Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cell biology tells us about the structure and functions of the cell and its organelles, whereas biochemistry addresses the chemical basis of the composition of the human body, its structure and its functions, and also the preservation and continuity of the structure and function through generations. The body is an open system and, thus, there are the interfaces with surroundings that are both sensory and metabolic, the latter through nutrition and excretion of metabolic products. The function of the organism may become disrupted during both the lack of nutrients (starvation, malnutrition) and their excessive intake (the diseases of excess: obesity, cardiovascular disease and diabetes).
Central to metabolism and homeostasis is the energy flow: metabolism includes reactions that are energy requiring (endergonic) and the ones that yield energy (exergonic). Some chains of reactions (pathways) accumulate energy in a range of biosynthetic products (anabolic pathways), and others release energy (catabolic pathways). Reactions take place in an aqueous environment, and electrolytes and ions are both bystanders and participants in chemical reactions. Most reactions happen at physiological temperature within a narrow range of pH, thanks to the action of biocatalysts (enzymes).
There has been an enormous acceleration in our understanding of the chemical aspects of body structure and function. Perhaps the most significant development in biochemistry in the last few years was the expansion of knowledge concerning cellular signalling systems and their links to the control of gene expression ( Fig. 2.1 ) . It is so important because the aim of a large number of medical therapies is to control these processes when the normal regulatory mechanisms fail.
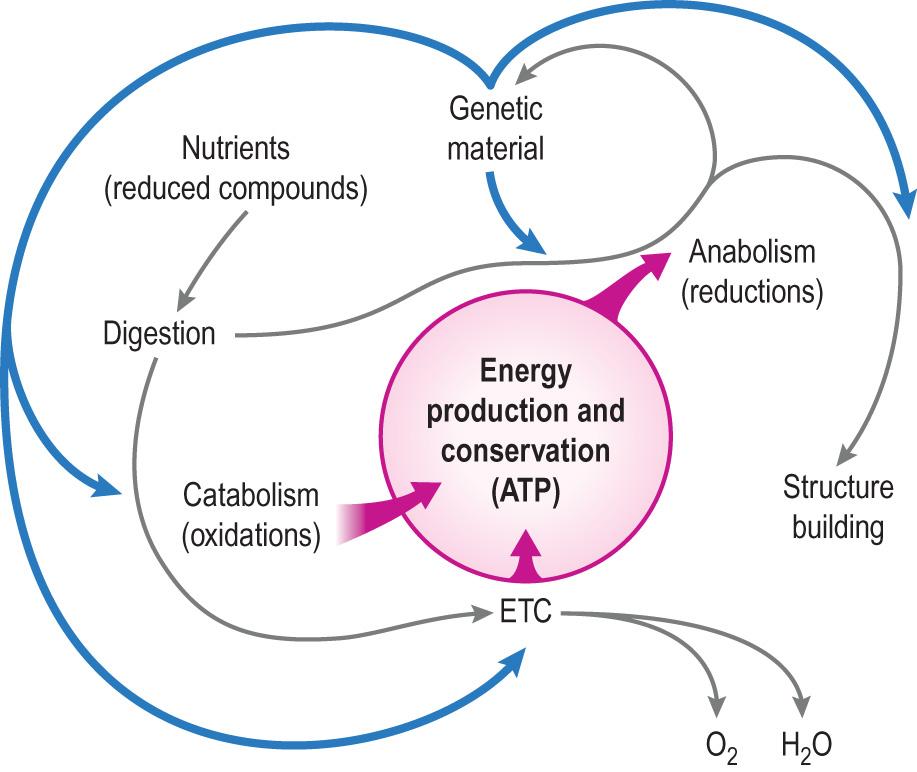
In this chapter, we will first consider the fundamentals of the atomic structure, chemical bonds and chemical reactions, highlighting the energy flow in biological systems. We will go on to discuss the chemical composition of the human body and the most important classes of biological compounds: carbohydrates, fats, proteins, nucleic acids and a range of ‘hybrid’ molecules, such as glycolipids or proteoglycans.
We will then discuss the cell and its organelles, highlighting the function of cell structures that enable cells to communicate, transport nutrients and interact with each other.
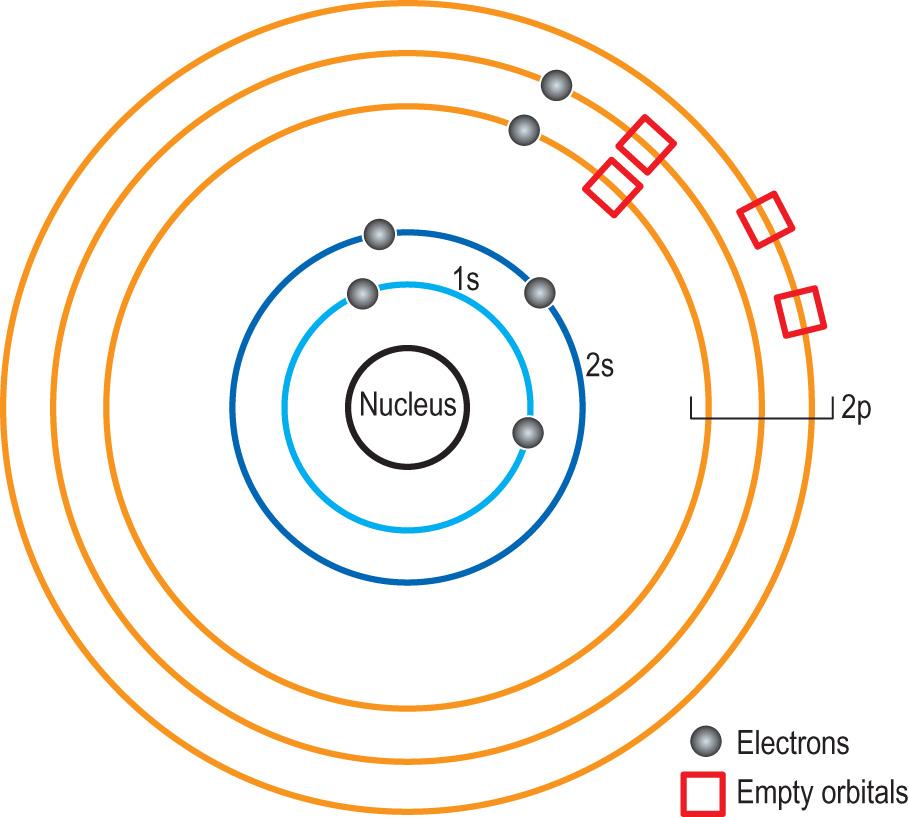
All atoms have a nucleus surrounded by shells of electrons. Each of these shells is characterised by a different energy level. Subshells (orbitals) exist within each shell. Atomic orbitals are described by quantum numbers. The principal quantum number (X) corresponds to the energy level, and the angular quantum number l (type, denoted by a small letter) describes the shape of the subshell. The superscript (y) in the convention Xtype y describes the number of electrons in an orbital.
The orbitals are designated as 1s, 2s, 2p, 3s, 3p and 4s. The 1s orbital is closest to the nucleus. Each orbital can be occupied by a maximum of two electrons, each of them having a different spin. The order in which the atomic orbitals are filled goes from the lowest energy level (closest to the nucleus) to the higher levels. The order (from first to last) is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d and 7p. When orbitals of the same energy are available, the electrons fill them singly first (this is known as the Hund rule).
Each of the electron shells contains a defined number of electrons in their orbitals:
The first (innermost) shell can have one orbital (1s) and a maximum of 2 electrons.
The second shell 2s2p has 4 orbitals and a maximum of 8 electrons.
The third shell can have 3 orbitals (3s3p3d), which together makes 9 orbitals and up to 18 electrons.
The fourth shell has 16 orbitals and up to 32 electrons.
Electrons always occupy orbitals of the lower energy first. Note the anomaly that occurs between the third and the fourth shell. The 4s orbital is filled before 3d (contravening the general rule, it has a lower than 3d energy level).
A fully occupied shell makes an atom chemically inert (examples are the noble gases such as helium and neon). Atoms that have incompletely filled outer shells can react until their shells become fully occupied. The outermost orbital of the atom contains the so-called valence electrons that participate in forming chemical bonds. The configuration of eight electrons (octet) in the outer shell is the most stable one ( Fig. 2.2 ) .
Atoms that possess an unpaired electron that is not shared with other atoms are known as free radicals and are highly reactive.
The nucleus contains protons and neutrons (the hydrogen atom has only a single proton as a nucleus). The number of protons is the atomic number of an element. The sum of the protons and neutrons is the atomic mass . Different isotopes of a given element differ with respect to the atomic mass ( Information box 2.1 ).
Carbon is the element present in all organic molecules. The carbon atom is assigned an atomic number of 6 because it has 6 protons. However, it can have 6, 7 or 8 neutrons, forming different isotopes with different atomic masses. Carbon isotopes show no differences in chemical reactivity ( Table 2.1 ).
In an atom, there is normally a balance between the positive charge of the protons and the negative charge of the electrons, rendering the atom electrically neutral. Consequently, when it gains or loses electrons, it acquires an electrical charge: such an atom is called an ion . The charge can also be associated with groups of atoms, forming ionised (functional) groups. Thus,
Anions are negatively charged ions and are generated by the gain of electrons.
Cations are positively charged ions and are generated by the loss of electrons.
Metals tend to form cations, and non-metals form anions. Formation of ionic bonds between atoms is one of the principal mechanisms of chemical reactions.
The concept of an acid and a base is associated with the movement of protons and electrons in aqueous solutions. According to the Brönsted-Lowry definition, an acid is a molecule that can donate protons and a base is a molecule that accepts protons. Therefore, an acid in a sense ‘contains’ a base: when an acid loses a proton, the remaining species (now negatively charged) is its conjugate base. Another definition of an acid, the Lewis definition, defines it as a molecule that accepts a pair of electrons and a base as a molecule that donates a pair of electrons ( Information box 2.2 )
The strength of an acid is the ease with which it donates proton or accepts electrons. Strong acids dissociate completely, whereas weak acids dissociate to a limited extent. This tendency is described by the acid's dissociation constant ( K a ), which is a ratio between its undissociated and dissociated forms:
The derivative of the dissociation constant is its negative logarithm, the p K a . The lower the p K a is, the more active a molecule is as a proton donor (a stronger acid). Examples of strong acids are inorganic acids such as hydrochloric or sulphuric acid. The weak acids are carbonic acid (an important blood buffer) and the carboxylic acids.
Conversely, increasing p K a means an increase in the alkalinity- of the conjugate base.
