Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
acyl-CoA oxidase
apical sodium bile acid transporter
cerebrotendinous xanthomatosis
fast atom bombardment ionization mass spectrometry
gas chromatography−mass spectrometry
γ-glutamyltranspeptidase
low-density lipoprotein
liquid secondary ionization mass spectrometry
progressive familial intrahepatic cholestasis
taurine-conjugated form of ursodeoxycholic acid
ursodeoxycholic acid
very long chain fatty acids
vertical sleeve gastrectomy
The importance of bile acid synthesis and metabolism to normal physiology and pathophysiologic states is well established. For a long time these small and relatively simple molecules, constructed on a steroid backbone, have been considered essential for cholesterol metabolism and bile flow and important for micelle formation for absorption of fats in the small intestine. More recently, bile acids have been further recognized as signaling molecules that regulate metabolism. This chapter provides an overview of the pathways of bile acid synthesis and metabolism. It will focus on specific inborn errors in bile acid synthesis because these highlight the important role of bile acids in maintaining hepatic bile flow and as signaling integrators of metabolism.
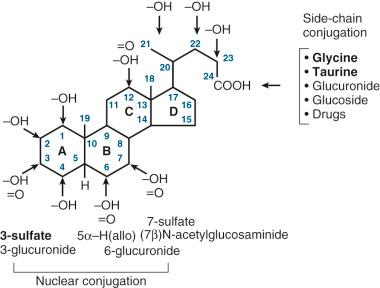
Structurally, bile acids possess a cyclopentanoperhydrophenanthrene (ABCD ring) nucleus and therefore belong to the chemical class of steroids. They differ from steroid hormones and neutral sterols, such as cholesterol, in having a five-carbon-atom side chain with a terminal carboxylic acid ( Fig. 2-1 ). Bile acids are synthesized in the liver from cholesterol by a complex series of reactions catalyzed by 17 different hepatic enzymes located in the endoplasmic reticulum, mitochondria, cytoplasm, and peroxisomes. Consequently there is considerable trafficking of intermediates between these subcellular compartments. Several of the enzymes are also found in extrahepatic tissues. The enzymes involved in bile acid biosynthesis have all been isolated and well characterized in pioneering work performed in the late 1960s and the 1970s. More recently the role that each enzyme plays in the regulation of bile acid synthesis has been elucidated from studies of gene knockout animal models and humans with genetic defects in bile acid synthesis. Complementary (c)DNAs have now been described for these enzymes, including the rate-limiting enzyme in the bile acid biosynthetic pathway, cholesterol 7α-hydroxylase, and these have provided important tools to examine the regulation of bile acid synthesis and to confirm genetic defects in bile acid synthesis.
Conjugated (glycine and taurine) cholic and chenodeoxycholic acids are the two primary bile acids synthesized in humans, but there is considerable variability in the qualitative pattern of bile acid synthesis among animal species. Rodents synthesize mostly cholic acid and the 6β-hydroxylated bile acid, β-muricholic (3α6β.7β-trihydroxy-5β-cholanoic acid), and these are predominantly taurine conjugated, whereas pigs synthesize a 6α-hydroxylated bile acid, hyodeoxycholic (3α,6α-dihydroxy-5β-cholanoic acid) and 6-oxo-lithocholic acid. Such differences need to be considered when working with different species and animal models of disease.
Although there is a tendency to illustrate the reactions in the bile acid synthetic pathway to occur in a linear fashion ( Fig. 2-2 ), moving from initiation of changes to the steroid nucleus through modification of the side chain, in reality there is considerable substrate promiscuity for the 17 enzymes catalyzing the various reactions, which consequently results in vast number of different bile acids and intermediates being synthesized. This is especially evident during early development, a period of physiologic cholestasis, and in pathologic conditions that interfere with the integrity of the enterohepatic circulation. Furthermore, intestinal bacterial modifications, resulting in the formation of “secondary” bile acids, add a further level of complexity to the bile acid composition of biologic fluids.
