Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bile acids have long been understood to be physiologic detergent molecules synthesized from cholesterol and critical for the absorption of intestinal lipids. More recently we have come to comprehend their broader role as signaling molecules in a variety of metabolic processes such as glucose homeostasis, immune cell function, and regulation of cell growth and proliferation. Thus, it is not surprising that bile acid physiology is a tightly regulated process of synthesis in the liver, transport and biotransformation in the intestines, followed by reabsorption in the ileum. Any alteration in this homeostasis has effects on hepatic metabolic processes, resulting in inflammation and development of diseases such as cholestatic liver diseases, dyslipidemia, diabetes, and even tumorigenesis. , This chapter provides an overview of bile acid synthesis and metabolism with a focus on disorders of bile acid metabolism that result in neonatal liver disease.
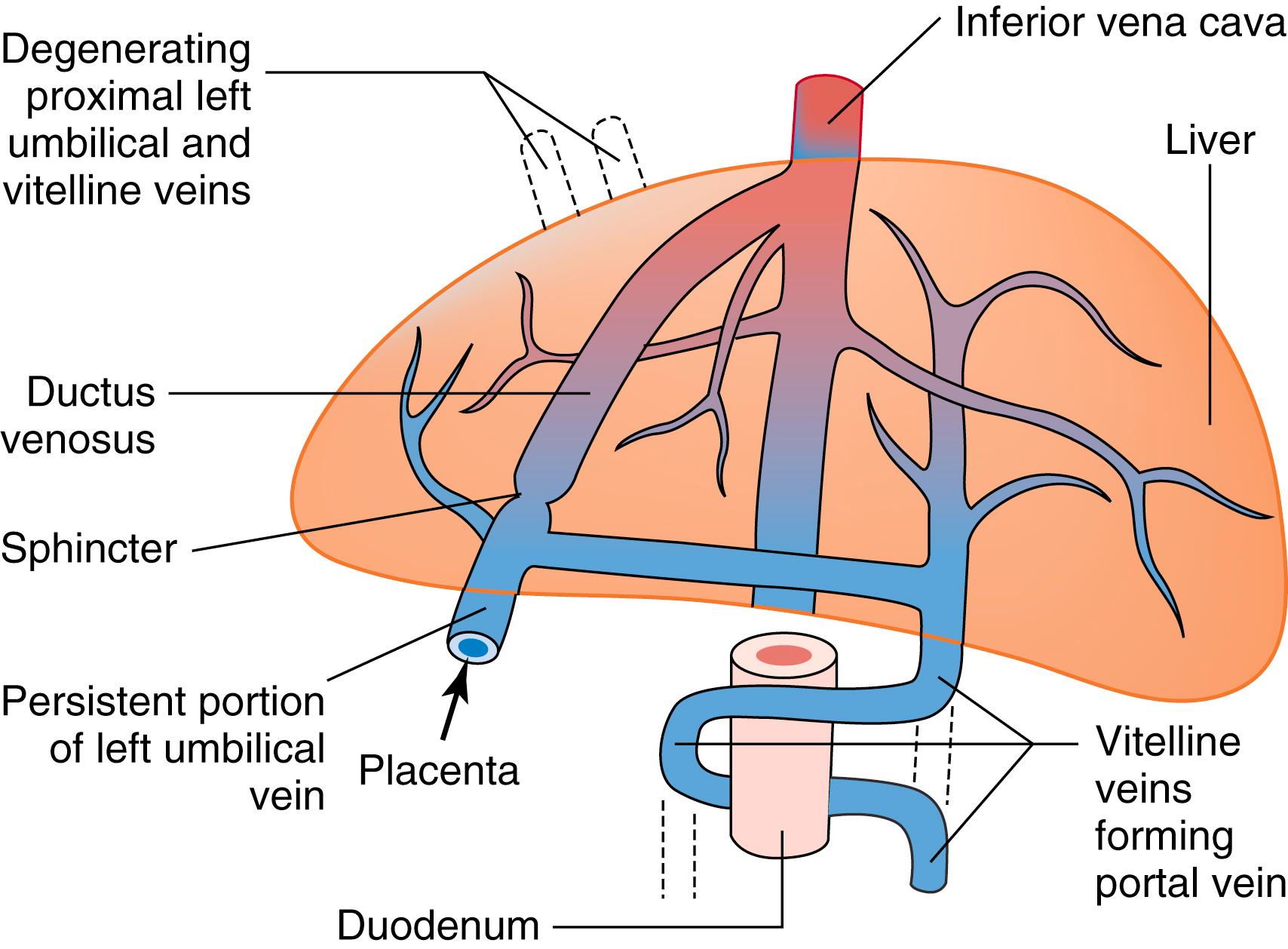
The liver begins as an endodermal bud from the ventral foregut above the yolk sac that forms around the third week of gestation. This bud enlarges and differentiates into a semblance of the adult lobulated structure by the sixth week of gestation. , Concurrently, the intrahepatic biliary tree develops from the cranial bud while the caudal portion gives rise to the extrahepatic biliary tree (gallbladder, common bile duct, and cystic duct) via differentiation of ductal plates. , The vitelline veins, which pass between the yolk sac and the sinus venosus of the heart, eventually form the portal vein by the seventh week. The portal vein then joins with the umbilical vein to form the ductus venosus ( Fig. 89.1 ). In contrast to adult circulation, in the fetus, the ductus venosus shunts blood from the left umbilical vein to the inferior vena cava directly. After birth, enteral feedings trigger the closure of this physiologic shunt, allowing systemic blood flow to instead reach the liver. , , The hepatic artery forms at the eighth week, after the venous system and the establishment of ductal plates. Intrahepatic arterial branches extend into the liver parenchyma and portal tracts between the 10th and 15th weeks of gestation. Therefore, the framework for the hepatic arterial circulation is dependent on the development of the intrahepatic portal and biliary system.
