Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As humans have ascended to higher and higher altitudes in aviation, mountain climbing, and space exploration, it has become progressively more important to understand the effects of altitude and low gas pressures on the human body. This chapter deals with these problems and acceleratory forces, weightlessness, and other challenges to body homeostasis that occur at high altitudes and in space flight.
Table 44-1 lists the approximate barometric and oxygen pressures at different altitudes, showing that at sea level, the barometric pressure is 760 mm Hg; at 10,000 feet, it is only 523 mm Hg; and at 50,000 feet, it is 87 mm Hg. This decrease in barometric pressure is the basic cause of all the hypoxia problems in high-altitude physiology, because as the barometric pressure decreases, the atmospheric oxygen partial pressure (P o 2 ) decreases proportionately, remaining at all times slightly less than 21% of the total barometric pressure; at sea level, P o 2 is about 159 mm Hg, but at 50,000 feet, P o 2 is only 18 mm Hg.
| Altitude (ft/m) | Barometric Pressure (mm Hg) | P o 2 in Air (mm Hg) | Breathing Air | Breathing Pure Oxygen | ||||
|---|---|---|---|---|---|---|---|---|
| P co 2 in Alveoli (mm Hg) | P o 2 in Alveoli (mm Hg) | Arterial Oxygen Saturation (%) | P co 2 in Alveoli (mm Hg) | P o 2 in Alveoli (mm Hg) | Arterial Oxygen Saturation (%) | |||
| 0 | 760 | 159 | 40 (40) | 104 (104) | 97 (97) | 40 | 673 | 100 |
| 10,000/3048 | 523 | 110 | 36 (23) | 67 (77) | 90 (92) | 40 | 436 | 100 |
| 20,000/6096 | 349 | 73 | 24 (10) | 40 (53) | 73 (85) | 40 | 262 | 100 |
| 30,000/9144 | 226 | 47 | 24 (7) | 18 (30) | 24 (38) | 40 | 139 | 99 |
| 40,000/12,192 | 141 | 29 | 36 | 58 | 84 | |||
| 50,000/15,240 | 87 | 18 | 24 | 16 | 15 | |||
Even at high altitudes, carbon dioxide (CO 2 ) is continually excreted from the pulmonary blood into the alveoli. In addition, water vaporizes into the inspired air from the respiratory surfaces. These two gases dilute the O 2 in the alveoli, thus reducing the O 2 concentration. Water vapor pressure in the alveoli remains at 47 mm Hg as long as the body temperature is normal, regardless of altitude.
In the case of CO 2 , during exposure to very high altitudes, the alveolar partial pressure of CO 2 (P co 2 ) falls from the sea level value of 40 mm Hg to lower values. In the acclimatized person, who increases ventilation about fivefold, the P co 2 falls to about 7 mm Hg because of increased respiration.
Now let us see how the pressures of these two gases affect the alveolar O 2 . For example, assume that the barometric pressure falls from the normal sea level value of 760 to 253 mm Hg, which is the usual measured value at the top of 29,028-foot Mount Everest. Of this, 47 mm Hg must be water vapor, leaving only 206 mm Hg for all the other gases. In the acclimatized person, 7 mm Hg of the 206 mm Hg must be CO 2 , leaving only 199 mm Hg. If there were no use of O 2 by the body, one-fifth of this 199 mm Hg would be O 2 and four-fifths would be nitrogen—that is, the P o 2 in the alveoli would be 40 mm Hg. However, some of this remaining alveolar O 2 is continually being absorbed into the blood, leaving about 35 mm Hg O 2 pressure in the alveoli. At the summit of Mount Everest, only the best acclimatized people can barely survive when breathing air. However, the effect is very different when the person is breathing pure O 2 , as we see in the following discussions.
The fifth column of Table 44-1 shows the approximate P o 2 values in the alveoli at different altitudes when one is breathing air for both the unacclimatized and the acclimatized person. At sea level, the alveolar P o 2 is 104 mm Hg. At 20,000 feet altitude, it falls to about 40 mm Hg in the unacclimatized person but only to 53 mm Hg in the acclimatized person. The reason for the difference between these two is that alveolar ventilation increases much more in the acclimatized person than in the unacclimatized person, as we discuss later.
Figure 44-1 shows arterial blood O 2 saturation at different altitudes while a person is breathing air and while breathing O 2 . Up to an altitude of about 10,000 feet, even when air is breathed, the arterial O 2 saturation remains at least as high as 90%. Above 10,000 feet, the arterial O 2 saturation falls rapidly, as shown by the blue curve of the figure, until it is slightly less than 70% at 20,000 feet and much less at still higher altitudes.
