Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Uterine fibroids (also called leiomyomas) are benign smooth muscle tumors, and about 80% are asymptomatic. They are very common, with an estimated prevalence of 70% by the sixth decade of life. Uterine tumors presenting as fibroids are rarely malignant, with less than 1 in 1000 leiomyosarcomas found at the time of surgical removal. Fibroids may cause abnormal uterine bleeding, pelvic discomfort, and pressure when they enlarge. They can cause pain (sometimes severe) if degeneration and infarction occur.
Fibroids arise within the myometrium (intramural) but may grow near the serosal surface (subserosal) or near the endometrium (submucosal). Some fibroids are pedunculated. About 40% of fibroids enlarge during the first trimester of pregnancy, but rarely thereafter.
Medical treatment with progestins, gonadotropin-releasing hormone (GnRH) analogues, or other hormones may be indicated initially for uterine bleeding and fibroid enlargement. Uterine artery embolization (UAE) and magnetic resonance directed ultrasound may be used as alternatives to surgery. Surgical treatment ranges from myomectomy (removal of one or several fibroids) for women who desire fertility or uterine preservation to hysterectomy when less invasive treatments fail.
Endometrial and cervical polyps may cause uterine bleeding and must be biopsied to rule out cancer. Complex atypical endometrial hyperplasia progresses to endometrial cancer in about 20-30% of cases. Simple hyperplasia may be treated medically.
Congenital anomalies of the uterus and cervix are most often due to incomplete fusion of the paramesonephric (müllerian) ducts, incomplete dissolution of the midline fusion of those ducts, or formation failures. Diagnosis of uterine defects can be made by hysterosalpingogram or magnetic resonance imaging. Some defects may be diagnosed and treated by hysteroscopy.
Congenital anomalies of the uterus and cervix are most often caused by incomplete fusion of the paramesonephric (müllerian) ducts, incomplete dissolution of the midline fusion of those ducts, or formation failures. Diagnosis of uterine defects can be made by hysterosalpingogram (HSG) or magnetic resonance imaging (MRI). Some defects may be diagnosed and treated by hysteroscopy.
Benign conditions of the uterine corpus and cervix are commonly encountered in gynecologic practice, because they may adversely affect a woman's fertility, cause abnormal uterine bleeding, or cause pelvic pain. In this chapter, benign neoplasms, epithelial changes, functional disorders of the uterus (corpus and cervix), and congenital anomalies are discussed along with both conventional and emerging therapies. Cervical dysplasia along with cervical cancer is covered in Chapter 38 .
Uterine fibroids are benign tumors derived from the smooth muscle cells of the myometrium. They are also referred to as leiomyomas or myomas, but “fibroid” is most often used today. Fibroids are the most common neoplasm of the uterus. Estimates are that more than 70% of women have fibroids by the age of fifty, but most are asymptomatic. However, fibroids may be associated with heavy menstrual bleeding or infertility, pelvic pressure related to uterine bulk and, rarely, pain secondary to degeneration. Fibroids are the primary indication for as many as one-third of the 500,000 hysterectomies performed in the United States each year. Although fibroids have the potential to grow to be large, they rarely become malignant. Leiomyosarcomas occur in less than 1 per 1000 women operated on for presumed fibroids. Rapid growth is not always a reliable sign of leiomyosarcoma.
Risk factors associated with developing fibroids include increasing age during the reproductive years, ethnicity, nulliparity, and family history. African American women have a 2- to 3-fold increased risk of developing fibroids compared with white women, and they may develop more numerous fibroids and at a younger age. Some studies have suggested that higher body mass index (BMI) may be associated with a greater risk of developing fibroids. In other studies, oral contraceptive pills have been associated with a reduced risk.