Approximately 80% of the blood reaching the liver is poorly oxygenated blood from the portal vein, which is composed of blood from the intestines, pancreas, and spleen. The other 20% is well-oxygenated blood from the hepatic artery. The two blood sources converge at the level of the hepatic sinusoids ( Fig. 89.2 ), a vascular channel with a fenestrated endothelium that allows the exchange of nutrients, toxins, endobiotics, and xenobiotics between the blood and the adjacent hepatocytes. These sinusoids are lined by endothelial cells that lack a basement membrane and perform endocytotic and synthetic activity. , Other cells present in the lumen of sinusoids include hepatic stellate cells, which play a role in regulation of sinusoidal blood flow, storage of vitamin A, and liver fibrogenesis ; pit cells, which are immunoreactive natural killer (NK) cells ; and phagocytic Kupffer cells. The sinusoids are separated from the hepatocytes by the perisinusoidal space, or the space of Disse, through which nerve fibers course on their way to the lobules. The sinusoids drain into the central veins, which join to form the hepatic veins. The hepatic veins comprise the outflow from the liver that eventually empties into the supra-hepatic inferior vena cava. ,
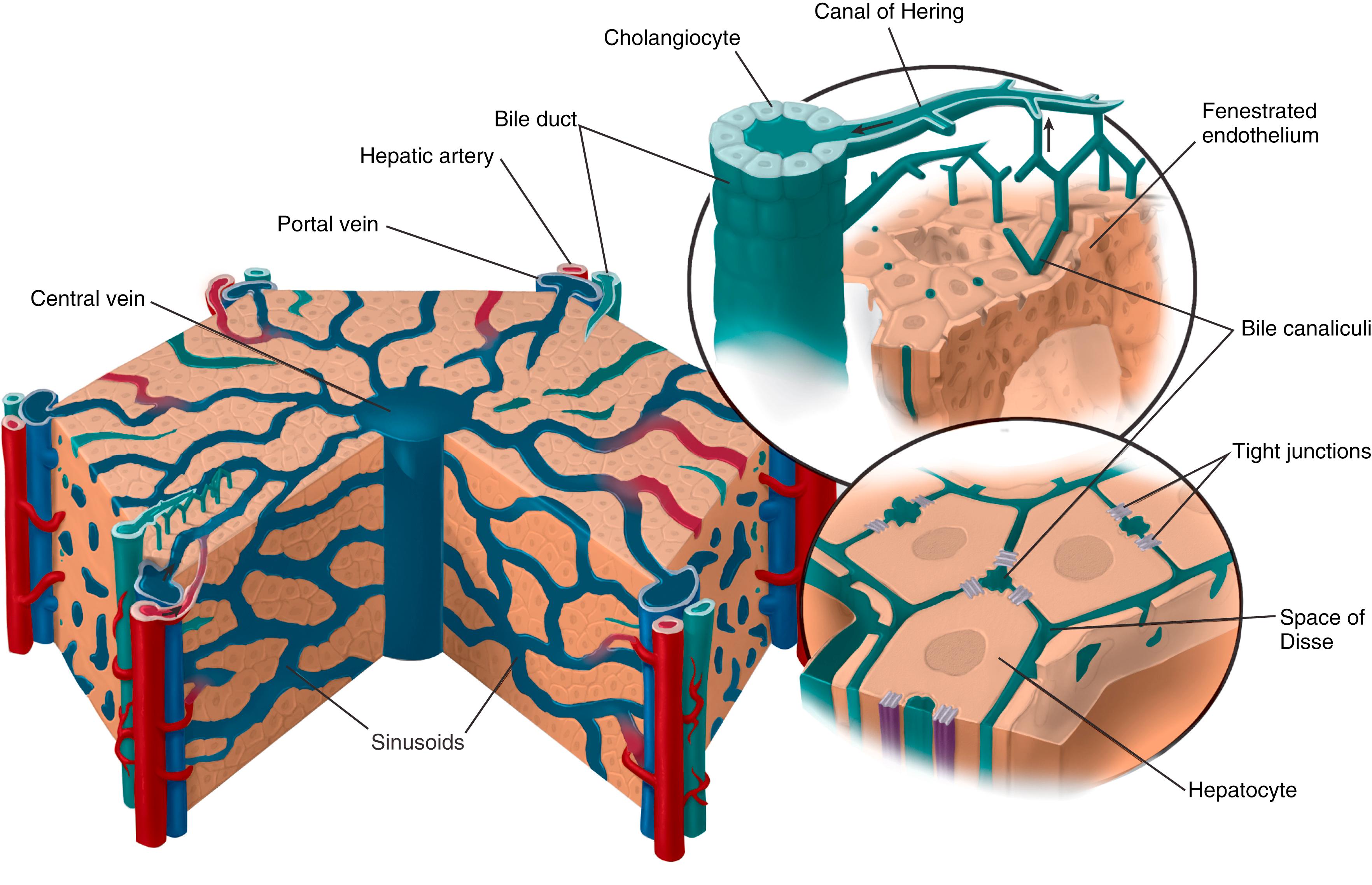
The functional unit of the liver is the hepatic lobule, a polygonal structure with a central vein as its axis and radially arranged sheets of hepatocytes with portal triads (portal vein, hepatic artery, bile duct) at the vertices of the imaginary transverse polygon (see Fig. 89.2 ). The mixed venous and arterial blood from the portal tract flow through the sinusoids past the sheets of hepatocytes, creating a zonal gradient. The periportal region is the closest to the vascular inflow and therefore exposed to the highest concentration of nutrients and is richest in oxygen. The pericentral region is most distal and thus has the lowest oxygen content. In addition, lobular heterogeneity in subcellular structure and gene expression creates a functional zonation with a distinct gradient in relation to fatty acid, glucose, toxin, and bile acid metabolism from periportal to the pericentral regions.
