Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bile is a complex aqueous secretion that originates from hepatocytes and is modified distally by the biliary epithelium. As a basic “humor” in the body, the significance of bile had been recognized since antiquity. However, our understanding of bile was originally restricted to knowledge of its composition, and the mechanism of bile formation remained elusive until the mid-20th century with the advent of techniques to perfuse isolated livers and to study isolated hepatocytes. The concept that bile cycles between the liver and the gut, a “motus circularis bilis,” dates back more than 300 years to the work of Mauritius van Reverhorstand the elegant kinetic modeling by the Neapolitan mathematician, Giovanni Borelli. As the major biliary solute and driving force for bile flow, much attention has been focused on the mechanisms responsible for bile acid biosynthesis and enterohepatic cycling, and the relationship of those mechanisms to hepatic and gastrointestinal physiology.
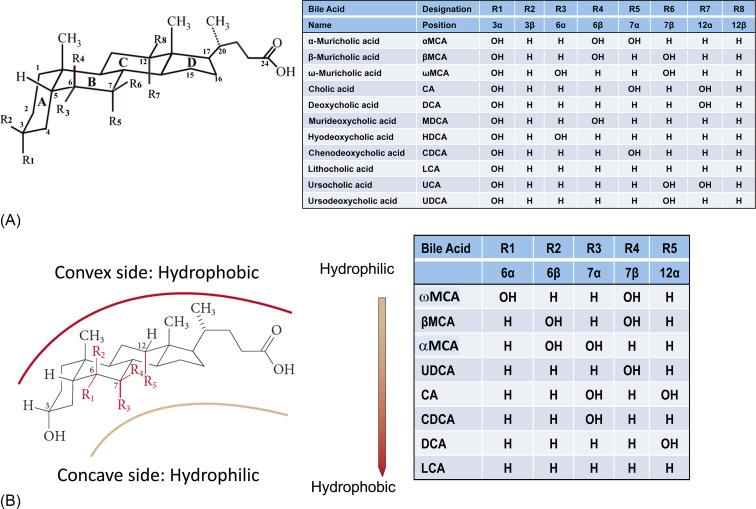
Bile acids are planar amphipathic molecules possessing a characteristic four-ring, 19-carbon perhydrocyclopentanophenanthrene nucleus and a multicarbon side chain. In all the vertebrates examined, cholesterol serves as the precursor for bile acid biosynthesis, whereby a water- insoluble, hydrophobic membrane lipid is converted into water-soluble derivatives that can be excreted in bile. As a group, these molecules are termed “bile salts” or “bile acids,” and are included in the ST04 category (sterol lipids: Bile acids and derivatives) ( http://www.lipidmaps.org ) of the LIPID MAPS Lipid Classification System. Most bile acids can be assigned to three general structural classes according to the length of the side chain and functionality of the terminal polar group. The three classes are 27-carbon (C27) bile alcohols, C27 bile acids, and 24-carbon (C24) bile acids, with C24 bile acids being the predominant form in mammals. Bile acids are not known to be made by invertebrates. In vertebrates, bile acids show a remarkable diversity in their chemical structures across the species, with modification to both the C19 steroid nucleus and the side chain. This large diversity is thought to be unique among classes of small molecule endobiotics, however, the evolutionary forces driving the variation remain poorly understood. In vivo, bile acids exist primarily as sulfate conjugates of bile alcohols and as taurine (or glycine) aminoacyl-amidated conjugates of bile acids. The general structure of the steroid nucleus and side chain, position of hydroxyl groups, and hydrophobicity for the major mammalian bile acid species are shown in Fig. 41.1 .
Bile formation and secretion is essential for life and fulfills a number of important functions in vertebrates. (1) Bile secretion is a major route for excretion of heavy metals, lipophilic endogenous compounds (endobiotics) such as bilirubin, cholesterol and steroids, and lipophilic exogenous compounds (xenobiotics) such as drugs, drug metabolites, and environmental toxins. (2) Bile is a critical digestive secretion and works in concert with saliva, gastric, and pancreatic secretions to facilitate the breakdown and assimilation of food. (3) Bile secretion plays a role in innate immunity and controlling intestinal microbes by serving as a conduit for the release of IgA antibodies. (4) Bile secretion is the vehicle for the excretion of bile acids, the major organic solutes in bile. As described in the next section, bile acids perform a variety of indispensable functions in the liver and gastrointestinal tract.