The acidity or alkalinity of a solution also relates to the concentration of hydrogen ions (H + ) measured as its negative logarithm, the pH . The neutral pH of pure water is close to 7.0. Solutions with a pH of less than 7.0 are acidic, and solutions with a pH of greater than 7.0 are alkaline. The normal range of human blood pH is 7.35–7.45. The maintenance of stable pH of the body fluids is necessary for survival ( Ch. 1 ).
Chemical bonds determine how molecules join together. Atoms can form chemical bonds with other atoms of the same or different kind ( Fig. 2.3 ) . Bonds differ in their strength and stability, and are also determine the spatial conformation of molecules. The bonds most relevant to biomolecules are the following:
Ionic bonds
Covalent bonds
Hydrogen bonds.
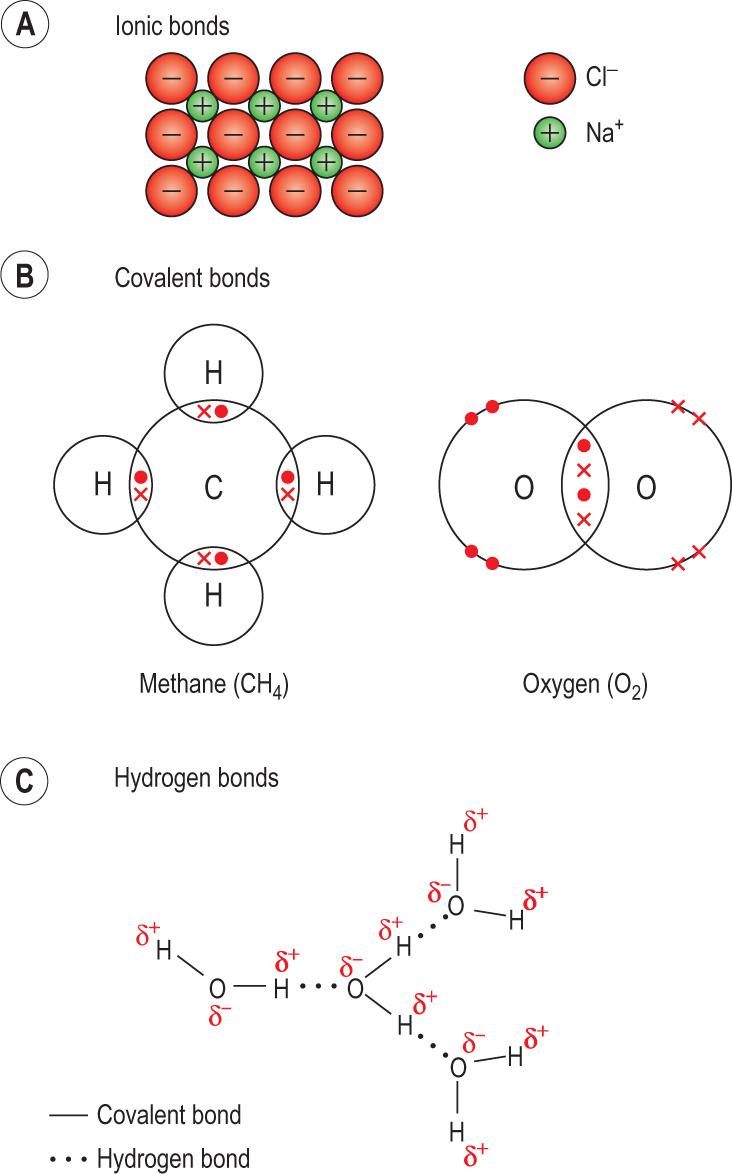
Ionic bonds form when ions are attracted to each other by their opposite electrical charge. An electron(s) from one atom move(s) closer to the nucleus of another, forming a molecule. Importantly, such molecules dissociate into their component ions in an aqueous solution. For instance, sodium chloride (table salt) is formed when sodium (Na) and chlorine (Cl) atoms attract each other. In this reaction, Na loses an electron, becoming a cation, Na + , whereas Cl acquires the electron, forming an anion, Cl − . A strong ionic bond forms sodium chloride (NaCl). When the water is removed, salt crystals consisting of Na + and Cl − held together by ionic bonds are formed (see Fig. 2.3A ).
The principle behind covalent bonds is electron sharing . A pair of electrons is shared between two atoms, making both atomic shells complete. This may occur between atoms of the same or different elements. Examples include oxygen (O 2 ), where two oxygen atoms form a double bond (O O); hydrogen atoms sharing electrons with a single bond (H–H); and carbon and hydrogen atoms sharing electrons to form methane (CH 4 ) (see Fig. 2.3B ).
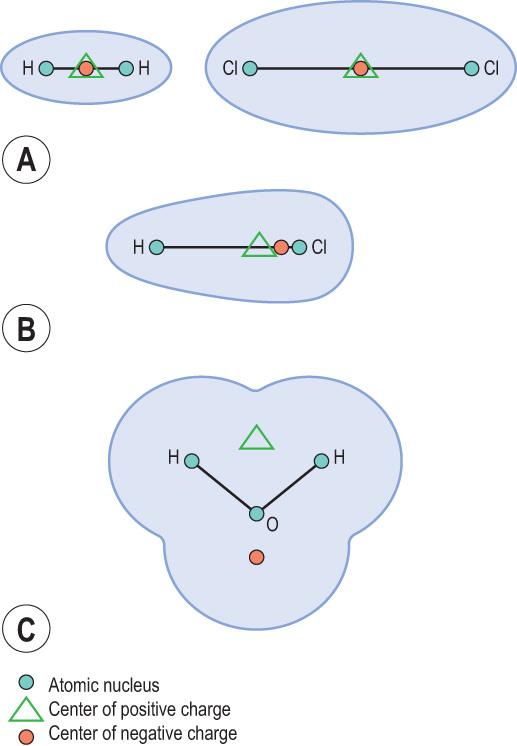
A covalent bond is electrically neutral when the electrons remain equidistant from the two participating atoms ( Fig. 2.4A ) . However, when the nuclei of two atoms differ in their positive charge, this distance becomes unequal, creating partial electric charges across the covalent bond. Such a bond becomes a polar covalent bond (see Fig. 2.4B ) and the resulting molecule becomes a dipole . The water molecule (H 2 O) is an example of a dipole (see Fig. 2.4C ). The strongly negative oxygen atom attracts electrons away from the two hydrogen atoms, which become positive. Because of this, they keep at a distance from each other. The water dipole can form hydrogen bonds with non-water molecules: this is the principle behind the solubilisation of substances by water. The hydrogen ions can also associate with other water molecules. This results in water dissociation into the hydronium ion (H 3 O + ) and the hydroxide ion (OH − ):
We normally simplify this in our notation and show water dissociation as
The hydrogen atom has a single proton as the nucleus, and only one electron shell, occupied by a single electron. When this electron is lost, a cation (H + ) forms. Such a cation can attract the negative pole of a dipolar molecule, forming a hydrogen bond (see Fig. 2.3C ). These bonds are weaker than covalent and ionic bonds, and are easily disrupted by pH and temperature changes. They are important in stabilising the spatial structures of proteins and nucleic acids. Many large molecules contain numerous hydrogen bonds.
Non-polar molecules are water-insoluble ( hydrophobic ). Such molecules tend to aggregate in polar solvents; this is known as hydrophobic interaction . An example is the behaviour of lipid molecules in the plasma membrane. Another type of weak interaction, the Van der Waals force , makes molecules align in an energetically optimal conformation. Van der Waals forces are effective at a relatively long range (up to 50 nm) and are easily reversible. For example, they contribute to the binding of substrates to enzyme molecules and to the binding of antibodies to antigens.
An organic compound is a compound containing carbon atoms linked by covalent bonds. This definition usually excludes some small molecules, such as carbon dioxide. Organic compounds may also contain oxygen, nitrogen and sulphur.
The carbon atoms commonly bond with each other and with hydrogen, forming hydrocarbon chains . The hydrogen atoms in hydrocarbons may be replaced by other atoms or functional groups. In addition, carbon atoms can share more than one electron with another atom, forming double or triple covalent bonds. Table 2.1 shows examples of the chemical groups that occur in different classes of organic compounds.
| Group | Formula | Class |
|---|---|---|
| Hydroxyl | R-OH | Alcohols |
| Aldehyde | R-COH | Aldehydes |
| Ketone | R-COR′ | Ketones |
| Carboxy | R-COOH | Carboxylic acids |
| Ester | R-COO-R′ | Esters |
| Amino | R-NH 2 | Amines |
| Imino | R-NH | Imines |
| Sulphydryl | R-SH | Thiols |
The carbon atoms may share four electrons with other atoms, including carbon (see Fig 2.2 ). For example, in methane (CH 4 ), the carbon atom links covalently with four hydrogen atoms. Importantly, the carbon can form long chains (e.g. in some fatty acids), which can also branch, or form rings (e.g. the steroids), containing either carbon only or carbon linked to other atoms, such as nitrogen.
Within an organic molecule, a carbon atom that shares its four available electrons with four different atoms (or groups) is known as the chirality centre or stereocentre . The chiral compound cannot be superimposed on its mirror image. Such a molecule can exist as variants that, while having the same formula, have different spatial orientations (known as stereoisomers ). Stereoisomers are identified by the way they rotate the plane of polarised light. Those that rotate the compound anticlockwise (to the left) are called L-isomers, whereas those that rotate it clockwise (to the right) are called D-isomers.
Another type of isomerism is cis–trans isomerism , which relates to the arrangement of atoms across the carbon–carbon double bonds. The cis configuration is when two linked atoms reside on the same side of the double bond, whereas the trans one is when they reside on the opposite sides. Cis–trans isomerism is particularly important in lipid chemistry.
Chemical reactions are exchanges of protons and electrons. They result in the formation, or breakage, of chemical bonds between atoms and molecules. Chemical reactions are associated with energy transfer – some require energy to proceed, whereas others release energy. The strength (potential energy) of different chemical bonds is shown in Table 2.2 .
| Type of bond | Energy (kJ/mol) |
|---|---|
| Ionic | 12.6–29.3 |
| Covalent (single) | 210–160 |
| Covalent (double) | 500–710 |
| Covalent (triple) | 815 |
| Hydrogen | 4.2–8.4 |
| Van der Waals interactions | 4.2 |
The main types of chemical reactions are the following:
Synthesis , when a larger molecule is formed from smaller substrates
Lysis , when a molecule is broken down into smaller compounds
Exchange reactions , where atoms, or groups of atoms, are exchanged between molecules (e.g. transamination reactions, where the amino group is transferred between molecules).