The liver parenchyma is composed primarily of hepatocytes (>70%). Hepatocytes themselves are polarized cells with a basolateral surface that faces the sinusoids and apical surface facing a network of tiny passages that form the bile canaliculi. Both surfaces of the cells have microvilli that increase the surface area available for exchange of different substrates between the spaces. , The lateral surface lies between adjacent hepatocytes and is contiguous with the basal. The basolateral surface has a number of plasma membrane transporters dedicated to the exchange of organic and inorganic solutes. The lateral plasma membrane contains gap junctions that facilitate signaling between hepatocytes. The apical surface also has transporters that allow for secretion of anions and solutes into bile.
The biliary tree begins at the canal of Hering, a transitional zone, wherein the bile canaliculi join the rest of the tree. The canals of Hering contain liver progenitor stem cells capable of regenerative activity when the liver sustains damage. The canals drain into bile ductules that combine to form interlobular ducts, which combine with other interlobular ducts to form septal ducts. These septal ducts coalesce into progressively larger ducts to eventually form segmental and, finally, the right and left hepatic ducts. These two lobular ducts then converge to form the common hepatic duct, which joins the cystic duct to form the common bile duct. The common bile duct combines with the pancreatic duct that empties bile into the intestine.
Cholangiocytes are polarized epithelial cells that line the canals of Hering, bile ducts, and gallbladder (see Fig. 89.2 ). They comprise approximately 5% of the cells of the liver. These cells modify bile composition by secreting electrolytes, organic solutes, and water in response to hormonal signals. As gastric contents enter the duodenum, gastric acid, fat, and protein stimulate the production of several hormones, including secretin. Secretin, in turn, binds to the secretin receptor on cholangiocytes, leading to stimulation of the cystic fibrosis transmembrane conductance regulator (CFTR) and transport of chloride out of the cell. The chloride gradient that is created drives the Cl − /HCO 3 − anion exchanger with final excretion of bicarbonate into bile, thereby alkalinizing the bile. Mutation of the gene encoding CFTR can lead to neonatal jaundice and liver disease in approximately 10% of patients with cystic fibrosis.
Cholangiocytes also absorb glucose, amino acids, and other molecules, further modifying the bile. A small fraction of bile acids in bile are absorbed and returned to the hepatocytes in a pathway known as the cholehepatic shunt , which is hypothesized to promote bile flow. , ,
Bile acids are synthesized from cholesterol through a complex multi-enzyme series of reactions in the hepatocytes ( Fig. 89.3 ). The enzymes for these reactions are found in the cytosol, peroxisomes, mitochondria, and endoplasmic reticulum. There are two main pathways responsible for bile acid synthesis. In the neutral or classic bile acid pathway, the rate-limiting cytochrome P 450 enzyme, cholesterol 7α-hydroxycholesterol (CYP7A1), initiates the conversion of cholesterol to the primary bile acid, cholic acid (CA). In the acidic or alternative pathway, cholesterol 27-hydroxylase (CYP27A1), a mitochondrial P 450 enzyme, catalyzes the first reaction that leads to the final production of chenodeoxycholic acid (CDCA). The acidic pathway contributes to less than 10% of the total bile acid production in humans. Of note, the acidic pathway has been found to be more important in those with liver disease and in neonates. , Mutations of CYP7B1 in neonates have been reported to result in significant cholestatic liver injury, with accumulation of hepatotoxic monohydroxy bile acids.