Although best known for their ability to form micelles and facilitate absorption of lipids in the gut, the physiological functions of bile acids extend well beyond their role as simple detergents. The recognized functions ascribed to bile acids in the liver and gastrointestinal tract are summarized in Table 41.1 .
| Tissue | Function |
|---|---|
| Whole body |
|
| Liver—hepatocyte |
|
| Liver—endothelial cell |
|
| Biliary tract—lumen |
|
| Biliary tract—cholangiocytes |
|
| Gallbladder epithelium |
|
| Small intestine—lumen |
|
| Small intestine—ileal enterocyte |
|
| Small intestine—other effects |
|
| Large intestine—colonic enterocyte |
|
| Large intestine—other effects |
|
| Gut microbiota |
|
The major functions of bile acids include: (1) Inducing bile flow and hepatic secretion of biliary lipids (phospholipid and cholesterol). The active vectorial movement of bile acids from blood to the bile canaliculus creates an osmotic gradient, allowing water and small solutes to enter the biliary space by solvent drag. This is a major driving force for bile formation. (2) Digestion and absorption of dietary fats such as long-chain fatty acids, cholesterol, and fat-soluble vitamins. Bile acids form mix micelles with lipids and lipid digestion products to increase their aqueous solubility in the gut lumen, thereby enhancing their diffusion across the unstirred aqueous layer at the surface of the intestinal epithelium. Fat-soluble vitamins (A, D, E, K) are poorly absorbed from the intestinal lumen in the absence of bile acid micelles, and disturbances in the secretion or enterohepatic cycling of bile acids cause fat-soluble vitamin deficiency. Along with their major role in dietary lipid absorption, bile acids may facilitate intestinal absorption of dietary protein by promoting protein denaturation and accelerating hydrolysis by pancreatic proteases. (3) Bile acids play a complex role in maintaining cholesterol homeostasis. On one hand, bile acids increase cholesterol intake by promoting intestinal absorption of biliary and dietary cholesterol. However, on the other hand, bile acids also promote cholesterol loss from the body. Bile acids are water-soluble end products of cholesterol catabolism and bile acid loss in the feces is quantitatively the second most important route for cholesterol elimination. Bile acids also promote hepatic secretion of cholesterol into bile by inducing bile flow and solubilizing biliary cholesterol, thereby enabling cholesterol to move from the liver to the intestinal lumen for elimination. (4) Bile acids contribute to the gut's antimicrobial defenses through direct bacteriostatic actions of bile acid-fatty acid mixed micelles and by signaling to induce expression of antimicrobial genes, thereby reducing small bowel bacterial translocation and intestinal inflammation. Bacterial overgrowth occurs in biliary fistula or bile duct-ligated animals, as well as in animals with experimental cirrhosis. In cirrhotic or cholestatic rats with bacterial overgrowth, feeding of conjugated bile acids or bile acid analogs ameliorates bacterial overgrowth, decreases bacterial translocation to intestinal lymph nodes, and decreases endotoxemia. (5) Bile acids regulate gut microbial diversity and vice versa under physiological and pathophysiological conditions. (6) Bile acids act to prevent the formation of calcium gallstones and calcium oxalate kidney stones. Conjugated bile acids, which are fully water soluble as calcium salts, prevent the formation of gallstone-enucleating precipitates of calcium bilirubinate or salts of calcium phosphate, calcium carbonate, or calcium palmitate by binding calcium in the biliary tract and gallbladder. In the small intestinal lumen, dietary oxalate is usually precipitated by calcium. However, in patients with severe ileal resection and in obese patients after bariatric surgery, excess amounts of dietary fatty acids and bile acids passing into the colon act as a sink for calcium, greatly lowering its intraluminal activity. As a result, oxalate remains in solution and is absorbed from the colon, leading to hyperoxaluria and an increased risk of renal stone formation. In the presence of an intact enterohepatic circulation, bile acids are present at sufficient luminal concentrations in the small intestine to facilitate fat absorption, thereby reducing steatorrhea, colonic fatty acid concentrations, and oxalate absorption. (7) Bile acids act as hormones to signal through nuclear and G-protein-coupled receptors in order to regulate the bile acid enterohepatic circulation, hepatic function, gut motility, and fat, glucose, and energy homeostasis.