Factors that initiate fibroids are not known. These benign growths are monoclonal, and about 40% are chromosomally abnormal. The remaining 60% may have as yet undetected mutations or epigenetic changes. Genetic differences between fibroids and leiomyosarcomas indicate that leiomyosarcomas do not result from malignant degeneration of fibroids. Ovarian sex steroids, both estrogen and progesterone, are important for the growth of fibroids. Fibroids rarely develop before menarche and seldom develop or enlarge after menopause, unless stimulated by exogenous hormones. Approximately 40% of fibroids enlarge during pregnancy. Most of the growth occurs in the first trimester and they seldom interfere with the course of the pregnancy.
Fibroids have increased levels of estrogen and progesterone receptors compared with other smooth muscle cells. Estrogen stimulates the proliferation of smooth muscle cells, whereas progesterone increases the production of proteins that interfere with programmed cell death (apoptosis). Many growth factors are over-expressed in fibroids, including those that stimulate the production of fibronectin and collagen, both of which are major components of the extracellular matrix that characterizes fibroids. Other growth factors include those that increase smooth muscle proliferation and DNA synthesis, as well as those that promote mitogenesis and angiogenesis.
Fibroids are usually elliptical or spherical, well-circumscribed, white, firm lesions with a whorled appearance on cut section. Although the fibroid appears discrete, it does not have a true cellular capsule. Compressed smooth muscle cells on the tumor's periphery provide the false impression of a true capsule. This pseudocapsule contains a rich network of blood vessels, but few blood vessels and lymphatics actually traverse the pseudocapsule into the fibroid.
Fibroids can undergo degenerative changes, most commonly hyaline acellularity, in which the fibrous and muscular tissues are replaced with hyaline tissue. If the hyaline substance breaks down from a further reduction in blood supply, cystic (fluid) degeneration may occur. Calcification may occur in degenerated fibroids, particularly after the menopause. Fatty degeneration may also occur but is rare. During pregnancy, 5-10% of women with fibroids undergo a painful red or carneous degeneration caused by hemorrhage into the tumor.