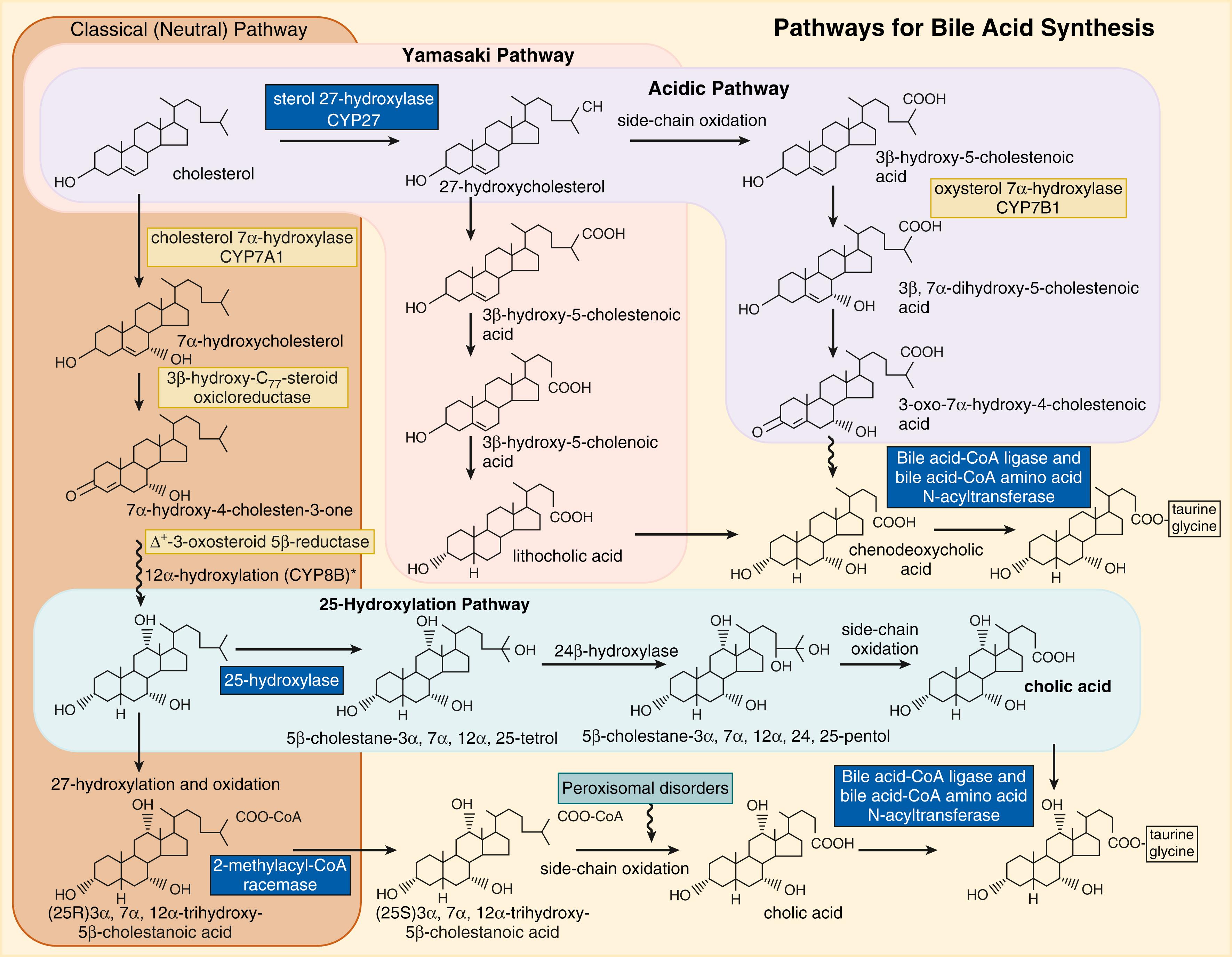
Typically, the primary bile acids, CA and CDCA, are conjugated to the amino acids glycine and taurine in a 3:1 ratio, depending on the availability of dietary taurine and species (e.g., in mice most bile acids are taurine-conjugated). , In contrast, in neonates, the majority (>80%) of bile acids are taurine-conjugated, due to an abundance of taurine stores in the liver. , Conjugation serves to increase the solubility of the bile acids and enables their transport via bile acid transporters on hepatocytes into the bile canalicular system and subsequently into the gallbladder. It also limits their passive reabsorption as they pass down the biliary system. After a meal, the secretion of cholecystokinin induces gallbladder contraction, which releases its contents into the gastrointestinal tract. Greater than 70% of the stored bile is expelled into the proximal small intestine.
Intestinal bile acids act as detergents to emulsify dietary fats and form mixed micelles. Dietary fats and lipid-soluble vitamins are then absorbed by the intestine.
Some of the bile acids are passively absorbed in the proximal small intestine. The vast majority of bile acids secreted into the intestines each cycle are reabsorbed in the ileum by active transport back into the portal system and circulated back to the liver. Transporters on the apical membrane of ileal enterocytes (the apical sodium-dependent bile acid transporter or ASBT, SLC10A2) and on the sinusoidal membrane of the hepatocyte (the Na⁺-taurocholate cotransporting polypeptide or NTCP, SLC10A1) are highly efficient in this process, with recovery of about 95% of secreted bile acids. , , In adults, this bile acid pool of approximately 4 to 5 g is recycled 6 to 10 times per day, with only a small fraction (averaging 0.5 g) lost into the stool. The fecal losses are replenished by de novo bile acid synthesis in the liver. Synthesis of bile acids is regulated by negative feedback by bile acids returning to the liver. (Please see the following discussion of feedback signals.) A small fraction (0.5 mg/day) of circulating bile acid spills over into the systemic circulation and is excreted in urine. ,
The enterohepatic circulation of bile acids serves to control bile acid synthesis through a negative feedback mechanism. Studies of rats fed with bile acids strongly reduced the activity of CYP7A1 and bile acid synthesis, whereas interruption of the enterohepatic circulation with use of bile acid binding resins (e.g., cholestyramine) increased the activity of CYP7A1.
In addition to their historically defined role as emulsifiers, bile acids are now well understood to be ligands for nuclear receptors. These include the farnesoid X receptors (FXR), the vitamin D receptor (NR1I1), the pregnane X receptor (NR1I2), and the liver X receptor (NR1H3), as well as G protein coupled transmembrane receptor protein, TGR5. FXR has been implicated in the regulation of the enterohepatic circulation through downstream activation of fibroblast growth factor-19 (FGF19) and its receptor FGFR4 leading to inhibition of CYP7A1. FXR knockout mice have increased bile acid synthesis and CYP7A1 expression. FXR has also been shown to induce a bile salt export pump (BSEP) on the canalicular membrane of hepatocytes and inhibit a sinusoidal sodium-dependent taurocholate cotransporter (NTCP) that helps in the uptake of bile acids into hepatocytes. ,
The small percentage of primary bile acids that reach the colon undergo significant structural modifications by intestinal bacteria, leading to the formation of secondary bile acids, deoxycholic acid (DCA), and lithocholic acid (LCA), respectively. DCA and LCA are both hydrophobic and known to be hepatotoxic but are mostly excreted into feces.