Beyond their roles as simple detergents to facilitate dietary lipid absorption and cholesterol homeostasis, bile acids also function as signaling molecules. Bile acids activate specific nuclear receptors (farnesoid X receptor alpha, FXR; pregnane X receptor, PXR; vitamin D receptor, VDR), G-protein-coupled receptors (Takeda G-protein-coupled receptor, TGR5; muscarinic receptors; sphingosine-1-phosphate receptor 2, S1PR2), integrins (α5,β1-integrin), and cell- signaling pathways (protein kinase C, PKC; c-jun N-terminal kinase 1/2, JNK 1/2; serine/threonine protein kinase, AKT/PKB; extracellular signal-regulated kinase, ERK 1/2; p38 mitogen-activated protein kinase, p38 MAPK; epidermal growth factor receptor, EGFR), with FXR and TGR5 being the best understood examples.
Evidence of their signaling properties began to emerge in the 1980s and 1990s, when bile acids were shown to activate protein kinase C isoforms and exhibit cell growth-modulatory effects. However, the role of bile acids as hormones/signaling molecules was not firmly established until 1999, when bile acids were identified as ligands for the orphan nuclear receptor FXR (gene symbol: NR1H4 ). The FXR (FXRα) should not be confused with the related nuclear receptor, FXRβ. FXRβ (gene symbol: NR1H5 ) is a widely expressed nuclear receptor that is activated by lanosterol, but not by bile acids. Although expressed by many vertebrates and mammalian species including mice, rats, rabbits, and dogs, FXRβ ( NR1H5 ) is a nonexpressed pseudogene in humans and primates. For FXR, many of the major mammalian bile acids (both unconjugated as well as glycine or taurine conjugated) function as ligands, with the following rank order of potency: chenodeoxycholic acid > lithocholic acid ≈ deoxycholic acid > cholic acid. Notable exceptions to the list of bile acid FXR agonists are ursodeoxycholic acid and 6-hydroxylated bile acids species such as muricholic acid, which do not activate FXR or function as FXR antagonists. There are four major isoforms of FXR in mice and humans, which are generated through the use of alternative promoters and alternative splicing. Although all four FXR isoforms encode identical ligand binding domains, the abundance of the isoforms vary between tissues and there are differences in their relative transcriptional activity. As noted above, vertebrates exhibit a remarkable diversity in their bile acid chemical structures, and the FXR ligand binding domain appears to have coevolved with its bile acid ligand. The FXR is mainly expressed in the liver intestine, kidney, and adrenal. Consistent with its gastrointestinal expression, FXR plays important roles in the regulation of enterohepatic cycling of bile acids, feedback regulation of bile acid biosynthesis, and protection against bile acid-associated toxicity. In the liver, these functions include stimulating bile acid conjugation and export across the canalicular membrane into bile. In the small intestine, activation of FXR protects the enterocyte from bile acid overload by inducing expression of the ileal cytosolic ileal bile acid binding protein (IBABP; gene symbol: FABP6 ), the basolateral bile acid transporter subunits, organic solute transporter (OST) alpha (OSTα), and OST beta (OSTβ), and the endocrine polypeptide hormone fibroblast growth factor (FGF) 19 (mouse ortholog: FGF15), a central regulator of hepatic bile acid synthesis. With regard to general functions in the gastrointestinal tract, FXR induces expression of genes important for intestinal barrier function and antimicrobial defense, and has important antiproliferative and antiinflammatory properties.
Bile acids also signal via the nuclear receptors PXR (gene symbol: NR1I2 ) and VDR (gene symbol: NR1I1 ). These receptors are activated by lithocholic acid, a hydrophobic and potentially cytotoxic secondary bile acid produced from chenodeoxycholic acid by intestinal anaerobic bacteria. With regard to ligand specificity, bile acids activate PXR with a rank order of potency: lithocholic acid > deoxycholic acid > cholic acid, and activate VDR with a rank order of potency: 3-oxo-lithocholic acid > deoxycholic acid > cholic acid. With regard to bile acid homeostasis, PXR or VDR primarily function to induce expression of enzymes involved in bile acid metabolism and detoxification, and likely play only a minor role in regulating bile acid biosynthesis.
In 2002, two groups independently identified TGR5 (also called membrane-type bile acid receptor, M-BAR; G-protein-coupled bile acid receptor 1, GPBAR1; gene symbol: GPBAR1 ) as a G αs -coupled bile acid receptor, which stimulates adenylate cyclase and increases intracellular cAMP levels. TGR5 is activated by conjugated and unconjugated bile acids, with the following rank order of potency: deoxycholic acid > lithocholic acid > chenodeoxycholic acid > cholic acid. Notably, there are bile acid ligand specificity differences between FXR and TGR5, which have been exploited to generate bile acid derivatives that selectively activate the individual receptors or serve as agonists for both. TGR5 is ubiquitously expressed, with highest levels of expression in gallbladder and moderate levels of expression in liver and intestine. In the liver, TGR5 is not expressed by hepatocytes. However TGR5 is expressed by Kupffer cells, where it is thought to play an immunomodulatory role, and by sinusoidal endothelial cells, where TGR5 functions to induce nitric oxide synthesis and regulate the hepatic microcirculation. With the growing appreciation of bile acids as signaling molecules, considerable study is being directed toward understanding the physiological functions of TGR5. For example, bile acid activation of TGR5 can regulate gallbladder filling, intestinal motility, and may have a role in bile acid-induced itch and the analgesia associated with cholestatic liver disease. There are also metabolic effects associated with TGR5 signaling in brown adipose, muscle, and macrophages.