During a chemical reaction, atomic structures of reacting molecules are modified by the transfer of electrons (or protons). The excess electrons from one atom may ‘invade’ the orbitals of another, forming new shared orbitals. The relevant terminology is as follows:
Nucleophiles are negatively charged atoms that are electron-rich. They tend to lose pairs of electrons.
Electrophiles are positively charged atoms that are electron-poor. They accept electrons.
A nucleophilic attack is a situation where the electrons from a nucleophile move into an electrophilic atom. Nucleophilic attack underpins, for instance, very common reactions of hydrolysis.
Oxidation–reduction (redox) reactions are paired reactions in which electrons pass from one molecule to another ( Fig. 2.5 ) . In the process, the energy trapped in the chemical bonds in the molecule being oxidised is transferred to the molecule being reduced.
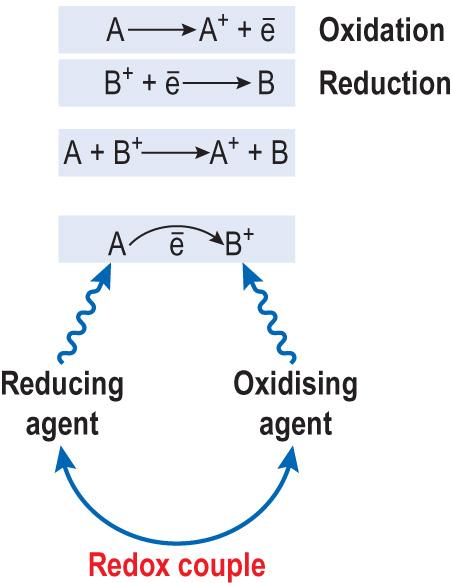
Oxidation is a net loss of electrons with an increase in oxidation state by a molecule (the complete oxidation state is when an atom is maximally charged after all its electrons have participated in ionic bonds).
Reduction is a net gain of electrons (or protons – hydrogen ions) with a decrease in oxidation state by a molecule.
Oxidation has also been defined as a loss of a hydrogen atom from a compound, and reduction as an addition of a hydrogen ion to a compound.
The oxidation–reduction (redox) reactions are key to the cellular flow of energy.
In redox reactions, electrons will flow from carrier A to carrier B, which has the higher redox potential (a tendency to be reduced or to accept electrons).
An electron added to an atom (a reductant) traps the energy. Reduced molecules possessing such trapped energy are more stable. The energy can then be released during oxidations.
Specific compounds in the cell transfer protons and electrons. The coenzyme nicotinamide adenine dinucleotide (NAD + ) is the most important electron acceptor. Another one is flavin adenine dinucleotide (FAD), which is an integral part (the prosthetic group) of several enzymes. Both are also hydrogen ion carriers. The structurally almost identical nicotinamide adenine dinucleotide phosphate (NADP) is a reductant that participates in many biosynthetic reactions.
Energy is the capacity to do work. There is potential energy and kinetic energy. Potential energy is the capacity of an object to do work (e.g. because of its position in space), whereas kinetic energy is the capacity to do work associated with the movement of an object. Each chemical reaction is associated with a change in free energy (Δ G ). Exergonic reactions are reactions that release energy and are characterised by a negative change in free energy (−Δ G ). Endergonic reactions are reactions that require energy to proceed (positive change in free energy (+Δ G ).
The energy required by living organisms is trapped in foodstuffs by plant photosynthesis, which consumes carbon dioxide and generates oxygen. Organic molecules thus produced then become metabolic fuel for animals. Their absorption and digestion by living organisms provide the energy necessary for their survival. This energy is released in a highly controlled, stepwise manner during biological oxidations, resulting finally in the production of carbon dioxide and water. The released energy is used for the building up of sophisticated biological structures. It is also used to support cellular transport, neural transmission and mobility, as well as growth, reproduction, defence and repair.
Formation of a chemical bond requires energy input, and some potential energy accumulates in the formed bond (see Table 2.2 ). This energy can be recovered when the bond is broken. An analogy is putting a bucket of water on a high shelf (inputting energy) and recovering the energy when the water is poured down to drive a wheel. The biologically important bonds that have a particularly high potential energy are the phosphoanhydride bonds formed between phosphate groups in molecules such as adenosine triphosphate (ATP).
Another mechanism of energy trapping is building up an ion gradient across a biological membrane. Energy input is required to create such a gradient; it is released when the ions ‘return’ across the barrier.
The content of free energy ( G ) changes during chemical reactions. We describe the energetics of reactions in relative terms, indicating free energy change (Δ G ), which is negative when the energy content decreases or positive when it increases. If a reaction releases energy, it is exergonic , and when it requires energy input to proceed, it is endergonic . The products of exergonic reactions have a lower potential energy than their substrates, and the products of endergonic reactions have a higher potential energy.
Reactions where products have less potential energy than the substrates tend to occur spontaneously, whereas those where the potential energy of substrates increases require energy input. The key concept in biochemistry is that the energetically favourable reactions are used to drive the unfavourable ones. Let us imagine two reactions:
A + B → X and (endergonic reaction)
X + Y → Z (exergonic reaction).
Reaction 1 is energetically unfavourable. It occurs only very slowly, yielding small amounts of the product X, according to its equilibrium. Reaction 2, on the other hand, proceeds spontaneously. As its substrate is X, it depletes it, shifting the equilibrium of reaction 1 and ‘forcing’ it to proceed. This is a common pattern in various metabolic pathways. Note that the hydrolysis of ATP has a highly negative G . Thus, ATP can drive the otherwise energetically unfavourable reactions.
Metabolic pathways are chains of chemical reactions, classed according to their purpose. The anabolic pathways use energy in order to build up large molecules and are usually associated with reductions; the catabolic pathways , on the other hand, break down large reduced precursor molecules. They are associated with oxidations.
Metabolism generates energy to support body functions, growth and regeneration. The required energy is acquired from nutrients. Cellular energy flow depends on biological oxidations. The overall oxidation process in living organisms is the multistep conversion of highly reduced organic compounds, in the presence of oxygen, to carbon dioxide and water. It is analogous to the combustion reaction, but in biology this ‘combustion’ proceeds by minute, carefully controlled, stages and under physiological conditions. The substrates for oxidation are highly reduced compounds, mainly carbohydrates and fats. We call them metabolic fuels ( Information box 2.3 ). A substantial part of the released energy is chemically trapped for subsequent use. The major stages in this process are as follows:
Ingestion of nutrients and their digestion in the gut, which results in the liberation of the molecules of metabolic fuels from more complex compounds. These are absorbed into plasma.
Conversion of the fuel molecules . Metabolic fuels are then oxidised. The electrons (and protons) are first transferred to intracellular coenzymes, such as NAD and NADP, forming the reduced NADH and NADPH, respectively (the NADPH is used in biosynthetic reactions). The NADH then carries the electrons, accompanied by protons, to the mitochondria and transfers them to the electron transport chain (ETC; Ch. 3 ).
In the ETC, electrons and protons are transferred along the chain, participating in the sequence of redox reactions involving ferric iron complexes, cytochromes and other electron carrier molecules. The final recipient of electrons is the oxygen atom, and a molecule of water is formed.
Metabolic fuels are highly reduced compounds that can be oxidised in the body to release energy. The main metabolic fuels are carbohydrates and fats. Among the carbohydrates, the most important fuel is glucose with the caloric value 16.7 kJ (4 kcal)/g. Under normal circumstances, glucose is the only fuel used by the brain. Red blood cells are also completely dependent on glucose for energy. Muscle preferentially uses glucose at the start of exercise, switching subsequently to the use of fatty acids. A limited amount of glucose, sufficient for approximately 12 hours, is stored in the body in the form of glycogen.
Fatty acids, which yield 37.7 kJ (9 kcal)/g, are the most efficient metabolic fuel. The amount of fat stored in the adipose tissue of a lean average human is sufficient for survival for more than 2 months without food.
Proteins can be converted to glucose when its supply is insufficient. Their caloric value is also 16.7 kJ (4 kcal)/g. The pathway of conversion of non-carbohydrate compounds to glucose is known as gluconeogenesis. The primary substrates for gluconeogenesis are the amino acid alanine, glycerol and lactate.
As the electrons proceed through the ETC, the ‘remaining behind’ protons are pumped out, creating a concentration gradient across the inner mitochondrial membrane. This an electrogenic pump system generates an electrochemical potential because it pumps ions across the membrane without the ion movement in the opposite direction. The formed gradient acquires potential energy. These protons are then returned to the mitochondrial matrix through a membrane channel, which constitutes part of the enzyme ATP synthase; as a result the released energy is trapped in high-energy bonds of the ATP, formed from ADP. The process is known as oxidative phosphorylation.
The body contains a vide variety of organic compounds. The skeleton contains substantial inorganic, mineral components. Carbon, oxygen, hydrogen and nitrogen make up 88.5% of the dry body mass. The most abundant mineral is calcium (4%), followed by phosphorus (2.5%), potassium, sulphur, sodium, chlorine and magnesium (0.1%). Other elements are present in much lesser amounts: iron, for instance, constitutes only 0.01%. The elements that are present in less than 0.01% are known as the trace elements (e.g. manganese or iodine). Despite their minute amounts, many perform important metabolic roles, particularly as prosthetic groups and cofactors of enzymes.
Sodium and potassium are fundamental for the maintenance of membrane potential and impulse transmission. Sodium and chloride also maintain osmolality and, therefore, fluid volume in the body compartments, including cell volume. Magnesium serves as a cofactor for multiple enzymes, including the ones participating in DNA replication and ATP synthesis. Calcium and phosphate are the main components of bone. Calcium is also fundamental for the transmission of impulses and cell signalling. Fluoride is present in bone and teeth.
Iron is a component of haem and, thus, of haemoglobin and myoglobin. Iodine is a component of thyroid hormones. Copper scavenges for free radicals, is associated with oxygenases and is important for collagen cross-linking. Zinc, like magnesium, is a cofactor for multiple enzymes and influences wound healing, tissue proliferation and growth, and immune function. Selenium is a cofactor for enzymes, including the ones participating in the metabolism of thyroid hormones (deiodinases). It also affects the immune function.