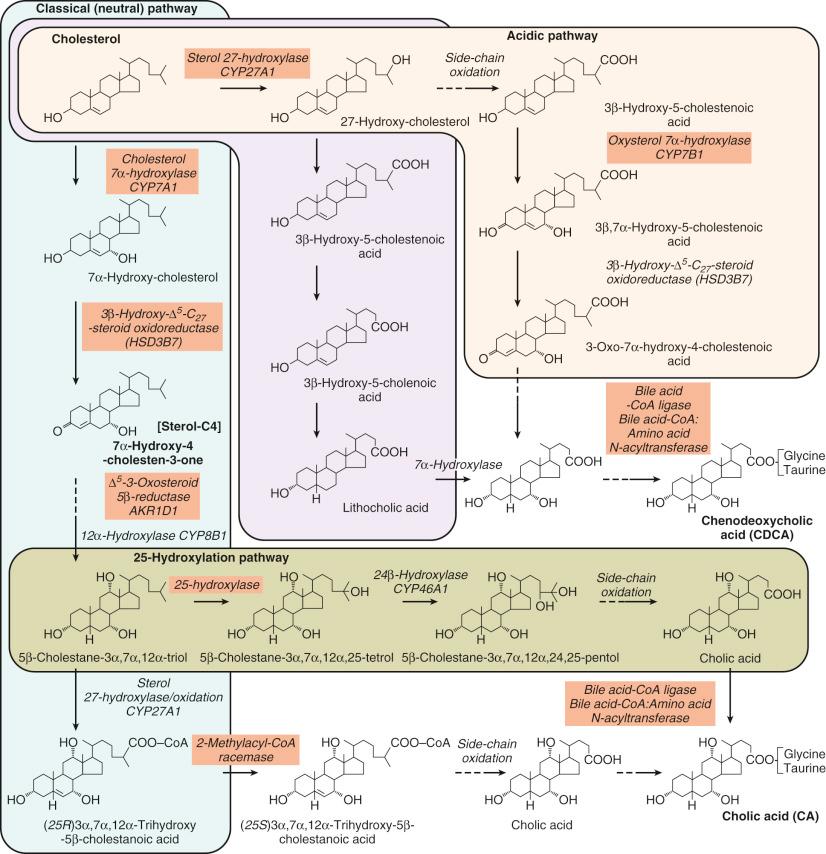
There are two main pathways leading to primary bile acid synthesis. These are termed the neutral and acidic pathways, the former being the classic one that is initiated by the rate-limiting cytochrome P 450 liver-specific enzyme, cholesterol 7α-hydroxylase (CYP7A1) leading to cholic acid synthesis, and the latter being initiated by the action of cholesterol 27-hydroxylase (CYP27A1) on the side chain to yield chenodeoxycholic acid. This acidic pathway leads to the formation of 3β-hydroxy-5-cholenoic and lithocholic acids as intermediates to chenodeoxycholic acid. These markedly hepatotoxic monohydroxy-bile acids are increased in early life and in cholestatic liver diseases. 27-Hydroxylation occurs in the liver and in many other tissues, including brain, alveolar macrophages, vascular endothelia, and fibroblasts, and its extrahepatic role appears related to the cellular regulation of cholesterol homeostasis by its ability to generate oxysterols that are potent repressors of cholesterol synthesis. It is now accepted that the acidic pathway contributes significantly to overall total bile acid synthesis, and especially to chenodeoxycholic acid synthesis. Normal levels of bile acids are synthesized in mice even when the gene encoding cholesterol 7α-hydroxylase is knocked out, and bile acid synthesis is sustained in rats when cholesterol 7α-hydroxylase is inhibited by continuous infusion of squalestatin. However, Cyp7a1−/− mice die within the first few weeks of life from liver failure and the consequences of fat-soluble vitamin malabsorption, unless fat-soluble vitamins and cholic acid are fed to these animals immediately after birth. Despite being deficient in cholesterol 7α-hydroxylase, primary bile acid synthesis occurs via the developmental expression of an oxysterol 7α-hydroxylase (CYP7B1) specific to the acidic pathway, and this enzyme is essential in early human life to protect the liver from hepatotoxic monohydroxy-bile acids that are formed as intermediates in this pathway. The aforementioned examples show that primary bile acid synthesis is not exclusively dependent on cholesterol 7α-hydroxylase, and under certain conditions, alternative pathways are induced. For some time it was evident that there were a number of different 7α-hydroxylases. This was confirmed by Russell et al. following the isolation and characterization of the oxysterol 7α-hydroxylases, CYP7B1 and CYP39A1. CYP7B1 has high activity in human liver and is also found in brain, kidney, and prostate, but its regulation is not fully understood. It has broad substrate specificity, being active on the oxysterols, 27- and 25-hydroxycholesterol, on the bile acids 3β-hydroxy-5-cholenoic and 3β-hydroxy-5-cholestenoic acids, and also on C 19 steroids. The CYP7B1 gene is localized to chromosome 8q21.3 and in close proximity to the CYP7A1 gene. Genetically engineered Cyp7b1 (−/−) mice lacking this enzyme have elevated levels of 27- and 25-hydroxycholesterol, but not 24-hydroxycholesterol. Similarly, extremely high levels of 27-hydroxycholesterol and the hepatotoxic monohydroxy bile acids hepatotoxic monohydroxy bile acid 3β-hydroxy-5-cholenoic and 3β-hydroxy-5-cholestenoic acids were found in an infant with a genetic defect in oxysterol 7α-hydroxylase. A mutation in the CYP7B1 gene will cause a phenotype of progressive and fatal liver disease and indicates the quantitative importance of the acidic pathway in early human life.