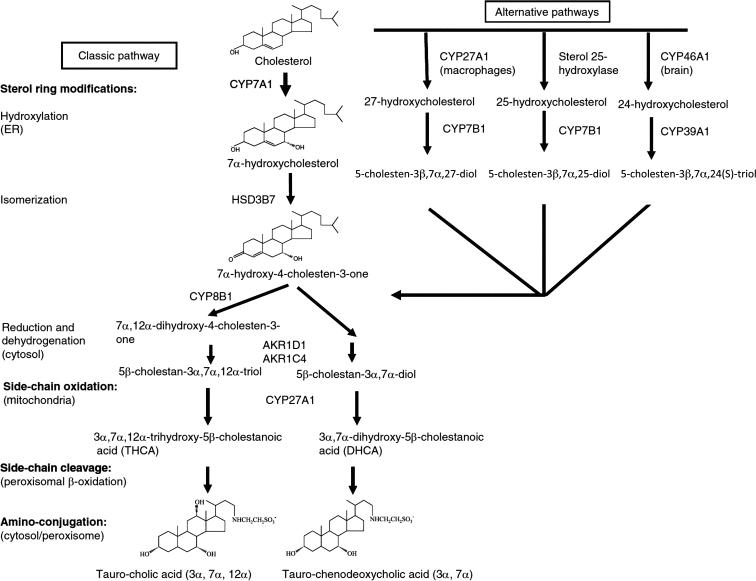
Bile acids are synthesized from cholesterol in the pericentral hepatocytes of the hepatic acini. In this process, the hydrophobic substrate, cholesterol, is converted to a water-soluble, amphipathic product through a series of sterol ring hydroxylations and side chain oxidation steps. Bile acids synthesized by the hepatocyte are designated primary bile acids to distinguish them from the secondary bile acids that are formed by the reactions carried out by the host or gut microbiota, which include dehydroxylation, dehydrogenation (oxidation of a hydroxy group to an oxo group), oxidation, epimerization (changing an α-hydroxy group to a β-hydroxy group or vice versa), and esterification. Bile acid synthesis was originally thought to involve one major pathway, the “classical” or neutral pathway (cholesterol 7α-hydroxylase pathway) that favors cholic acid biosynthesis. This paradigm was later modified by the discovery of a second pathway, the “alternative” or acidic pathway (oxysterol 7α-hydroxylase pathway) that favors the biosynthesis of chenodeoxycholic acid in humans and 6-hydroxylated bile acids such as muricholic acid and hyocholic acid in mice and rats. Details of the hepatocellular and biochemical mechanisms responsible for the metabolic channeling of cholesterol toward cholic acid versus chenodeoxycholic acid/6-hydroxylated bile acids are still not clear, and the specificity is not absolute. The cholesterol 7α-hydroxylase pathway produces some chenodeoxycholic acid/6-hydroxylated bile acid whereas the alternative pathway can yield cholic acid. In the alternative pathway, the first step involves modification of the cholesterol side chain by C-24 (sterol 24-hydroxylase; gene symbol: CYP46A1 ), C-25 (sterol 25- hydroxylase; gene symbol: CH25H ), or C-27 (sterol 27-hydroxylase; gene symbol: CYP27A1 ) sterol hydroxylases present in liver and extra-hepatic tissues such as brain. This reaction is then followed by an oxysterol 7α-hydroxylation, which is mediated primarily by CYP7B1 in liver. Of these alternative hydroxylation pathways, the contribution of 27-hydroxycholesterol to bile acid synthesis is quantitatively most important. Although quantitatively a minor contributor to bile acid synthesis, the conversion of cholesterol to 24S-hydroxycholesterol functions as a major mechanism for cholesterol elimination from brain by facilitating sterol transfer across the blood-brain barrier into the systemic circulation for excretion by the liver.