Water makes up approximately 60% of the lean body mass (see Chs 1 and 16 ). A 70-kg adult male has approximately 42 L total body water. Two-thirds of it is in the intracellular fluid (ICF) and one-third is in the extracellular fluid (ECF). ECF is further compartmentalised into the interstitial fluid, plasma and transcellular fluids, such as lymph and cerebrospinal fluid (CSF) ( Table 2.3 ). The main cations, i.e. sodium and potassium, are differentially distributed across the plasma membrane, with higher concentrations of sodium in the ECF and higher concentration of potassium in the ICF. ICF also has a higher concentration of proteins and phosphates than ECF. The transfer of water, ions and metabolites across cell membranes is fundamental to maintaining body functions.
| Ion | Approximate extracellular concentration (mmol/L) | Approximate intracellular concentration (mmol/L) |
|---|---|---|
| Na + | 140 | 12 |
| K + | 4 | 140 |
| Cl - | 110 | 4 |
| Bicarbonate, HCO 3 − | 25 | 12 |
| Phosphates | 2 | 13 |
| Protein anions | 9 | 138 |
| Ca 2+ | 2.4 | <0.0002 |
| Mg 2+ | 1.0 | 0.8 |
The concentration gradient of sodium and potassium ions across the cell membrane is the key driver of cellular transport systems. The distribution of sodium and potassium ions across the cell membrane creates an electrical potential known as the resting membrane potential . In most cells, it is of the order of −60 mV, the inside being negative ( Ch. 8 ). Changes in the electrical potential underlie the electrical signals generated by excitable cells and nerve impulses ( Clinical box 2.1 , see also Information box 2.4 ).
Changes in the extracellular K + concentration alter the resting membrane potential of cardiac myocytes. This changes their excitability. Normal extracellular potassium concentration is 3.5–5.0 mmol/L, but if levels fall below this, the myocytes become hyperpolarised and cardiac excitation is reduced. On the other hand, if the K + concentration rises above 5.5 mmol/L, cardiac excitation increases. I both situations there is a potential risk of arrhythmias and cardiac arrest ( Ch. 11 ).
The water and electrolyte balance of a patient is assessed by recording the patient’s intake and output of fluids (the record includes the amount drunk, the volume of given intravenous solutions, the urine volume, the volumes of fluids obtained during surgical drainage, etc.).
This is complemented by measurements of the ionic balance of the plasma. The set of common measurements, known in hospital jargon ‘urea and electrolytes’, includes measurements of sodium and potassium as the main cations, and chloride and bicarbonate as the main anions. The measurements of plasma urea and creatinine are normally included because they reflect kidney function – the most important determinant of the ionic balance.
Normally, the sum of sodium and potassium is greater than the sum of bicarbonate and chloride. The difference, which is normally approximately 10 mmol/L, is known as the anion gap (AG):
The anion gap includes anions that are not routinely measured, such as lactate or ketones. The gap can increase substantially when these anions accumulate (e.g. ketones in poorly controlled diabetes) and, thus, is of diagnostic significance for the physician.
Another ionic concentration gradient fundamental for cell function is the gradient of calcium ion (Ca 2+ ) concentration. Calcium concentration outside the cell is approximately 10,000 higher than that inside. Therefore, a cell can be flooded with calcium very quickly. After an increase in concentration, calcium can be compartmentalised within the cell, for instance, in the endoplasmic reticulum. This decreases its concentration in the cytoplasm. Sudden calcium ion concentration changes are very important for intracellular signalling and are essential, for muscle contraction (see Ch. 9 ), neurotransmission and most secretory processes. For details of laboratory assessment of water and electrolyte balance see Information box 2.4 .
Vitamins are essential organic nutrients. They are structurally diverse and are different from the main nutrient groups. They are only required in very small amounts. Vitamins act as coenzymes, and some as signalling molecules. Most vitamin deficiencies lead to specific diseases. Vitamin A and vitamin D are also toxic in excess.
Vitamins are classified into fat soluble (A, D, E and K) and water soluble (vitamin B, folate, biotin and vitamin C).
Vitamin D and vitamin A (retinol) act in a steroid hormone-like way through intracellular receptors. Vitamin D (calciol) plays a crucial role in bone metabolism. Vitamin D can be synthesised in the skin, and vitamin A is key to the process of vision and also affects cell growth and proliferation. Vitamin E is an important antioxidant, and vitamin K (phylloquinone) participates in the clotting of blood and is required for the synthesis of prothrombin and several other coagulation factors.
The B vitamins serve predominantly as coenzymes. Vitamin B 1 (thiamine) is important for carbohydrate metabolism, and B 2 (riboflavin) participates in FAD synthesis. Vitamin B 3 (niacin) is required for the synthesis of NAD. Vitamins B 6 (pyridoxine) and B 7 (biotin) participate as coenzymes in carbohydrate, lipid and amino acid metabolism. Biotin is also required for the synthesis of some neurotransmitters.
Vitamin B 9 (folic acid) participates in the so-called single-carbon transfer reactions and is necessary for the synthesis of purines and pyrimidines, and, thus, of nucleic acids. Vitamin B 12 is part of the haem structure. Panthotenic acid is important for the synthesis of coenzyme A (CoA). Vitamin C is a reducing agent and is necessary for the synthesis of collagen.
The main classes of organic biomolecules are carbohydrates, fats and proteins, and also nucleotides and nucleic acids. They may combine in forming ‘hybrid’ molecules such as glycoproteins or glycolipids.
Carbohydrates and fats are the main energy substrates. Lipids are also a major structural component of cell membranes.
Proteins are required to maintain body structure and functions, but can be transformed into fuel when there is a large demand for energy and/or when the supply of carbohydrates is poor. Proteins in the form of enzymes, signalling molecules and antibodies underpin most body functions. They are synthesised, maintained and regulated according to the genetic information contained in the DNA, which itself is a molecule containing amino acid–derived structures (the purine and pyrimidine bases), carbohydrates and phosphate.
Amino acids, the basic units of proteins, and lipid molecules such as cholesterol, also serve as precursors of a wide range of biologically important molecules, including hormones and neurotransmitters.
Carbohydrates form only approximately 2% of the body mass. They consist of carbon, oxygen and hydrogen, with a ratio of hydrogen to oxygen of approximately 2 : 1. Carbohydrates have the general formula (CH 2 O) n , where n is the number of carbon atoms. They are single molecules (simple sugars, monosaccharides , or their polymers, polysaccharides ), which may contain thousands of units.
Monosaccharides are present in the diet or may be obtained by digestion of more complex carbohydrates. Because they can also be synthesised in the body from non-carbohydrate sources, their external supply is not required for survival. The simplest monosaccharides are the three-carbon sugars (trioses; Fig 2.6 ). Those that contain an aldehyde group are known as aldoses , and those with a ketone group in their structure are called ketoses (see Fig. 2.6 ).
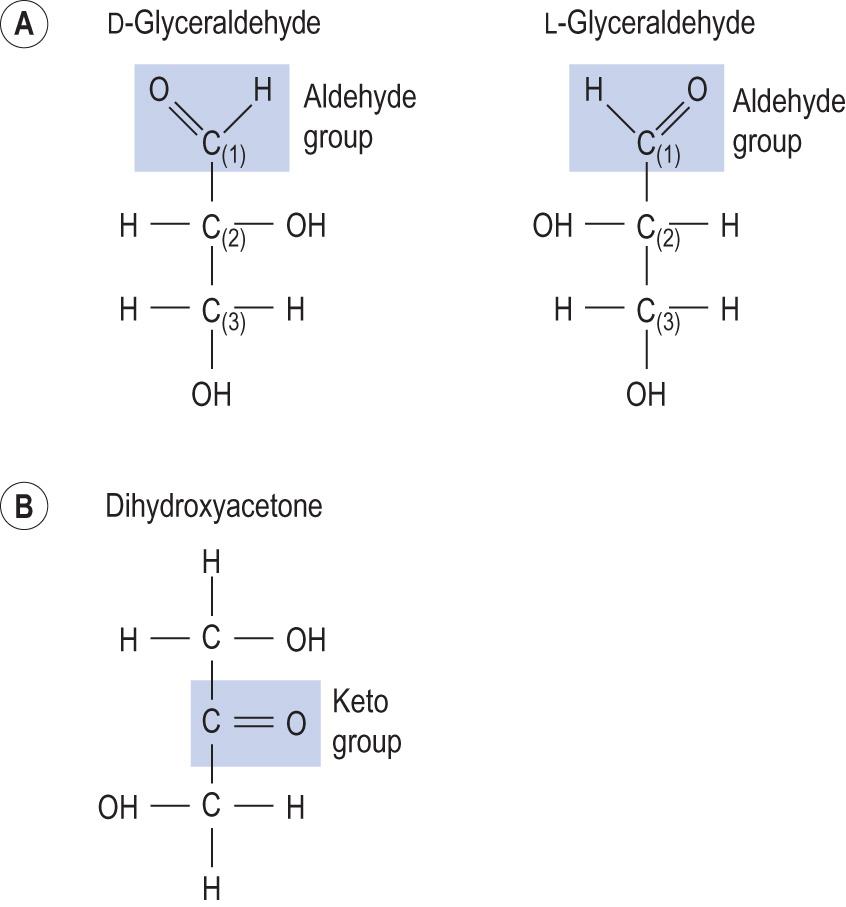
Like the other types of organic molecules, carbohydrates exhibit isomerism , i.e. variability of structure with the same atomic content. For instance, in glyceraldehyde, which is a triose, the central carbon atom is chiral and therefore it can exist as two (D- and L-) stereoisomers. All sugars that have chiral groups are divided into the D- and L-series, according to the isomer of glyceraldehyde from which their asymmetrical carbon farthest from C1 derives, Virtually all sugars found in biological systems are D-isomers. There is also optical isomerism, which makes the molecules rotate polarised light to the right (dextrorotatory; d/D) or to the left (laevorotatory; l/L).
Two important pentoses are ribose and deoxyribose , the components of the nucleic acids RNA and DNA, respectively.