Following the synthesis of 7α-hydroxycholesterol, modifications to the steroid nucleus take place; these result in oxidoreduction and C-12 hydroxylation, consequently preparing the sterol intermediates for direction into either the cholic acid (3α,7α,12α-trihydroxy-5β-cholan-24-oic) or chenodeoxycholic acid (3α,7α-dihydroxy-5β-cholan-24-oic) pathways. According to convention, 7α-hydroxycholesterol is converted to 7α-hydroxy-4-cholesten-3-one, a reaction catalyzed by a microsomal NAD-dependent 3β-hydroxy-Δ 5 -C 27 -steroid oxidoreductase (HSD3B7) enzyme (C 27 3β-HSD), formerly referred to as a 3β-hydroxy-Δ 5 -C 27 -steroid dehydrogenase/isomerase . This enzyme shows substrate specificity toward 7α-hydroxylated sterols and bile acids possessing a 3β-hydroxy-Δ 5 nucleus and is inactive on 7β-hydroxylated analogs. Comparable reactions occur in steroid hormone synthesis; however, the enzyme active on bile acid intermediates is a distinct single enzyme that shows absolute specificity toward C 27 -sterols, differing from the isozymes active on C 19 and C 21 neutral steroids. 3β-Hydroxy-Δ 5 -C 27 -steroid oxidoreductase is not exclusive to the liver but is also expressed in fibroblasts, which enables its activity to be determined in patients with a genetic defect in this enzyme. Mutations in the gene encoding this enzyme are associated with progressive intrahepatic cholestasis, and this is often the cause of late-onset chronic cholestasis.
12α-Hydroxylation of the product of the above reaction will direct the Δ 4 -3-oxo intermediate into the cholic acid pathway. This reaction is catalyzed by a liver-specific microsomal cytochrome P 450 12α-hydroxylase (CYP8B1), which is highly expressed in rabbit and human liver, two species where deoxycholic acid is quantitatively important. When the gene encoding this enzyme is knocked out in mice, there is loss of cholic acid and reduced cholesterol absorption. The primary structures of the rabbit, mouse, and human enzymes have been established by molecular cloning of their cDNAs. The activity of 12α-hydroxylase enzyme determines the relative proportion and synthetic rate of cholic relative to chenodeoxycholic acids and appears in humans to be up-regulated by interruption of the enterohepatic circulation and in animals by starvation. It is possible that in utero there may be reduced activity of this enzyme because fetal bile has a predominance of chenodeoxycholic acid. In contrast, the ratio of cholic acid to chenodeoxycholic acid is very high in neonatal bile compared with adult bile. The neonatal period of life is associated with a phase of physiologic cholestasis, which may lead to an up-regulation in 12α-hydroxylase activity with a consequent increase in cholic acid synthesis.
7α-Hydroxy-4-cholesten-3-one and 7α,12α-dihydroxy-4-cholesten-3-one both undergo reduction with formation of a 3-oxo-5β(H)-structure, and this generates the basic trans configuration of the A/B-rings of the steroid nucleus that is common to the majority of bile acids in most mammalian species. Allo(5α-H)-bile acids are often major bile acid species of lower vertebrates but are found in small proportions in biologic fluids from humans. These are formed by an analogous reaction but catalyzed by a hepatic 5α-reductase. The K m of 5α-reductase is high, and consequently under normal conditions 5β-reduction is favored. The Δ 4 -3-oxosteroid 5β-reductase, a cytosolic aldo-keto reductase (AKR1D1), is a protein of approximately 38 kDa comprising 326 amino acids. It differs significantly in structure from the 5α-reductase and has broad substrate specificity. Its crystal structure, and the effect of a number of point mutations on the substrate binding sites and enzyme activity, was recently reported. Although under normal conditions this enzyme does not appear to be of regulatory importance for bile acid synthesis, its activity parallels the activity of cholesterol 7α-hydroxylase, and therefore measurement of the plasma concentration of 7α-hydroxy-4-cholesten-3-one (often referred to as C4 , or sterol C4 , see Fig. 2-2 ) can be used as an indirect assessment of hepatic cholesterol 7α-hydroxylase activity. The finding of elevated proportions of 3-oxo-Δ 4 bile acids in biologic fluids during early life and in advanced cholestatic liver disease suggests that under pathologic conditions it is this enzyme that becomes rate limiting for bile acid synthesis, rather than cholesterol 7α-hydroxylase. Mutations in the gene encoding AKR1D1 are clinically manifest as progressive intrahepatic cholestasis and biochemically by the production of large amounts of C 24 -3-oxo-Δ 4 -bile acids and allo-bile acids.