Fibroids always arise within the myometrium (intramural) but may develop near the serosal surface (subserosal) or the endometrium (submucosal), as depicted in Figure 19-1 . Fibroids very near the serosal or endometrial surfaces may develop pedicles. The submucosal fibroids can be propelled by uterine contractions until they extend through the endocervical canal and deliver through the cervical os. This process can be associated with significant bleeding and cramping pain. A subserosal fibroid on a long pedicle can present as a mass that feels separate from the uterus. MRI is often helpful to differentiate a pedunculated fibroid from other types of pelvic masses. Very rarely, pedunculated subserosal fibroids attach to the blood supply of the omentum or bowel mesentery and lose their uterine connections to become parasitic growths. Fibroids can also arise in the cervix, between the leaves of the broad ligament (intraligamentous), and very rarely in the various supporting ligaments (round or uterosacral) of the uterus.
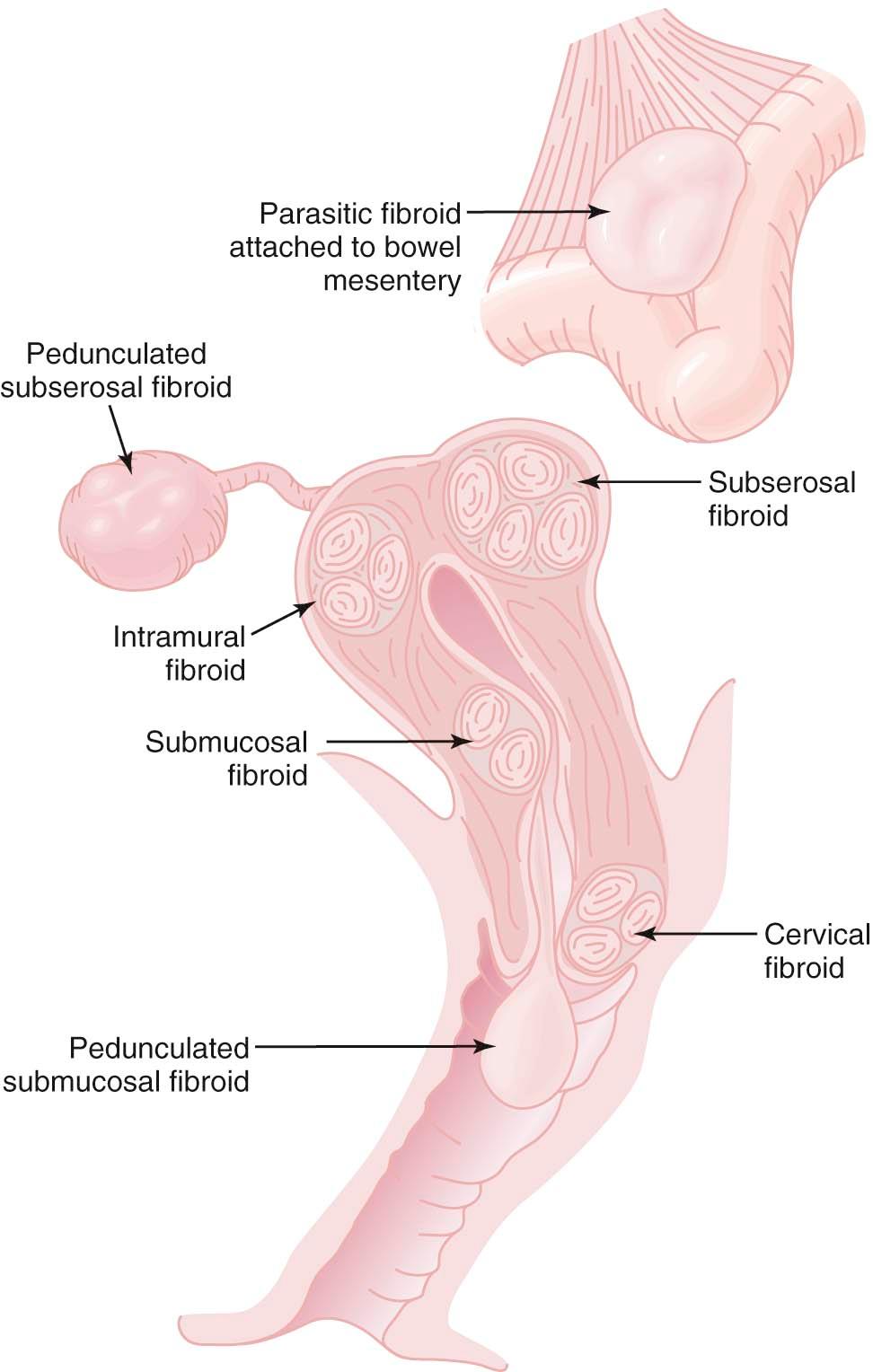
The majority of uterine fibroids (approximately 80%) cause no symptoms. Occasionally, a woman may be able to feel a lower abdominal mass when the fibroid protrudes above the pelvis. For women with fibroids that are asymptomatic or mildly to moderately symptomatic, “watchful waiting” may allow treatment to be deferred, perhaps indefinitely. Symptomatic women may complain of pelvic pressure, congestion, bloating, or a feeling of heaviness in the lower abdomen. Rarely, lower back pain may be associated with fibroids. If the fibroid presses upon the bladder, it may cause frequency of urination or nocturia. A large fibroid in the lower uterine segment or near the cervix may compress the vesicourethral junction and lead to urinary retention. Compression of the ureters with resultant hydronephrosis is very rare and, if suspected, can be ruled out with a renal ultrasound.
Prolonged or heavy menstrual bleeding may be associated with submucosal fibroids. Growth factors secreted by fibroids interfere with the blood-clotting cascade. Intermenstrual bleeding is not characteristic of these tumors but may occasionally occur with submucosal fibroids ulcerating through the endometrial lining. Excessive bleeding may result in anemia, weakness, and even dyspnea.