Bacterial deconjugation creates unconjugated mono- or dihydroxy bile acids, which can be passively absorbed through colonic membrane and recycled to the liver. The enzymes that catalyze the transformations are found in bacterial organisms such as Bacteroidetes , Clostridium species, Bifidobacteriaceae , and Enterococcus . , Gut bacteria are thought to benefit from bile metabolism through acquisition of glycine and taurine, which can be utilized as an energy source in metabolism. , These alterations in intestinal microbiota can also have effects on bile acid pool size and composition, relevant to multiple chronic disease states.
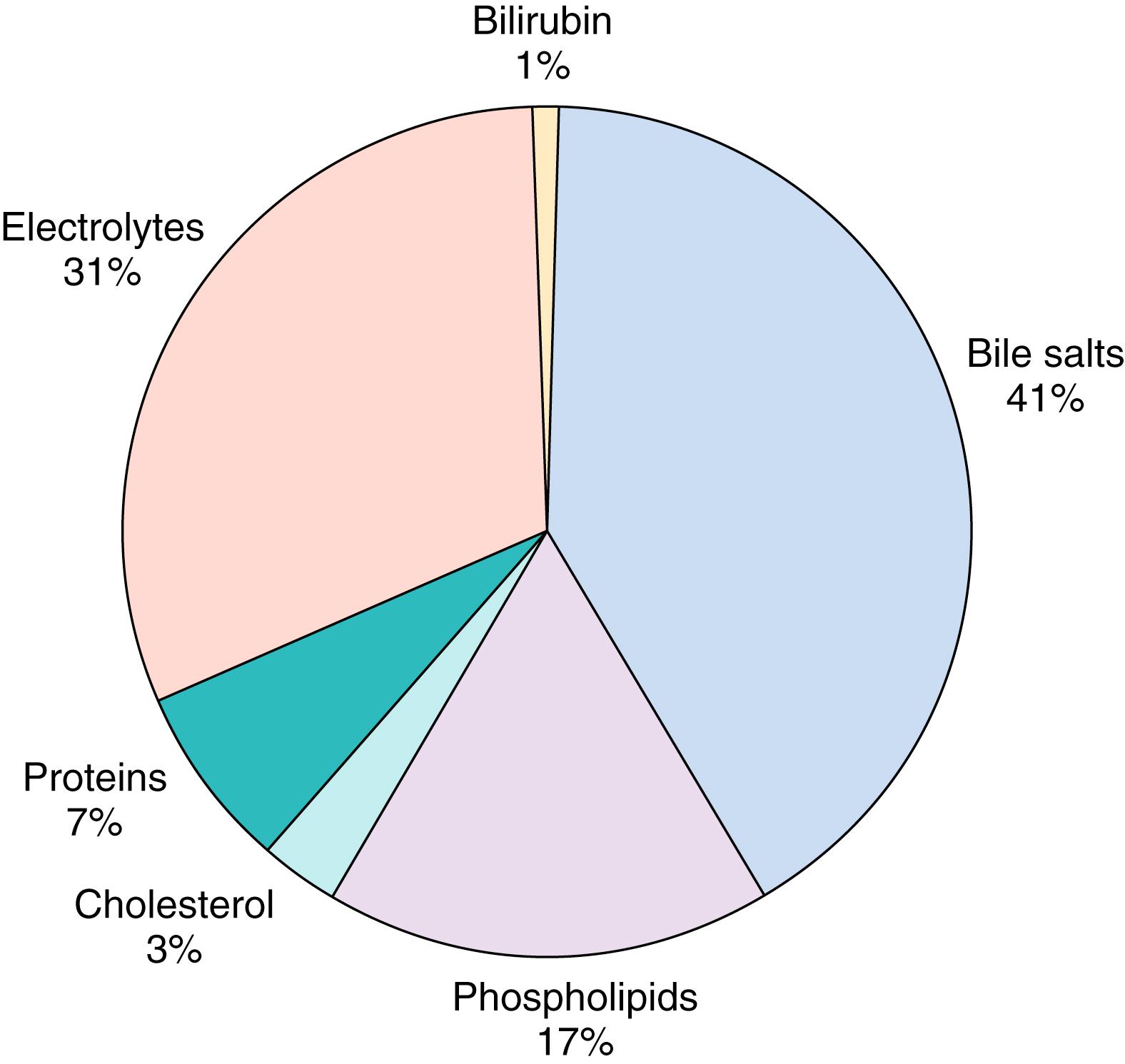
Bile is a complex aqueous secretion that consists of 95% water and organic and inorganic solutes, the most abundant being bile acids, which are found at concentrations of 20 to 30 mmol/L ( Fig. 89.4 ). Adult human bile acids consist of approximately 40% CA, 40% CDCA, 20% DCA, and trace amounts of LCA. Phosphatidylcholine is the major phospholipid in bile, whereas cholesterol, which makes up approximately 3% of biliary solute, is the predominant sterol. The formation of micelles with phosphatidylcholine and bile acids/cholesterol (2:1 ratio) lowers the free bile acid concentration, thereby protecting the canalicular membranes from the detergent action of bile acids. This point is made clear through animal models wherein the absence of phosphatidylcholine is associated with rapid development of liver injury. Imbalances in the bile acid/cholesterol to phospholipid ratio can result in the formation of sludge and gallstones. Finally, waste products such as bilirubin (from heme degradation, which gives bile its characteristic yellow color) are also eliminated via bile secretion.
Heavy metals such as iron, copper, manganese, and zinc are also excreted in bile. Retention of these substances and impairments in the processing of these potentially toxic components due to inherited mutations in genes involved in these pathways can contribute to liver disease, as seen in adult-onset hemochromatosis (iron) and Wilson disease (copper), but impairments in metal-handling processes are not clinically relevant in infants and young children. ,
The average human adult liver produces up to 600 mL of bile per day. Bile flow into the canalicular system is divided into two components: bile acid–dependent bile flow and bile acid–independent flow ( Fig. 89.5 ). The major driving force for bile secretion is the active transport of bile acids into the canalicular lumen. There is a linear relationship between the secretion of bile acids and bile flow.