The enzyme catalyzing the conversion of the 3-oxo-5β(H)-sterols to the corresponding 3α-hydroxy-5β(H) intermediates is a soluble 3α-hydroxysteroid dehydrogenase (AKR1C4). This enzyme catalyzes the oxidoreduction of a number of substrates, and several cDNA clones with sequence similarity to other aldo-keto reductases have been described, which suggests the existence of multiple isozymes. This final step in modification of the steroid nucleus results in the formation of the key intermediates, 5β-cholestane-3α,7α-diol and 5β-cholestane-3α,7α,12α-triol (bile alcohols), which then undergo a sequence of reactions leading to side chain oxidation and consequent shortening by three carbon atoms (see Fig. 2-2 ).
The initial step in side chain oxidation of the bile alcohols involves hydroxylation of the C-27 carbon atom, a reaction that is catalyzed by a mitochondrial cytochrome P 450 27-hydroxylase (CYP27A1) and leads to the formation of 5β-cholestane-3α,7α,12α,27-tetrol. It is now known that CYP27A1 is also responsible for the complete oxidation reaction, which yields directly 3α,7α,12α-trihydroxy-5β-cholestanoic acid. 5β-Cholestane-3α,7α,12α,27-tetrol may also undergo oxidation by the combined actions of soluble or mitochondrial alcohol and aldehyde dehydrogenases, but the relative importance of these reactions compared with the complete 27-hydroxylase-catalyzed reaction is not known. cDNAs encoding the rat, rabbit, and human sterol 27-hydroxylase have been isolated. This enzyme is expressed in many extrahepatic tissues, and its function appears to be important in facilitating the removal of cellular cholesterol. It shows substrate specificity toward many sterols, including cholesterol and vitamin D, and is the same enzyme that catalyzes the formation of 27-hydroxycholesterol, the first step in the acidic pathway. When the sterol 27-hydroxylase gene is disrupted in the mouse, bile acid synthesis is markedly reduced; however, mutations in this gene that cause the rare lipid storage disease of cerebrotendinous xanthomatosis (CTX) have only a modest effect on bile acid synthesis, partly because alternative pathways for bile acid synthesis support the production of compensatory levels of cholic acid.
Studies using radiolabeled precursors have shown that 5β-cholestane-3α,7α,12α-triol can be first 25-hydroxylated in the microsomal fraction, then 24β-hydroxylated, and finally oxidized to cholic acid. This pathway is specific for cholic acid because little or no hydroxylation of 5β-cholestane-3α,7α-diol has been demonstrated. Based on studies of patients with CTX, it was proposed that the C-25 hydroxylation pathway may be a major pathway for cholic acid synthesis in humans. The quantitative importance of this pathway was later reevaluated in vivo by measuring the production of [ 14 C]acetone after labeling the cholesterol pool with [26- 14 C]cholesterol. This approach showed that the C-25 hydroxylation pathway accounted for less than 5% of the total bile acids synthesized in healthy adults and less than 2% in adult rats. Hydroxylation of cholesterol also occurs at the C-24 and C-25 positions in addition to the aforementioned cholesterol 27-hydroxylation to yield oxysterols, which are potent repressors of cholesterol synthesis. Cholesterol 24-hydroxylase (CYP46A1) is expressed in the brain to a greater extent than in the liver where it is considered to play a role in cholesterol secretion. In gene knockout mouse models of cholesterol 24- and 25-hydroxylases, bile acid synthesis is unaffected.
The cholestanoic acids are next converted to CoA esters by the action of a bile acid–CoA ligase (synthetase) of which two forms have been identified: one activates newly synthesized C 27 cholestanoic acids, and the other activates cholanoic acids formed as secondary bile acids returning to the liver for reconjugation. The product of this reaction is the formation of the CoA esters of (25R) -3α,7α-dihydroxy-cholestanoic and (25R) -3α,7α,12α-trihydroxy-cholestanoic acids. The (25R) -diastereoisomers must be racemized to their (25S) -forms in order to penetrate the peroxisome for subsequent oxidation. This reaction is catalyzed by a 2-methylacyl coenzyme A racemase enzyme, the same enzyme that is also active on branched-chain fatty acids such as phytanic acids. A mutation in the gene encoding this enzyme leads to the accumulation of (25R) -cholestanoic acids and phytanic acids and presents with neurologic and liver disease.