The overall process of bile acid biosynthesis is complex, requiring the action of 16 enzymes that catalyze as many as 17 different reactions. In the classical pathway, the steroid nucleus is modified before the side chain, whereas in the alternative pathways, side chain modifications occur before or coincident with changes to the steroid nucleus. Cholesterol 7α-hydroxylase (gene symbol: CYP7A1 ) is the rate-limiting enzyme for bile acid synthesis via the classical pathway. However, the step catalyzed by the sterol 12α-hydroxylase (gene symbol, CYP8B1 ) controls the amount of cholic acid synthesized and is an important determinant of the ratio of cholic acid to chenodeoxycholic acid and cholic acid to muricholic acid in human and mouse bile, respectively. In this capacity, CYP8B1 plays a critical role in modulating the composition and hydrophobicity of the bile acid pool.
It should be noted that humans and mice have substantially different bile acid pool compositions. This reflects differences in bile acid conjugation (discussed below), synthesis of ursodeoxycholic acid as a primary bile acid in mice, and hydroxylation at the 6-position of the bile acid steroid nucleus in mice. In contrast, hydroxylation of bile acids at the 6-position is rare in humans, and detectable under only specialized circumstances, such as in fetal/early neonatal development or in certain cholestatic conditions. It has long been known that 6- hydroxylation of bile acids alters their physicochemical and detergent properties, with mice having a more hydrophilic bile acid pool. With the recognition of bile acids as signaling molecules that act through nuclear and G-protein-coupled receptors, the human-rodent bile acid structural differences have gained additional importance. The 6-hydroxylation of bile acids dramatically alters their signaling properties, potentially limiting the human relevance of mouse models for the study of bile acid-related disease. The cytochrome P450 enzyme(s) (CYPs) responsible for 6-hydroxylation of bile acids were very recently identified. Correlative data had implicated members of the murine CYP3A family, particularly CYP3A11. However, analysis of knockout mice lacking CYP3A enzymes or all members of the CYP1A, CYP2C, CYP2D, or CYP3A families found that members of the murine CYP2C family, including at least CYP2C70, are required and directly mediate the first step in 6-hydroxylation of chenodeoxycholic acid to alpha-muricholic acid and ursodeoxycholic acid to beta-muricholic acid. In contrast, the major human CYP2C enzyme, CYP2C9, was unable to oxidize bile acids, in agreement with the finding that 6-hydroxylated bile acid species are absent in humans under physiological conditions. The major bile acid biosynthetic pathways are summarized in Fig. 41.2 .
After their biosynthesis, bile acids are conjugated via a two-step process involving the generation of a bile acid-CoA by bile acid-CoA synthase and then amidation with taurine or glycine by bile acid-CoA: amino acid N-acyltransferase (BAAT). In most nonmammalian vertebrate species, bile acids are typically modified on their side by sulfation (for C27 bile alcohols) or conjugation with taurine or a taurine derivative (for C27 and C24 bile acids). In mammals, bile acids are primarily conjugated on their side chain to either taurine or glycine. Notably, the conjugation pattern varies considerably between different mammalian species, ranging from almost exclusively taurine in the rat, cat, mouse, sheep, and dog, to mostly glycine in the pig, hamster, guinea pig, and human, to exclusively glycine in the rabbit. The amino acid specificity for the conjugation of bile acids is controlled by the BAAT enzyme, and to a lesser degree, by the availability of the taurine precursor. However, the evolutionary forces driving the selection of a particular amino acid in different animal species are unclear. Taurine conjugated bile acids have a lower p K a than their respective glycine conjugates and are more likely to remain ionized and membrane impermeant. However, both the glycine and taurine amide linkages are more resistant to hydrolysis by the pancreatic carboxypeptidases, as compared to other amino acids. As such, both taurine and glycine-conjugated bile acids largely escape cleavage by host proteases in the intestinal lumen during the digestive process.
Of the two major biosynthetic pathways, the classical (CYP7A1) pathway is quantitatively more important in rodents and humans. In mice, the classical pathway accounts for ~ 70% of the total bile acid synthesis in adults, and is the predominant pathway in neonates. In humans, the classical pathway accounts for more than 90% of bile acid synthesis, as evidenced by approximately 96% reduction in fecal bile acid excretion in an adult patient with an inherited CYP7A1 defect. In contrast to mice and adult humans, the alternative pathway is the predominant biosynthetic pathway in human neonates, as evidenced by low to undetectable CYP7A1 expression in newborns and the finding of severe cholestatic liver disease in infants with inherited oxysterol 7α-hydroxylase ( CYP7B1 ) gene defects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here