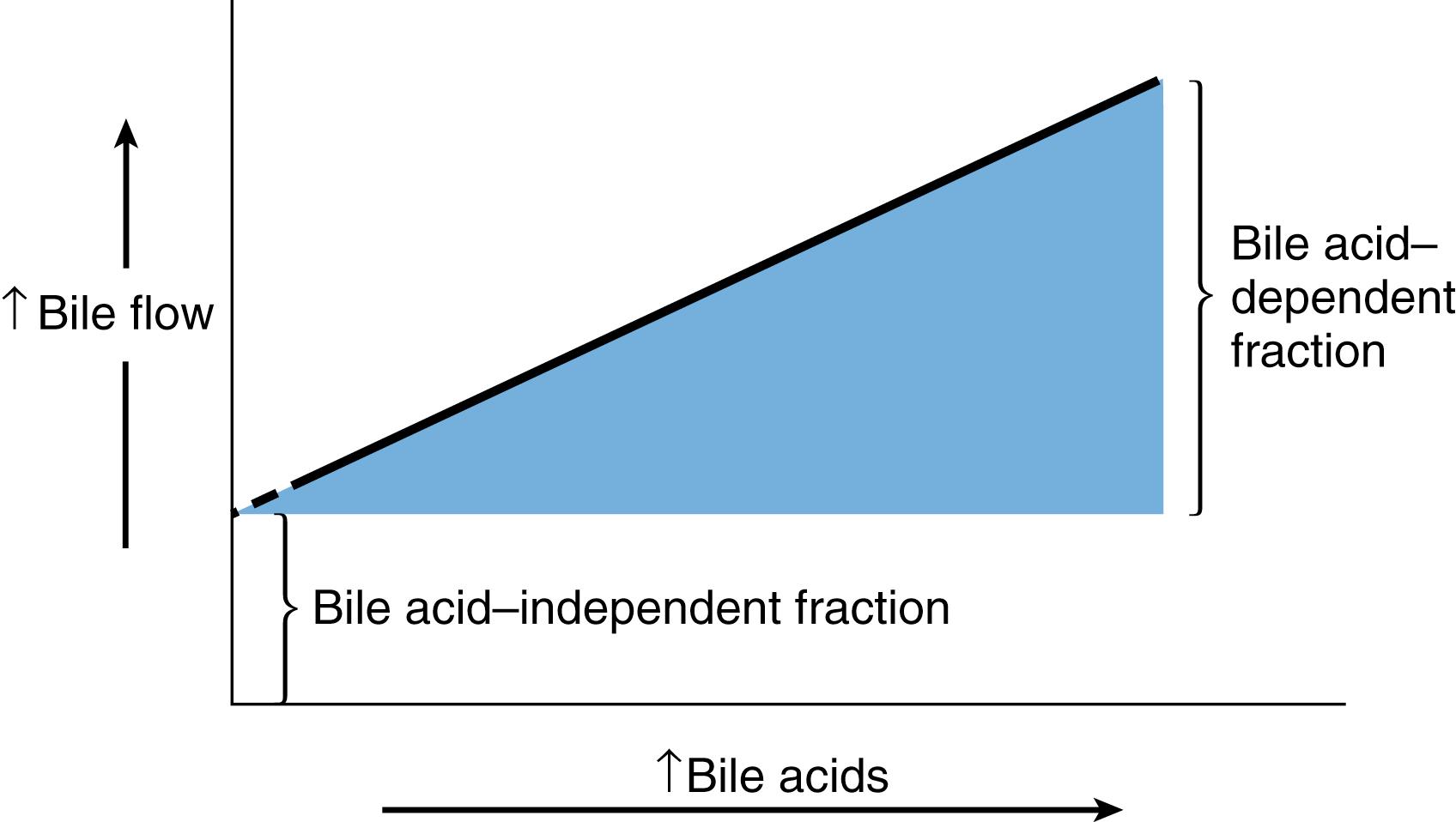
Bile acid–independent bile flow is largely dependent on the secretion of other solutes such as glutathione and glutathione conjugates, which are present in hepatocytes at relatively high concentrations of 5 to 10 mmol/L. Water, electrolytes, and other solutes follow passively in response to the osmotic gradient primarily by way of the paracellular route via tight junctions, although aquaporins are also present. In patients with cholestasis, administration of bile acids such as ursodeoxycholic acid will increase bile acid pool size and promote increased bile flow through the biliary system.
Canalicular bile is further modified by bicarbonate secretion from cholangiocytes in response to stimulation by hormones such as secretin. The binding of secretin to the bile duct cell results in phosphorylation of a chloride channel that increases chloride diffusion into the bile duct lumen. Chloride is then transported back to the cell via a chloride/bicarbonate antiporter at a ratio of 2Cl − /3HCO 3 − . ,
Disruption of normal flow of bile at any level results in cholestasis, an abnormal retention of bile acids and bilirubin in the blood and liver. Cholestatic disorders can be separated into extrahepatic or biliary causes (e.g., structural abnormalities such as biliary atresia) and intrahepatic or hepatocellular causes (e.g., impairment in bile transport, genetic or metabolic disorders, infection). , Cholestasis in infants manifests as jaundice, acholic or hypopigmented stools, pruritus, and fat-soluble vitamin deficiencies. Long-standing cholestasis can result in injury to hepatocytes, leading to hepatocellular synthetic dysfunction and eventual liver failure. In in vitro experiments, exposure of the basolateral membrane of hepatocytes and cholangiocytes to high bile acid concentrations caused disruption of cellular membranes, apoptosis, and necrosis. In reaction to chronic cholestasis, human hepatocytes undergo a number of adaptations mediated by FGF19 (Fgf15 in mice). In normal conditions, bile acids reaching the terminal ileum activate FXR and produce FGF19. During cholestasis, interruption of the enterohepatic cycle results in decreased bile acid absorption in the ileum. However, the serum level of FGF19 is actually elevated in cholestatic patients, due to ectopic production of FGF19 by the liver. FGF19 subsequently binds to its receptor FGFR4 causing downregulation of CYP7A1, thereby decreasing bile acid synthesis. In mice that have undergone bile duct ligation, adaptations of the canalicular system occur with increases in diameter of the canaliculi and length of spine-like protrusions into hepatocytes. This is similar to morphologic changes that occur in the obstructive disease, biliary atresia.