The final stage in modification of the side chain involves the β-oxidation of the cholestanoic acids, which occurs by a multiple-step reaction within peroxisomes. The sequence of these reactions is analogous to the β-oxidation of fatty acids. The CoA esters of the cholestanoic acid are acted on by a specific peroxisomal acyl-CoA oxidase (ACOX2). This reaction is rate limiting, and the enzyme has been partially purified from rat liver and found to differ from the analogous acyl-CoA oxidase (ACOX1) utilizing fatty acids as substrates. The situation in humans is somewhat different in that a single peroxisomal oxidase acts on both branched-chain fatty acids and bile acid intermediates. Formation of a C-24 hydroxylated derivative occurs by the action of a bifunctional enoyl-CoA hydratase/β-hydroxyacyl-CoA dehydrogenase, a reaction that goes through a Δ 24 -intermediate. Photoaffinity labeling experiments have shown that this enzyme is the same one that is involved in the peroxisomal β-oxidation of fatty acids. The dehydrogenase activity of the bifunctional enzyme yields a 24-oxo derivative that, following thiolytic cleavage by peroxisomal thiolase 2, releases three carbon atoms in the form of propionic acid. This results in the formation of the C 24 bile acid CoA end product. With the exception of the acyl-CoA oxidase, defects in any of the other enzymes responsible for the β-oxidation of very-long-chain fatty acids (VLCFAs) exhibit abnormalities in primary bile acid biosynthesis.
Some mention of allo (5α-reduced)-bile acids is warranted even though under physiologic conditions they account for a relatively small proportion of the total bile acids in human biologic fluids. These are major bile acid species of many lower vertebrates. In humans, 5α-reduced bile acids are usually formed by the action of intestinal microflora on 3-oxo-5β-bile acids during their enterohepatic circulation and consequently are found in significant amounts in feces. In rodents, these 5α-reduced bile acids can be formed in the liver from 5α-cholestanol. This pathway begins with 7α-hydroxylation of 5α-cholestanol and the product is then converted to 5α-cholestane-3α, 7α-diol via the intermediate 7α-hydroxy-5α-cholestan-3-one. Hepatic 12α-hydroxylation of 5α-sterols is very efficient in the rat and readily leads to formation of allo-cholic acid. A further pathway for allo-bile acid formation involves the hepatic 5α-reduction of 7α-hydroxy- and 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acids, a reaction catalyzed by a Δ 4 -3-oxosteroid 5α-reductase, and the finding of large proportions of allo-bile acids in infants with severe cholestatic liver disease due to a AKR1D1 deficiency indicates these to be primary bile acids of hepatic origin in humans. Both 5α-reductase isozymes are expressed in the liver beginning from birth.
A striking feature of bile acid synthesis and metabolism during early life is the relatively large proportion of polyhydroxylated, unsaturated, and oxo-bile acids that are synthesized and not typically found in the biologic fluids of healthy adults. Although frequently referred to as atypical , this moniker is a misnomer because they are in fact very typical of the developmental phase of hepatic metabolism. Interestingly, the qualitative and quantitative bile acid composition of biologic fluids in early life closely resembles that of adults with severe cholestatic liver disease, suggesting that in the diseased liver there is a reversion to more primitive pathways of synthesis and metabolism. The most notable distinction in ontogeny is the prevalence of cytochrome P450 hydroxylation pathways that rapidly decline in importance over the first year of life. The most important hydroxylation reactions are 1β-, 4β-, and 6α-hydroxylation that are of hepatic origin. The concentrations of several of the metabolites, in particular hyocholic (3α,6α,7α-trihydroxy-5β-cholanoic) and 3α,4β,7α-trihydroxy-5β-cholanoic acids, exceed that of cholic acid in fetal bile. The role of these hydroxylation pathways is uncertain, but additional hydroxylation of the bile acid nucleus will increase the polarity of the bile acid and facilitate its renal clearance, while also decreasing its membrane-damaging potential. In early life, and particularly in the fetus, an immaturity in canalicular and ileal bile acid transport processes leads to a sluggish enterohepatic circulation and hydroxylation serves as a hepatoprotective mechanism.
Irrespective of the pathway by which cholic and chenodeoxycholic acids are synthesized, the CoA thioesters of these primary bile acids are ultimately conjugated to the amino acids glycine and taurine. This two-step reaction is catalyzed by a rate-limiting bile acid-CoA ligase enzyme followed by a bile acid CoA:amino acid N-acyltransferase (EC 2.3.1.65). The genes encoding both enzymes, SLC27A5 and BAAT, were cloned decades ago. The conjugation reaction was originally believed to take place in the cytosol, but the highest activity of conjugating enzymes was later found to be in peroxisomes.