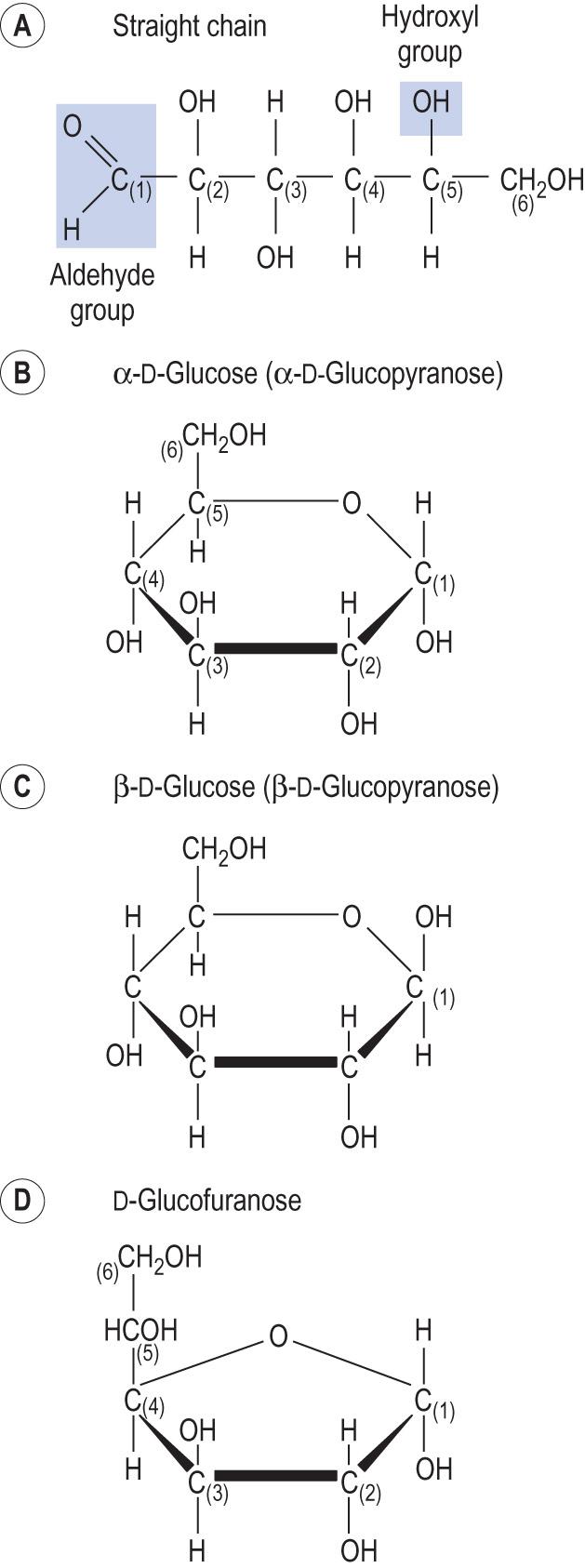
The most important hexose is glucose, C 6 H 12 O 6 . Glucose is the central molecule in human energy metabolism. In an aqueous solution, it can form ( Fig. 2.7 ) both straight chain (less than 1% of all glucose molecules) and ring structures. Furthermore, the so-called anomeric carbon in ring structures forms α- or β-glucose anomers that differ by the arrangement of the hydrogen atoms and hydroxyl (−OH) groups on C1.
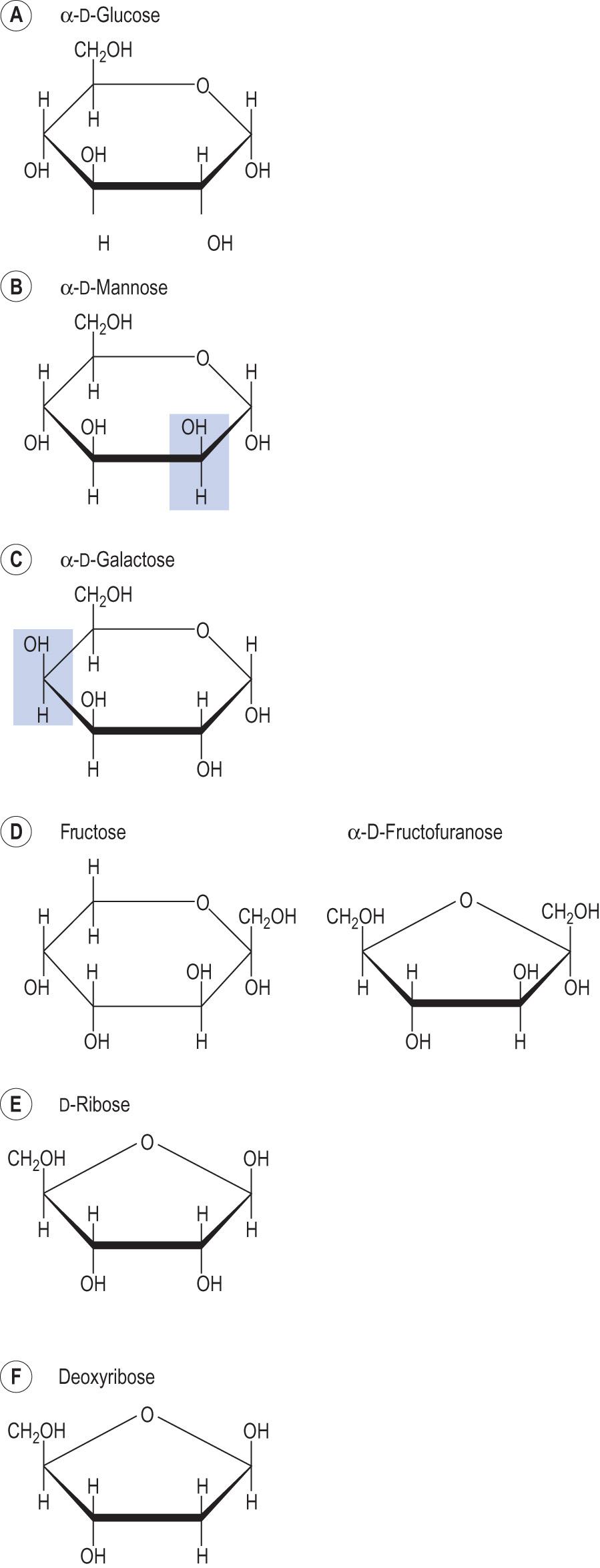
Other common hexoses are mannose, galactose and fructose ( Fig. 2.8 ) . Mannose and galactose only differ from glucose in the configuration of one of the carbons (mannose around C2 and galactose around C4) and are called epimers .
Monosaccharide molecules can be modified by other chemical groups. An introduction of the amino group on C2 of glucose and galactose yields the amino sugars glucosamine and galactosamine , respectively. The insertion of carboxyl groups creates glucuronic and sialic acids. The sulphate group is a component of the proteoglycans, which are key substances present in the extracellular matrix (ECM).
Disaccharides form from two either identical or different monosaccharide units linked together by a glycosidic bond . These bonds link C1 of one sugar and the hydroxyl group of another. They are either α or β linkages, depending on the orientation around C1 ( Table 2.4 ). Sucrose, the ordinary sugar, is a disaccharide made up of α-glucose and β-fructose linked by an α1→2 linkage. Two other dietary disaccharides are maltose , a dimer of glucose molecules, and lactose (milk sugar), made from glucose and galactose.
| Disaccharide | Carbon-1 sugar | Other sugar | Linkage | Digesting enzyme |
|---|---|---|---|---|
| Sucrose | α-Glucose | β-Fructose | 1→2 | Sucrase-isomaltase |
| Lactose | β-Galactose | β-Glucose | 1→4 | Lactase |
| Maltose | α-Glucose | β-Glucose | 1→4 | Maltase |
| Isomaltose | α-Glucose | β-Glucose | 1→6 | Sucrase-isomaltase |
Starches , the polymers of glucose, serve as storage carbohydrates in plants. The simplest, amylose , has long linear glucose chains linked by α1→4 bonds. Amylopectin , which makes up approximately 80% of the starch found in foods, has 1→4 linked glucose chains, which branch by forming 1→6 bonds. The starches found in grains such as wheat, rice and potatoes are the major source of dietary carbohydrates. Amylases secreted by the salivary glands and the pancreas digest them, releasing glucose, maltose and isomaltose.
Cellulose is an unbranched glucose polymer linked by β1→4 bonds. It is the principal component of plant cell walls and is the most common organic compound on the planet. Humans have no enzymes capable of hydrolysing these bonds. Cellulose and other non-metabolised polysaccharides form dietary fibre .
In human tissues, the carbohydrate storage molecule is glycogen . It is a large, branched polymer of glucose. The high level of glycogen branching means that the molecule has a large number of free ends. This facilitates its rapid degradation to glucose.
Glycogen stores glucose in a highly concentrated form, without the osmotic problems (attraction of large amounts of water, and thus increased volume) associated with a large number of free glucose molecules. However, the size of glycogen stores is modest in humans, with approximately 75 g present in the liver and 250 g in skeletal muscle. This can supply glucose for 12–18 hours, after which time the glucose needs to be synthesised from non-carbohydrate sources, such as amino acids, through gluconeogenesis .
Complex carbohydrates are carbohydrates covalently linked to proteins or lipids.
Thus, glycoproteins contain carbohydrate chains linked to serine and threonine residues of proteins. Their carbohydrate content is 10%–15%. The carbohydrates involved are most commonly mannose, galactose, fucose, xylose, amino sugars such as N -acetylglucosamine and N -acetylgalactosamine, and glucuronic and sialic acids. Carbohydrate chains in the glycoproteins are relatively short, containing 10–15 molecules, and often look like two- or three-pronged forks. They form highly variable structures important for cell recognition and immune recognition: such as, a range of membrane receptors. Together with glycolipids (see below), they constitute blood group antigens on the surface of red cells, the most important being the ABO blood group system .
The formation of a bond between the hydroxyl group of the amino acid side chain and C-1 of N -acetylgalactosamine is known as O-glycosylation , and the linkage between the amide group of the amino acid asparagine and C-1 of N -acetylglucosamine is known as N-glycosylation .
Complex carbohydrates linked to proteins, the proteoglycans , are the key components of the ECM. They typically contain a large proportion (95%) of carbohydrate. The carbohydrates are attached to a polypeptide chain, forming long, linear chains. These molecules are known as glycosaminoglycans (GAGs) or mucopolysaccharides . Many GAGs are negatively charged and thus attract cations and large amounts of water, forming a gel-like substance. Thus, they impart a degree of flexibility to the tissue and act as shock absorbers.
Lipids make up approximately 20% of body mass in adults of normal weight, more in women. They play a number of essential roles:
They are the key components of cell membranes.
They are the major form of energy storage.
They play important roles in cell signalling.
Fatty acids are hydrocarbon chains ( Fig. 2.9A, B ) , with a carboxy group at one end (the α-carbon) and a methyl group (CH 3 ) at the other (the ω-carbon):
S aturated fatty acids have all the carbon atoms in the chain linked by single bonds.
Monounsaturated fatty acids possess one double bond.
Polyunsaturated fatty acids have more than one double bond.
Many of the fatty acids found in cells have 16, 18 or 20 carbon atoms and up to 3 double bonds. Most of the double bonds in biological molecules are in the cis configuration. Such a configuration puts a kink in the hydrocarbon chain. The most common fatty acid is the C18 palmitic acid ( Information box 2.5 ). Some fatty acids have one of the double bonds placed three carbons from the ω-carbon (they are known as the ω-3 fatty acids). An example is the eicosapentaenoic acid, the precursor of some prostaglandins.