Serum levels of bilirubin and bile acids are used as clinical markers of cholestasis, and tests are cost effective. To distinguish cholestasis from benign causes of jaundice, serum bilirubin should be separated into unconjugated and conjugated fractions. Unconjugated hyperbilirubinemia is commonly seen and may be due to physiologic jaundice, breastmilk or breastfeeding jaundice, Gilbert syndrome, Crigler-Najjar syndrome, and other systemic diseases. Conjugated hyperbilirubinemia, however, is likely pathologic and is defined by a conjugated bilirubin level greater than 1 mg/dL (if total bilirubin is less than 5 mg/dL) or greater than 20% of the total bilirubin (if total bilirubin is greater than 5 mg/dL). If conjugated hyperbilirubinemia is found, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (AP), prothrombin time (PT) and international normalized ratio (INR), and albumin levels should be checked. Elevations of AST and ALT indicate damage to hepatocytes. Elevated levels of GGT and AP suggest damage to cholangiocytes; however, some cholestatic disease (e.g., PFIC 1) may present with normal or low GGT. , Disturbances in PT, INR, and albumin levels are indicators of liver synthetic function.
Imaging studies can be obtained to assess liver and biliary structures, detect abnormalities in arterial or venous flow, or identify splenic malformations. A percutaneous liver biopsy remains integral to the diagnostic workup of infants with cholestasis. In addition to providing potential diagnostic data, a liver biopsy can also offer prognostic information to predict outcomes.
In the fetus, the placenta is the primary site of bile acid metabolism and detoxification and elimination of lipophilic organic anions. In fact, the fetal liver functions as the major site of hematopoiesis and does not play a major part in bile acid metabolism. At birth, there is an immediate transition wherein the role of metabolism and detoxification transfers from placenta to the liver. However, the relative immaturity of the neonatal liver at birth is seen in studies demonstrating low bile acid synthesis and clearance. This hepatic immaturity is further compounded if the birth is premature.
Having said that, bile acids have been detected in the fetal liver and gallbladder as early as 14 to 16 weeks gestation, and by 22 to 26 weeks gestation, the principal bile acids found in fetal gallbladder bile are taurine-conjugated dihydroxy bile acids. After approximately 28 weeks’ gestation, taurocholic acid (TCA), a trihydroxy bile acid, and glycocholic acid are also present. , , In newborn infants, analysis of meconium and bile reveal a predominance of CDCA, which may indicate utilization of an alternative pathway favoring CDCA production. By 2 to 7 months of age, the proportion of glycine conjugates increases and reaches adult levels.
Another distinction in bile acid composition in the fetus is the lack of secondary bile acid production. Secondary bile acids are unable to be produced in the fetus as this process requires enzymatic cleavage by colonic bacteria, which are not present in the relatively sterile fetal intestine. The presence of secondary bile acids in human fetal circulation implies transfer occurring from the maternal pool to the fetus. Specific transporters found on the brush-border and basolateral membranes of the placental syncytiotrophoblast may therefore provide bidirectional transport of bile acids between the fetal and maternal circulations ( Fig. 89.6 ).