Genetic defects in the bile acid amidation have been associated with fat-soluble vitamin malabsorption states with variable degrees of liver disease. Recently, we identified and treated five patients (one male, four females) from four families with defective bile acid amidation caused by a genetically confirmed deficiency in BAAT with the conjugated bile acid, glycocholic acid. The bile acid CoA:amino acid N-acyltransferase enzyme utilizes glycine, taurine, and interestingly β-fluoroalanine, but not alanine, as substrates. It will also conjugate VLCFAs to glycine. The specificity of the enzyme has been examined in detail and found to be influenced by the length of the side chain of the bile acid; bile acids having a four-carbon-atom side chain, that is, nor(C 23 )–bile acids and homo(C 25 )–bile acids, are both poor substrates for amidation. In contrast, cholestanoic (C 27 ) acids are predominantly taurine conjugated. Significant species differences in substrate specificity are observed and should be considered when working with animal models. The human bile acid-CoA:amino acid N-acyltransferase conjugates cholic acid with both glycine and taurine; whereas the mouse enzyme shows selectivity only toward taurine. This is consistent with the mouse being an obligate taurine conjugator of bile acids, as is the rat and the dog. In humans, the final products of this complex multistep pathway are the two conjugated primary bile acids of cholic and chenodeoxycholic acids (see Fig. 2-2 ), and these are then secreted in canalicular bile and stored in gallbladder bile. In humans, glycine conjugation predominates with a ratio of glycine to taurine conjugates of 3.1 : 1 for normal adults. In early human life, more than 80% of the bile acids in bile are taurine conjugated due to the abundance of hepatic stores of taurine.
Although the principal bile acids of humans and most mammalian species are amidated, other conjugates occur naturally and these include sulfates, glucuronide ethers and esters, glucosides, N-acetylglucosaminides, and conjugates of some drugs. These conjugates account for a relatively large proportion of the total urinary bile acids. Conjugation significantly alters the physicochemical characteristics of the bile acid, and it serves an important function by increasing the polarity of the molecule, thereby facilitating its renal excretion, and by minimizing the membrane-damaging potential of the more hydrophobic unconjugated species. Under physiologic conditions, these alternative conjugation pathways are quantitatively less important. However, in cholestatic liver disease, or when the liver is subjected to a bile acid load, as in ursodeoxycholic acid (UDCA) therapy, the concentrations of these conjugates in biologic fluids may change. Detailed knowledge of these metabolic pathways is limited, but it is evident that there is significant localization of bile acid-conjugating enzymes in the kidneys.
Sulfation of bile acids, most commonly at the C-3 position but also at C-7, is catalyzed by a bile acid sulfotransferase, an enzyme that in the rat, not in the human, exhibits sex-dependent differences in activity. Although much has been written about the potential importance of sulfation in early life, it is evident from the finding of a relatively small proportion of bile acid sulfates in fetal bile that hepatic sulfation is negligible in the fetus and neonate. Indeed, it is most probable that urinary bile acid sulfates originate mainly by renal sulfation ; 60% to 80% of urinary bile acids are sulfated and their excretion increases in cholestasis. Only traces of bile acid sulfates are found in bile despite efficient canalicular transport of perfused bile acid sulfates.
A number of glucuronosyltransferases catalyze the formation of glucuronide ethers and esters. The enzymes show substrate selectivity in that bile acids possessing a 6α-hydroxyl group are preferentially conjugated at the C-6 position forming 6-O-ether glucuronides, whereas short-chain bile acids form mainly glucuronides. Purification of the hyodeoxycholic acid-specific human UDP-glucuronosyltransferase and subsequent cloning of a cDNA indicate that this enzyme is highly specific toward hyodeoxycholic (3α,6α-dihydroxy-5β-cholanoic) acid; no glucuronidation of hyocholic (3α,6α,7α-trihydroxy-5β-cholanoic) acid could be detected. It is probable that there is a family of isozymes that catalyze the glucuronidation of different bile acids.
Glucosides and N-acetylglucosaminides of nonamidated and amidated bile acids have been identified in normal human urine with quantitative excretion comparable to that of glucuronide conjugates. A microsomal glucosyltransferase has been isolated and purified from human liver but is also present in extrahepatic tissues. The enzyme responsible for N-acetylglucosaminide formation exhibits remarkable substrate specificity in that it preferentially catalyzes the conjugation of bile acids having a 7β-hydroxyl group and consequently these conjugates account for more than 20% of the urinary metabolites of patients administered ursodeoxycholic acid. Finally, the full extent to which drugs may compete for the conjugating enzymes is not known, although bile acid conjugates of 5-fluorouracil have been identified. The 2-fluoro-β-alanine conjugate of cholic acid was found to be a major metabolite in bile following administration of this therapeutic agent.
