germ capsule termed the trichilemmal sac forms around its base. This club fiber lacks pigmentation and is formed from cortical and cuticle cells only. The electron-dense, non-nucleated layer of trichilemmal keratin around its base has a characteristic “brush” structure that protrudes outwards and between surrounding ORS cells . Abundant desmosomes between the trichilemmal keratin and surrounding tissue are thought to physically anchor the club fiber within its trichilemmal sac until it receives the signal for release .
As the club fiber moves up the follicle, the dermal papilla, which is initially still in the subcutaneous fat, becomes separated from the club by an epithelial strand that is surrounded by basement membrane (the glassy membrane) . Apoptosis in the epithelial strand creates an “apoptotic force” that draws the dermal papilla up to just beneath the club fiber in the dermis, leaving behind a trailing dermal sheath called the fibrous streamer or stele . FGF-5 produced by macrophage-like cells facilitates regression of human HFs , and FGF5 mutations underlie familial trichomegaly characterized by abnormally long eyelashes . Macrophages also participate in the clearance of cellular debris during catagen .
Loss-of-function mutations in the hairless gene ( HR ) can result in atrichia with papular lesions (APL) , a rare autosomal recessive disease characterized by complete hair loss early in life and later eruption of papular lesions. Hairless is a transcription factor that is required for apoptosis in the epithelial strand, which draws the dermal papilla up beneath the club fiber and secondary hair germ in a position to activate the next anagen phase. Thus, individuals with APL are born with hair, which never grows back after it is shed. Activating mutations in the upstream open reading frame of HR lead to another form of sparse hair, Marie Unna hypotrichosis . Hairless interacts with the vitamin D receptor (VDR), which has ligand-independent functions in hair cycle control . Patients with hereditary vitamin D-resistant rickets caused by VDR mutations present with alopecia that is not corrected by vitamin D supplementation .
Telogen begins when the dermal papilla reaches the base of the permanent portion of the HF. The duration of telogen can be as short as 2 days in mice and as long as 3 months for human scalp hairs. The hair germ is derived from the bulge during the catagen-to-telogen transition and lies inferior to the bulge, separating the latter from the dermal papilla . As in HF morphogenesis, epithelial–mesenchymal cross-talk is required to activate the new anagen phase. Anagen progression recapitulates the middle and later stages of HF development, and many factors that drive follicle morphogenesis (e.g. WNT, SHH, noggin) also play important roles in telogen-to-anagen transition ( Fig. 68.4B ). The bulge is relatively quiescent during telogen, traditionally referred to as the “resting” phase, due to inhibitory signals (e.g. FGF-18, BMP-6) from keratin 6-positive inner bulge cells . The apparent dormancy of the HF during telogen may be an energy-efficient adaptation allowing for the maintenance of stem cells in preparation for the next anagen phase .
The macro-environment surrounding the HF also takes part in regulating cycle transitions . BMPs in the subcutaneous fat maintain HFs in a “refractory” telogen, and cessation of this inhibitory signal enables the follicle to progress to a “competent” telogen with a hair germ (biochemically distinct from the bulge) that is responsive to anagen-initiation signals and capable of entering a new anagen phase . Fluctuations of microRNA levels (e.g. microRNA-31) in the follicle and its macro-environment may also silence certain gene expression patterns, promoting a switch from telogen to anagen . After secondary hair germ activation, the germ cells begin to contribute to the hair matrix and concentric layers of the follicle via an upward movement, while bulge cells generate the ORS through a downward movement . In follicles with a longer anagen duration, the proliferative capacity of the matrix is not sufficient to sustain growth. The “stem cell trafficking theory” proposes that a continuous migration of precursor cells from the bulge down the ORS prevents exhaustion of the germinative matrix during anagen .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here