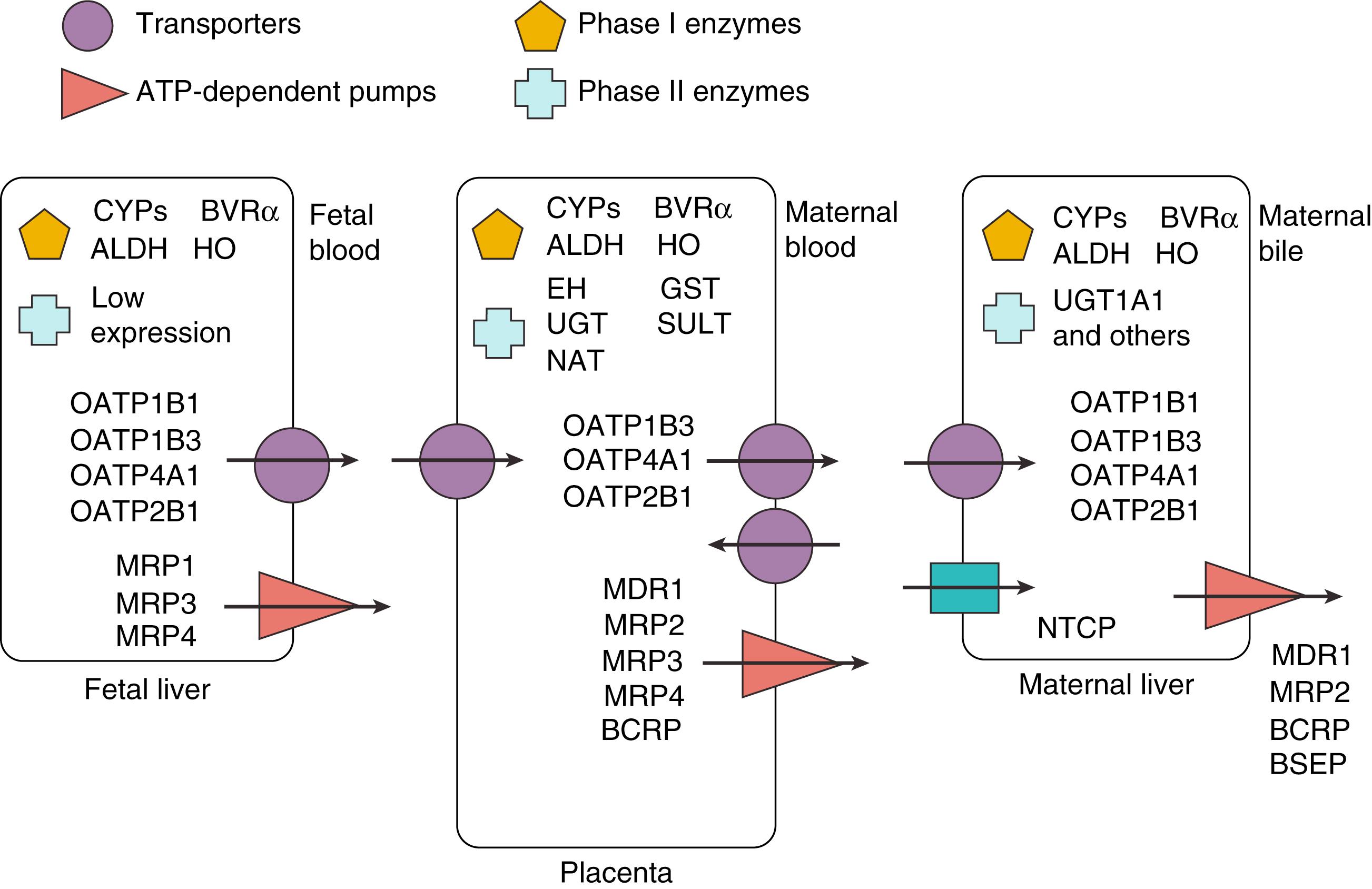
Elimination of bile acids from the fetal liver occurs first with hydroxylation of bile acids into more hydrophilic forms by phase I enzymes CYP3A4 and CYP3A7 present in hepatocytes. Export of these bile acids across the basolateral membrane occurs via transporters belonging to the organic anion-transporting polypeptide (OATP) family as well as adenosine triphosphate (ATP)-dependent pumps of the ATP-binding cassette (ABC) superfamily, including multidrug resistance protein (MRP)1 and isoforms, MRP3 and MRP4. Next, carrier proteins such as albumin and lipoproteins shuttle bile acids through the fetal circulation to the basolateral (fetal-facing) membrane of the placenta.
The enterohepatic circulation is relatively immature in the developing fetus. Perfusion studies performed in near-term fetal dog using radiolabeled taurocholate suggest that bile reabsorption occurs passively in the jejunum, but active bile salt reabsorption in the ileum is poorly developed. In fact, the ability to excrete bile acids into the canaliculus does not mature until the first year after birth. The poor reabsorptive capacity of the fetus coupled with the immaturity of hepatic synthetic mechanisms for bile acid production and efficient clearance through the maternal placenta leads to a small bile acid pool size in the fetus and neonate. , The term neonate has a reduced bile acid pool size that is approximately half of that of an adult. The premature neonate’s bile acid pool size is even more reduced at one-third that of a term neonate. It is of note that term neonates are capable of responding to increased fecal losses with the synthesis of new bile acids. This may represent a step-up in bile acid synthesis in order to grow an effective bile acid pool.
Further, the adrenal axis appears to play a role in maturation of the synthesis and enterohepatic recirculation of bile acids in the fetus. This is likely through an increase in transporter expression and function as the fetus develops. The administration of prenatal dexamethasone to healthy premature infants has been shown to increase the bile acid pool size in preterm neonates to levels equivalent to those for full-term infants and nearly four times those for untreated premature infants. Additional animal studies demonstrate increase in bile acid synthesis after treatment with cortisone.
The immaturity of the fetal liver and the reduced bile acid pool size may result in an intraluminal bile acid concentration too low to support normal lipid absorption efficiently. Effects of this are manifest as fat malabsorption, poor growth, and deficiencies in fat-soluble vitamins, such as vitamin D.
Despite a lower bile acid synthesis rate and small bile acid pool size, serum bile acid levels are notably increased in term and preterm neonates, reaching values as high as those found in adults with cholestasis. This degree of “physiologic cholestasis” improves over the first years of life with improvement in hepatic uptake of bile acids from the portal circulation.
Bile acids can easily pass through the placenta in a bidirectional process that is dependent on relative concentration gradients between the fetal and maternal circulation. As the bile acid concentration in the fetus is higher than in maternal serum because there is no significant excretion through the normal hepatobiliary systems, transfer of bile acids typically occurs across the placenta toward the mother and is subsequently eliminated via the maternal liver. However, in cases of maternal cholestasis (e.g., intrahepatic cholestasis of pregnancy), the increased levels of maternal bile acids counterbalance or even reverse bile acid flux across the placenta. However, accumulation of bile acids in the fetal compartment is potentially toxic and can result in fetal distress and even sudden intrauterine death. Thus, the maintenance of a normal fetal-maternal bile acid steady state favoring bile acid flux from fetus to mother is crucial to normal fetal physiology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here