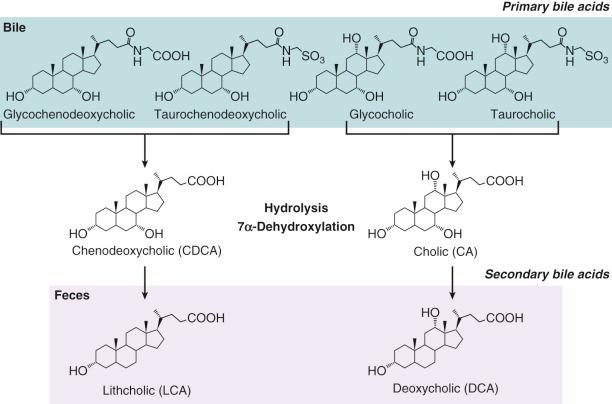
Intestinal microflora play an important role in bile acid synthesis and metabolism. Bacterial enzymes metabolize primary bile acids, altering significantly their physicochemical characteristics and influencing their physiologic actions during enterohepatic recycling. The result is the formation of a spectrum of secondary bile acids that are mainly excreted in feces ( Fig. 2-3 ). Deconjugation of conjugated bile acids, followed by 7α-dehydroxylation, are quantitatively the most important reactions, but bacterial oxidoreduction and epimerization at various positions of the bile acid nucleus also take place along the intestinal tract. This is evident from bile acid profiles along the entire length of the human intestine obtained at autopsy that show relatively high proportions of secondary bile acids in the proximal jejunum, mid-small bowel, ileum, and cecum. The enzymes that catalyze these reactions are found in a variety of organisms, such as Bacteroides, Clostridia, Bifidobacteria, Escherichia coli, and some of these reactions occur in the proximal small intestine. Deconjugation precedes 7α-dehydroxylation, and the bacterial peptidases responsible for this reaction exhibit remarkable substrate specificity in that the length of the side chain is a crucial factor influencing this reaction. The enzyme kinetics, factors influencing the reactions, and molecular biology of a number of the bacterial enzymes have been extensively studied by Stellwag et al and Coleman et al. 7α-Dehydroxylation of cholic and chenodeoxycholic acids, a reaction that proceeds via a 3-oxo-Δ 4 -intermediate, results in the formation of deoxycholic and lithocholic acids, respectively, and these secondary bile acids make up the largest proportion of total fecal bile acids. Lithocholic and deoxycholic acids are relatively insoluble and consequently poorly absorbed. However, both bile acids are returned to the liver to regulate bile acid synthesis. It should be noted that in rats, deoxycholic acid is very efficiently 7α-hydroxylated in the liver and converted back to cholic acid, but this reaction does not take place in humans. Serum concentrations of deoxycholic acid therefore provide a useful means of assessing the extent of impairment of the enterohepatic circulation in cholestatic liver diseases. Recent interest in gut dysbiosis with respect to gastrointestinal and systemic disease has further linked the dysmetabolism of bile acids to changes in the so-called gut-microbiome.
The major factor influencing bile acid synthesis is negative feedback by bile acids returning to the liver via the portal vein during their enterohepatic recycling. There are marked differences in the ability of different bile acids to regulate cholesterol 7α-hydroxylase. For example, whereas the primary bile acids cholic and chenodeoxycholic acids down-regulate synthesis, bile acids possessing a 7β-hydroxy group, such as ursodeoxycholic acid, do not, and the latter may actually increase synthesis rates. This observation has relevance to the treatment of inborn errors of bile acid synthesis. Interruption of the enterohepatic circulation by biliary diversion or the feeding of anion exchange resins that bind bile acids in the intestinal lumen results in an up-regulation of cholesterol 7α-hydroxylase activity. In general, factors that influence cholesterol 7α-hydroxylase activity cause concomitant changes in the activity of HMG-CoA reductase, the rate-limiting enzyme for cholesterol synthesis, and this serves to regulate cholesterol synthesis and maintain a constant cholesterol pool size. Interestingly, cholesterol 7α-hydroxylase exhibits a diurnal rhythm that is synchronous with the activity of HMG-CoA reductase and is reflected by diurnal changes in bile acid synthesis rates. A significant nocturnal rise in bile acid synthesis takes place that may be regulated by glucocorticoids because this regulation can be abolished by adrenalectomy or hypophysectomy.