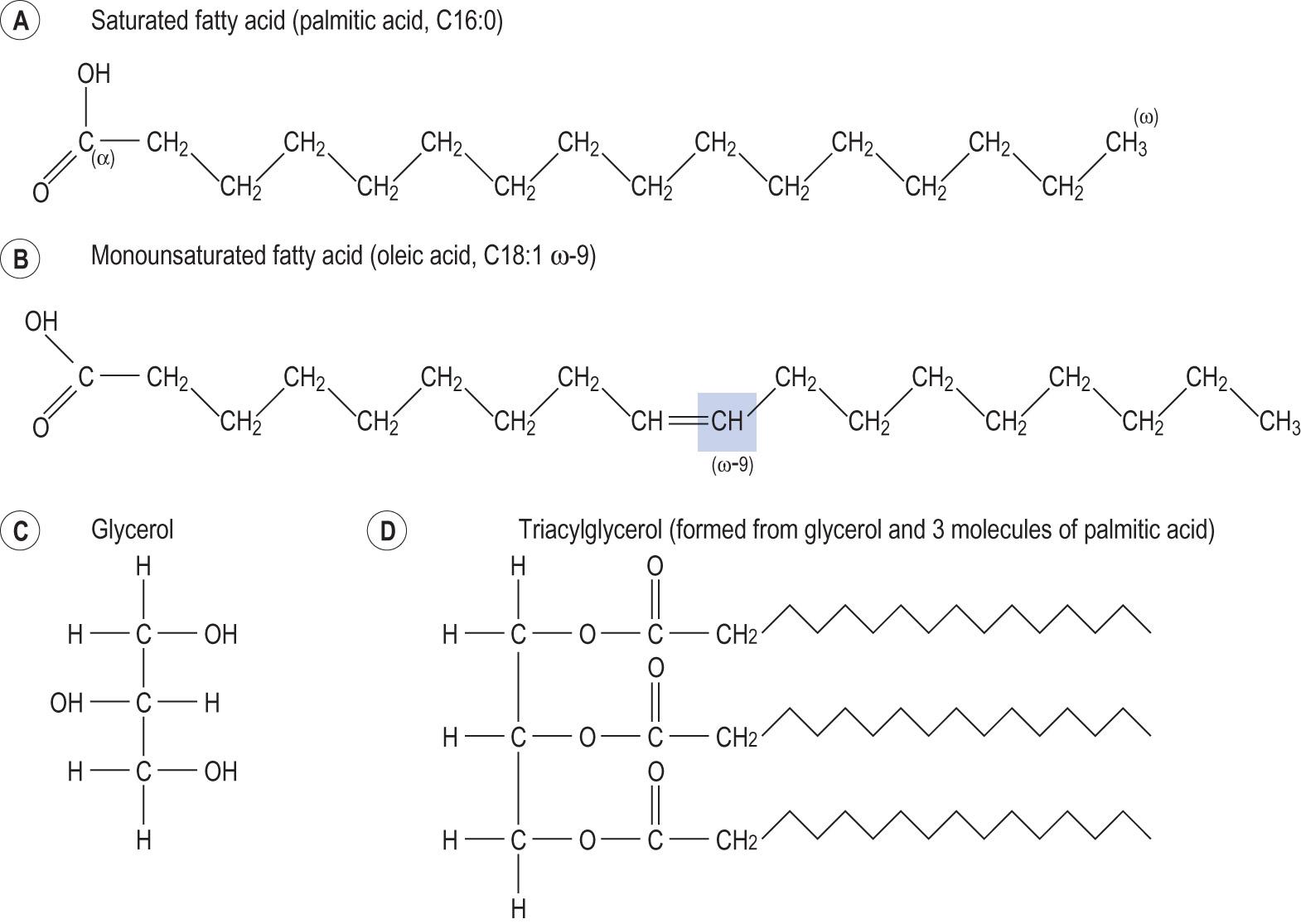
Fatty acids can be classified according to the number of carbon atoms in the chain and the number and position of the double bonds.
A notation that is widely used gives the number of carbon atoms, the number of double bonds and the position of the first double bond, counting from the ω-carbon. For example, the saturated fatty acid found in palm oil is called palmitic acid. The formula for palmitic acid is CH 3 (CH 2 ) 14 COOH, its chemical name is n -hexadecanoic acid and the shorthand notation is C16:0, indicating that it has 16 carbon atoms and no double bonds.
For arachidonic acid, the cis- 5,8,11,14-eicosatetraenoic acid, the shorthand notation is C20:4 ω-6. The positions of all of the double bonds are noted as C20:4 all cis- Δ 5 ,Δ 8 ,Δ 11 ,Δ 14 (see Fig. 2.7 ).
The ω-3 fatty acids are found in fish oil. Their ingestion confers some cardiac benefits; for instance, it prevents some disturbances of the heart rate (arrythmias).
Triacylglycerols (also called triglycerides ) are esters of glycerol and fatty acids and are the storage form of lipids. Glycerol is a C3 sugar alcohol, formed by reduction of the aldehyde group of a triose to the hydroxyl group ( Fig. 2.9C ) . Triacylglycerols are formed by dehydration reactions between the carboxyl group (COOH) of a fatty acid at each of the three hydroxyl (OH) groups of the glycerol molecule (see Fig. 2.9D ). Triacylglycerols are stored in the adipose tissue as subcutaneous fat and as visceral fat, which surrounds the abdominal organs.
Triacylglycerols are the main components of solid and liquid dietary fats. Fatty acids that make up the triacylglycerol molecules determine the physical properties of a particular fat. Triacylglycerols composed of short-chain fatty acids, or of unsaturated fatty acids, are liquid at room temperature. Examples of these are olive oil (containing oleic acid) and sunflower oil (containing polyunsaturated fatty acids). Triacylglycerols that contain more saturated fats and longer fatty acid chains are fats solid at room temperature (e.g. butter).
Most of the fatty acids used in the body are supplied in the diet. Saturated fatty acids can be synthesised from carbohydrates ( Ch. 3 ). They can be converted to unsaturated fatty acids. However, linoleic acid (ω-6 fatty acid C18:2; cis , cis 9,12) and linolenic acid (ω-3 C18:3; cis , cis , cis 9,12,15) are essential fatty acids . They cannot be manufactured in the body because none of the human enzymes can insert double bonds beyond C-10 in a fatty acid molecule.
Eicosanoids are derived from C20 fatty acids with between three and five double bonds. Arachidonic acid , synthesised from linoleic acid, is the precursor of a large number of eicosanoids. It can be released from the membrane by the action of the enzyme phospholipase A 2 (PLA 2 ) on phosphatidylcholine, one of the membrane phospholipids. Different groups of eicosanoids include prostaglandins, thromboxanes and leukotrienes. They are locally acting hormones with a very short half-life. They are important in the response to inflammation and in the control of vascular smooth muscle contraction ( Information box 2.6 ).
Prostacyclin released from the blood vessel wall is a vasodilator and thus encourages blood flow. It also inhibits the aggregation of blood platelets, preventing clot formation.
Conversely, thromboxane A 2 acts as a vasoconstrictor, thus reducing flow (and thus blood loss from a damaged vessel), and stimulates platelets to aggregate, facilitating coagulation.
The enzyme that acts on arachidonic acid in the first step in the synthesis of prostanoids is cyclo-oxygenase (COX). Aspirin (acetylsalicylic acid) belongs to a group of drugs called non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit COX and reduce inflammation and pain. Aspirin is also used in cardiovascular prevention, to inhibit blood clotting.
The cholesterol molecule has a four-ring structure known as the cholestane structure ( Fig. 2.10 ) . There is a hydroxyl group attached to ring number 1, and it can interact with water. Cholesterol is the major sterol in animals, whereas plants produce related sterols such as sitosterol and campesterol. Cholesterol is an essential structural component of the cell membranes. It decreases the fluidity of cell membranes. It is also an important precursor of a range of biologically important substances: bile salts, vitamin D and steroid hormones.
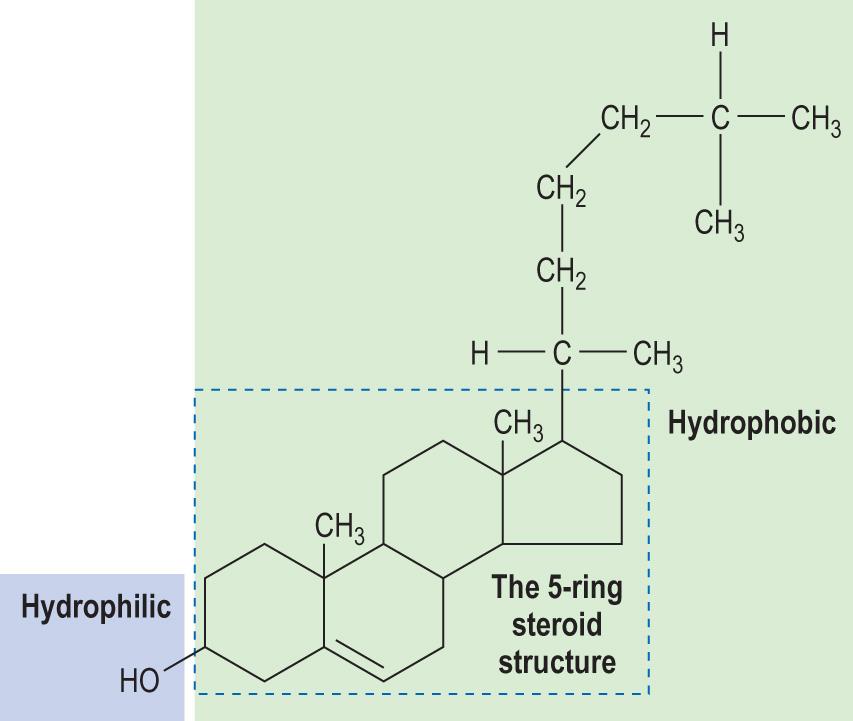
Most cells are capable of synthesising cholesterol. It can also be absorbed from the diet. It is distributed to tissues by lipid-transporting particles known as lipoproteins.
Cells regulate their cholesterol supply, balancing the rate of synthesis against the external provision. The key regulatory points are the enzyme HMG-CoA reductase (a key enzyme in cholesterol synthesis and a target of statin drugs) and the LDL receptor (which controls LDL, and thus cholesterol, uptake from plasma). Dysregulation of cholesterol balance leads to its high concentrations in plasma and contributes to the formation of atherosclerotic plaques ( Clinical box 2.2 ).