Fibroids do not usually cause pain, but severe pain may occur when degeneration (acute infarction) occurs within a fibroid. Dyspareunia can occur with posterior fibroids near the vaginal cul-de-sac. There is an increased incidence of secondary dysmenorrhea in women with uterine fibroids, generally caused by heavy menstrual bleeding. Although many women with uterine fibroids become pregnant and carry their pregnancies to term, submucosal fibroids may be associated with an increased incidence of infertility, possibly due to growth factor secretion by the fibroid that may interfere with implantation.
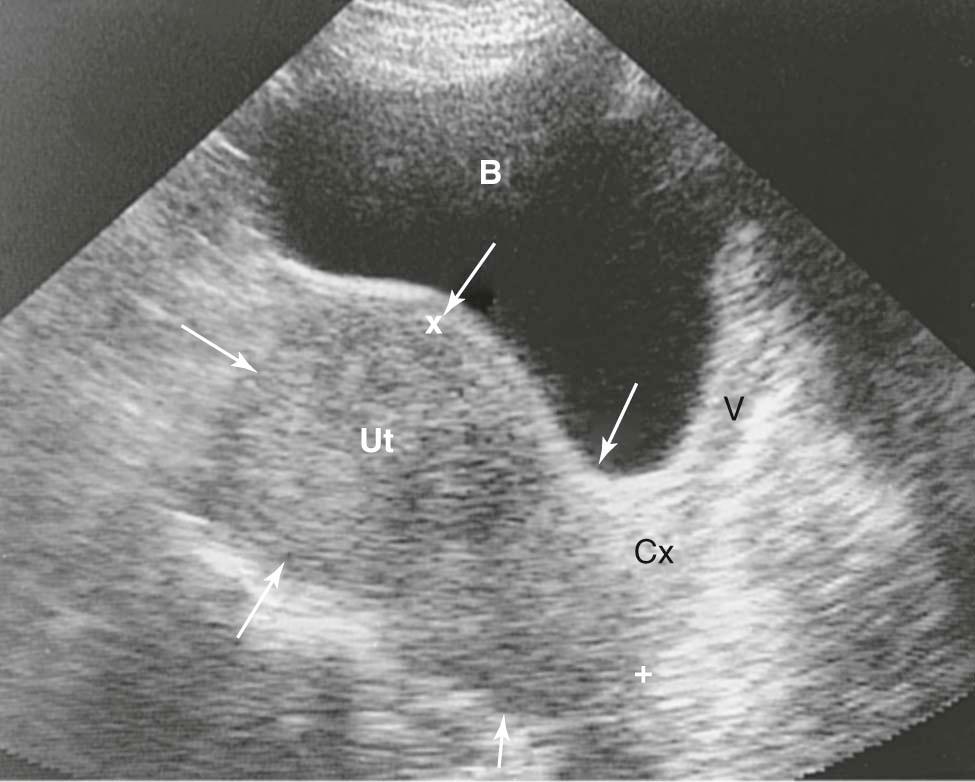
Fibroids smaller than a 12- to 14-week gestation are usually confined to the pelvis, but larger fibroids can be palpated abdominally. Before examination, the bladder should be emptied because a full bladder will alter the examiner's impression of uterine size. On bimanual pelvic examination, a firm, irregularly enlarged uterus with smoothly rounded or bosselated protrusions may be felt if the fibroids are subserosal or intramural. The tumors are usually nontender, although degenerating fibroids can be tender to palpation. Their consistency may vary from rock hard, as in the case of a calcified postmenopausal leiomyoma, to soft or even cystic, as in the case of cystic degeneration. In general, the fibroid uterus is in the midline, but sometimes a large portion of the fibroid lies in the lateral aspect of the pelvis and may be indistinguishable from an adnexal mass. If the mass moves with the cervix, it is suggestive of a fibroid. Often the presence of a fibroid precludes a proper evaluation of the adnexa, but ultrasonic imaging, as seen in Figure 19-2 , can help to distinguish adnexal masses from laterally placed fibroids.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here