The mechanism involved in regulating cholesterol 7α-hydroxylase activity and therefore bile acid synthesis is complex and mediated through an ever-increasing discovery of nuclear receptors and transcription factors that have specificity for bile acids and oxysterols. Bile acids have been shown to enter the nucleus of the hepatocyte, and nuclear concentrations increase with bile acid feeding. These nuclear receptors include the farnesoid X receptor (FXR, NHR1H4), short heterodimer partner (SHP, NR0B2), liver receptor homolog-1 (LRH-1, NR5A2), hepatocyte nuclear factor 4α (HNF-4α), liver X receptor α (LXRα, NR1H3), pregnane X receptor (PXR, NR112), constitutive androgen receptor (CAR, NR13), and fibroblast growth factor-19 (FGF19) and its receptor FGFR4, and the G protein–coupled receptor TGR5. Much has been learned about the regulation of cholesterol and bile acid synthesis from gene knockout models of these nuclear receptors. For instance we now understand that a novel enterocyte protein, Diet1, is a regulator of FGF19 production at the posttranscriptional level. The Diet1 and Fgf15 (the mouse homologue of FGF19) appear to have overlapping subcellular localization in murine enterocytes. Diet1-deficient mice constitutively convert cholesterol to bile acids and are resistant to diet-induced hypercholesterolemia and atherosclerosis. Thus, Diet1 appears to be a control point for the production of FGF15/19 in enterocytes and a key regulator of bile acid and lipid homeostasis. New bile acid molecules have been recently synthesized as specific agonists for these receptors in order to devise ways of regulating cholesterol homeostasis and glucose metabolism, and these are now in clinical trials. In addition to influencing the transcriptional regulation of cholesterol 7α-hydroxylase in the liver, these receptors also induce transcription of IBABP, the ileal bile acid binding protein that is involved in the ileal uptake and conservation of the bile acid pool.
Defects in bile acid synthesis have profound effects on hepatic and gastrointestinal function and on cholesterol homeostasis, especially when the cause is a genetic mutation encoding the enzymes responsible for primary bile acid synthesis. Such defects lead to an overproduction of hepatotoxic atypical bile acids that are synthesized from intermediates accumulating in the pathway proximal to the inactive enzyme and a progressive cholestasis exacerbated by the lack of primary bile acids that are critical for promoting bile flow. Marked alterations in urinary, serum, and biliary bile acids are found in all infants and children with liver disease, and it can be difficult to determine whether such changes are primary or secondary to the liver dysfunction. The first bile acid synthetic defect causing liver disease was discovered as a result of applying the liquid secondary ionization mass spectrometry (LSIMS) technique of fast atom bombardment ionization mass spectrometry (FAB-MS). This permitted the direct analysis of bile acids from a small drop of urine. Whereas FAB-MS is still a definitive technique for diagnosing bile acid synthetic defects, newer mass spectrometric approaches have since been used, including electrospray ionization tandem mass spectrometry and gene sequencing techniques. However, mass spectrometry continues to offer the fastest and most accurate method of screening for these disorders because the mass spectra generated permit accurate identification of the lack of primary bile acids and presence of atypical bile acids specific to each defect. To date, nine defects in bile acid synthesis have been described i (see Fig. 2-2 ), and all have a highly variable phenotypic expression of familial and progressive infantile or late-onset cholestasis, of syndromes of fat-soluble vitamin malabsorption, and of variable degrees of neurologic involvement ( Fig. 2-4 ). An international screening program at Cincinnati Children's Hospital Medical Center found bile acid synthesis defects to account for 2% of 13,500 screened cases of idiopathic cholestatic liver disease in infants and children. Broadly, these defects can be categorized as deficiencies in the activity of enzymes responsible for catalyzing reactions to the steroid nucleus or to the side chain.
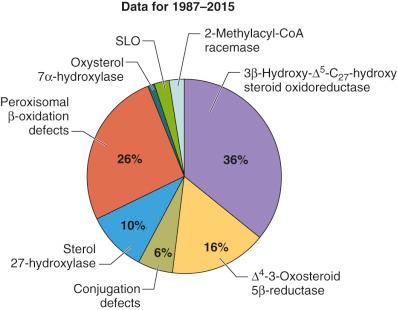
i See .
Three defects involving enzyme-catalyzing reactions that modify the ring structure of the steroid nucleus have been identified. Two reports of a 12α-hydroxylase (CYP8B1) defect have been proposed, but neither has been definitively confirmed. The clinical presentations of the 3β-hydroxy-Δ 5 -C 27 -steroid oxidoreductase (HSD3B7), Δ 5 -3-oxosteroid 5β-reductase (AKR1D1), and oxysterol 7α-hydroxylase (CYP7B1) deficiencies are of progressive cholestatic liver disease. Typical biochemical abnormalities include elevations in serum liver enzymes, conjugated hyperbilirubinemia, and evidence of fat-soluble vitamin malabsorption. A normal γ-glutamyltranspeptidase (GGT) is highly associated with, although not an exclusive feature of, all of the bile acid synthetic defects. Serum cholesterol concentrations are generally normal. The early clinical history of these patients often shows fat-soluble vitamin malabsorption, and in some cases rickets precedes any evidence of liver dysfunction. These abnormalities are usually responsive to oral vitamin supplementation, but these patients eventually present later in life with hepatosplenomegaly and elevated serum liver enzymes. The 3β-hydroxy-Δ 5 -C 27 -steroid oxidoreductase deficiency is the most common of the bile acid synthetic defects, frequently accounting for cases of late-onset chronic cholestasis ( Fig. 2-5 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here