There is a link between raised plasma cholesterol or low-density lipoprotein (LDL-cholesterol; Ch. 16 ) and the increased risk of coronary events such as heart attacks. It is beneficial to reduce plasma cholesterol levels, particularly in persons who already suffer from heart disease. Diets rich in polyunsaturated fats reduce serum cholesterol to some extent. To obtain greater reductions, one needs to use the statins, drugs that inhibit the regulatory enzyme in cholesterol synthesis pathway, the 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase).
The human body cannot metabolise cholesterol. It is excreted by the liver in bile.
Bile acids emulsify fats in the intestine and thus are essential for their absorption and digestion. They are synthesised in the liver from cholesterol and are secreted as conjugates with the amino acid taurine or glycine. They are stored in the gallbladder and are eventually secreted into the duodenum ( Ch. 15 ). The secreted bile acids (known as the primary bile acids ) are further modified by the intestinal bacteria, yielding the secondary bile acids . Bile acids are conserved through reabsorption in the large intestine. This recirculation process is known as the enterohepatic circulation .
Steroid hormones are all derived from cholesterol after its conversion to another key metabolite, pregnenolone. The subsequent reactions in the pathway yield glucocorticoids such as cortisol, mineralocorticoids , such as aldosterone, and oestrogens and androgens , including progesterone, the hormone that is important in maintaining pregnancy (see also Ch. 10 ).
Steroids are lipid soluble; they cross the plasma membranes and bind to cytoplasmic receptors. They are usually carried in the plasma bound to specific binding proteins ( Table 2.5 ).
| Protein | Ligand |
|---|---|
| Albumin | Non-specific transport protein: metal cations, free fatty acids, steroids, bilirubin, haem, therapeutic drugs |
| Transferrin | Iron |
| Thyroid-binding globulin | Thyroxine (T 4 ), tri-iodothyronine (T 3 ) |
| Cortisol-binding globulin | Cortisol |
| Sex-hormone-binding globulin | Androgens and oestrogens |
Vitamin D is a hormone with action mediated through the intracellular receptor. It is essential for calcium and bone metabolism. Its precursor (cholecalciferol; calciol) is synthesised from 7-dehydrocholesterol in the skin under the influence of ultraviolet light. It then undergoes two hydroxylation reactions. The first one, in the liver, yields 25-hydroxycalciferol (calcidiol), and the second one, in the kidney, generates the active form, 1,25-dihydroxycholecalciferol (calcitriol). Vitamin D facilitates the absorption of calcium in the intestine and its reabsorption in the kidney, and stimulates bone resorption by increasing the number of osteoclasts ( Ch. 16 ).
Complex lipids are lipid molecules with incorporated non-lipid (particularly carbohydrate) components. Their lipid component is hydrophobic, whereas the non-lipid part is often hydrophilic. Therefore, these molecules are amphipathic (both hydrophilic and hydrophobic) and orient themselves at the lipid/water interfaces. They are important components of cell membranes. Their hydrophilic part faces the ‘outside’ of the membrane, and the hydrophobic part orients towards the membrane core. Complex lipids also play a major role as components of the ECM.
Glycerophospholipids are formed from phosphatidic acid, a molecule where glycerol phosphate is esterified with two acyl residues.
Choline linked to the phosphate group of the phosphatidic acid produces lecithin , another cell membrane phospholipid. Instead of choline, serine or ethanolamine or inositol can also be linked to the phosphate group. The structure of phosphatidylserine is shown in Fig. 2.11A . The hydrophilic groups of phospholipids are negatively charged and, thus, the overall surface charge of the membrane is negative.
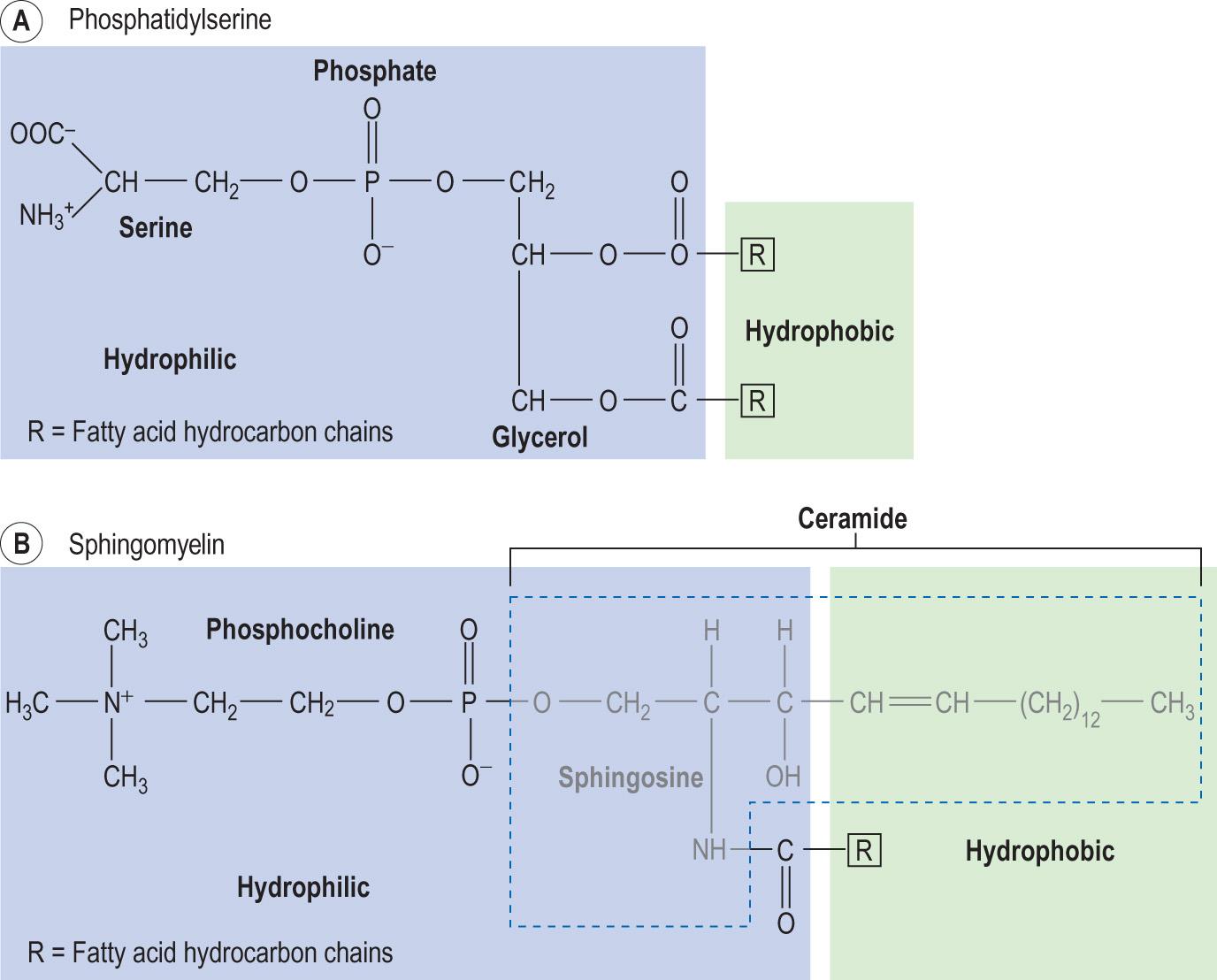
Sphingolipids are structurally similar to phospholipids, but instead of glycerol they contain the C18 sphingosine (an alcohol formed from palmitic acid and serine) (see Fig. 2.11B ). A molecule consisting of sphingosine linked to a fatty acid (acylsphingosine) is known as a ceramide . In turn, when ceramide is linked to a choline, it forms sphingomyelin . When a ceramide binds a sugar, it forms a cerebroside , and when it binds sialic acid, it forms a ganglioside .
Sphingomyelin, cerebrosides and gangliosides are components of cell membranes abundant in nerve tissues and the brain. Defects in the degradation of cerebrosides and gangliosides result in several rare clinical disorders known as lysosomal storage diseases .
Sphingolipids also contribute to the structure of blood group substances. Phosphorylated sphingosine is a signalling molecule.
Purines and pyrimidines contain nitrogen in their carbon rings. They also have basic amino groups (hence the alternative name ‘nitrogenous bases’). They are components of nucleotides and nucleic acids ( Fig. 2.12 ) .
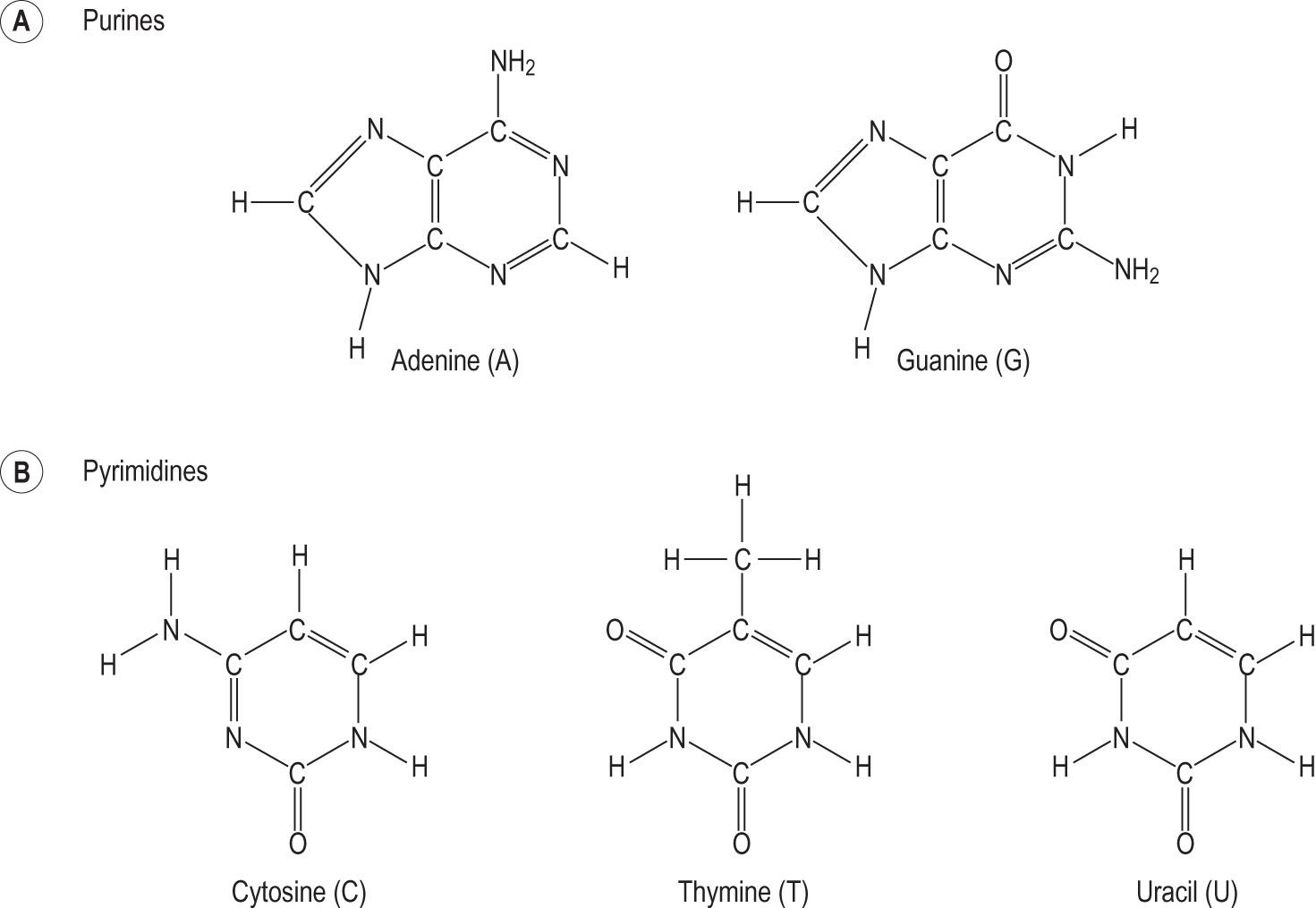
Purines are two-ring structures. The major purines are adenine and guanine . Pyrimidines consist of a single ring, and the major pyrimidines are cytosine, thymine and uracil .
Nucleosides are molecules in which a sugar ( ribose or deoxyribose ) phosphate is linked to a purine or a pyrimidine. Nucleotides are phosphorylated nucleosides ( Fig. 2.13 , Fig. 2.14 and Table 2.6 ). There could be one, two or three phosphates attached to C5 of the sugar, each via phosphoanhydride bonds, forming nucleotide mono-, bi- or tri-phosphates, respectively. Nucleotides are the building blocks that form nucleic acids. In addition, free nucleotides play important roles in energy transfer and cell signalling. Purines are components of nucleotide coenzymes such as ATP and GTP ( Fig. 2.14 and Information box 2.7 ). Cyclic nucleotides derived from them, also containing purines, are cyclic adenine monophosphate ( cAMP ) and cyclic guanosine monophosphate ( cGMP ), both being fundamental for signal transduction ( Table 2.6 ). Other key coenzymes, such as NAD , NADP , FAD and coenzyme A , are all derived from adenine nucleotide.
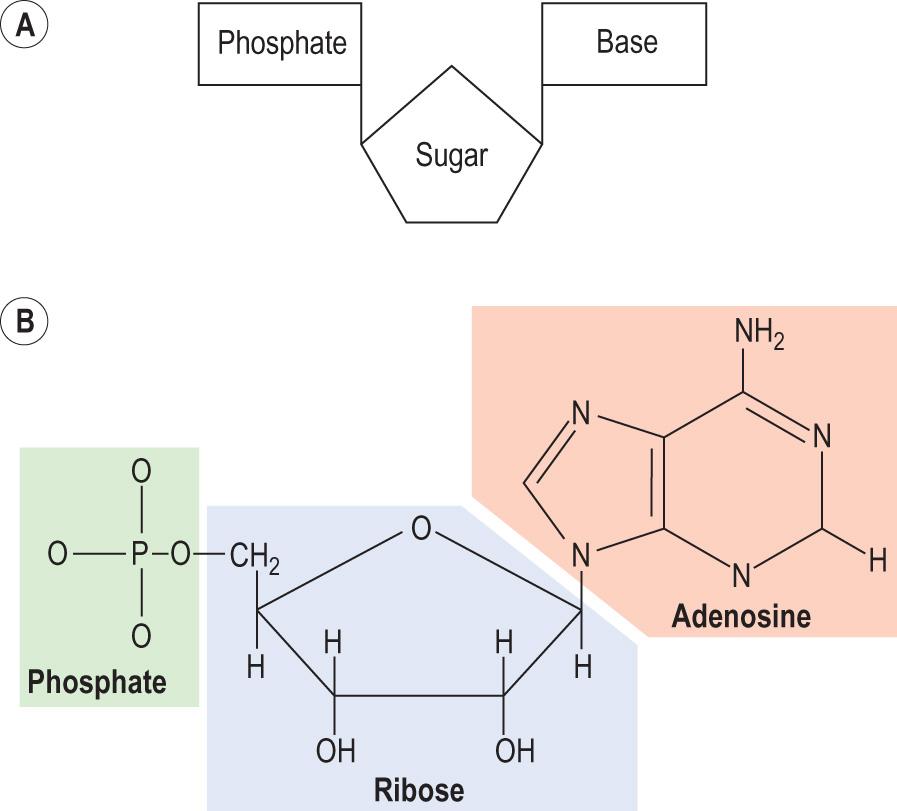
| Base | Nucleoside | Nucleoside monophosphates | Nucleoside diphosphates | Nucleoside triphosphates | Cyclic nucleotides |
|---|---|---|---|---|---|
| Adenine | Adenosine | AMP | ADP | ATP | cAMP |
| Guanine | Guanosine | GMP | GDP | GTP | cGMP |
| Cytosine | Cytidine | CMP | CDP | CTP | |
| Uracil | Uridine | UMP | UDP | UTP | |
| Thymine | Thymidine | TMP | TDP | TTP |
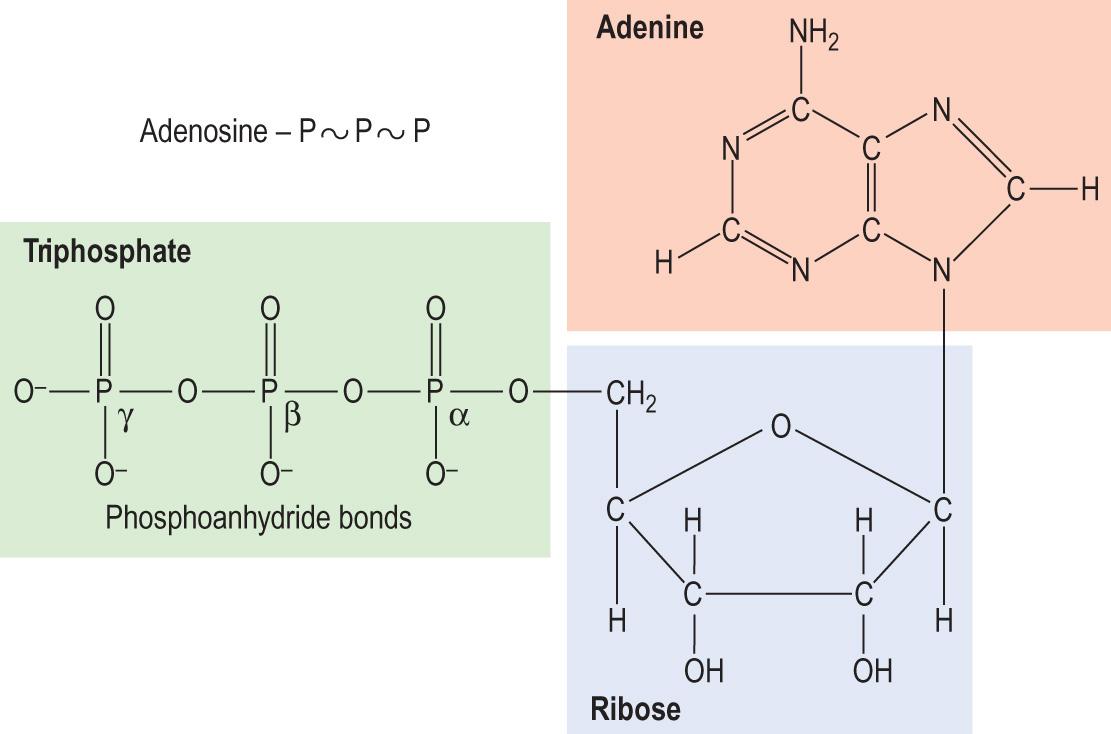
Adenosine triphosphate or ATP ( Fig. 2.14 ) is a highly mobile cellular energy store. It also participates in many metabolic regulatory loops, controlling the activity of key enzymes.
The phosphoanhydride bonds between the second (β) and the third (γ) phosphate group of the ATP release energy on hydrolysis, yielding about 7.3 kcal/mol each. These bonds are usually depicted by ~, indicating that they are high-energy bonds.
Most of the ATP in the cell is formed during oxidative phosphorylation in the mitochondrial electron transfer chain (ETC). A reaction that results in ATP synthesis by the transfer of a phosphate group from another phosphorylated compound outside the ETC is known as substrate-level phosphorylation.
The key intermediate in purine synthesis is 5-phosphoribosyl pyrophosphate (PRPP), a derivative of ribose supplied by the pentose phosphate pathway, a pathway that branches off from glycolysis. Reactions in the synthetic pathway require the amino acids glutamine, aspartate and glycine; CO 2 ; and the coenzyme tetrahydrofolate, which transfers single-carbon residues between molecules. The product is the nucleotide inosine monophosphate (IMP), the precursor of purines.
The precursor of pyrimidines is a 1-carbon molecule, carbamoyl phosphate. The synthetic pathway involves the amino acids glutamine and aspartate, and also the bicarbonate anion. The pathway yields uridine monophosphate (UMP), from which other pyrimidines are formed.
Ribonucleotides are reduced to deoxyribonucleotides by nucleotide reductase, producing purine and pyrimidine nucleotides that are used in DNA synthesis. Folic acid (a B vitamin) is required for the synthesis of the deoxyribonucleotide TMP (a thymidine nucleotide) from deoxyuridine monophosphate (dUMP) ( Information box 2.8 ).
One of the ways of treating cancer is by blocking the rapid proliferation of the cancer cells. Many of the side-effects of anti-cancer drugs can be accounted for by their effect on cells that are normally rapidly dividing, such as hair and intestinal cells.
A commonly used anti-cancer drug, methotrexate, acts by inhibiting an enzyme involved in the production of thymidine nucleotides. Methotrexate is an analogue of dihydrofolate, which acts as a competitive inhibitor of the enzyme dihydrofolate reductase. Other cytotoxic agents act by inhibiting other points in the production of nucleic acids.
Purines are degraded to uric acid, and pyrimidines to a Krebs cycle metabolite, succinyl-CoA.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here