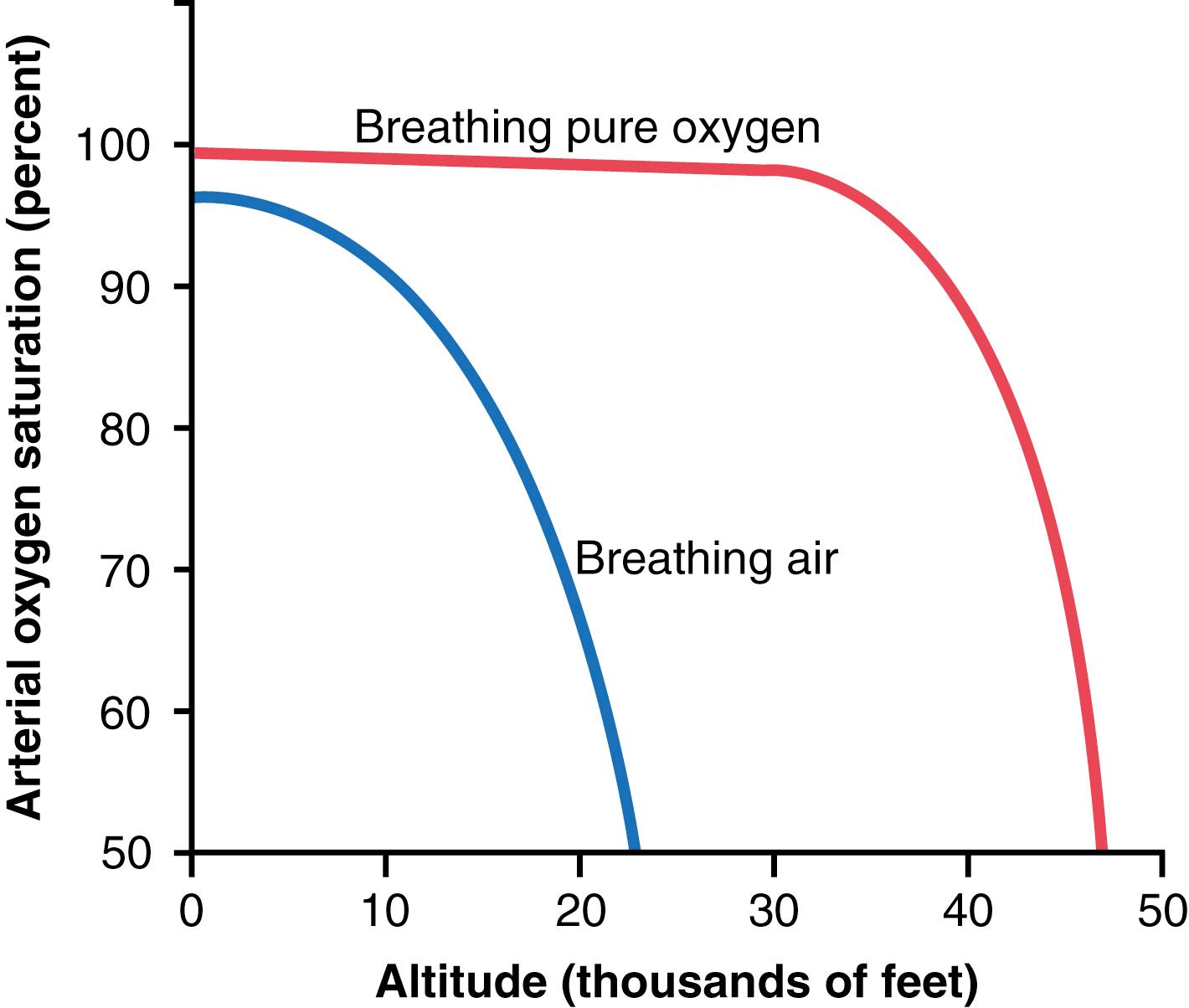
When a person breathes pure O 2 instead of air, most of the space in the alveoli formerly occupied by nitrogen becomes occupied by O 2 . At 30,000 feet, an aviator could have an alveolar P o 2 as high as 139 mm Hg instead of 18 mm Hg when breathing air (see Table 44-1 ).
The red curve of Figure 44-1 shows arterial blood hemoglobin O 2 saturation at different altitudes when a person is breathing pure O 2 . Note that the saturation remains above 90% until the aviator ascends to about 39,000 feet; then it falls rapidly to about 50% at about 47,000 feet.
When comparing the two arterial blood O 2 saturation curves in Figure 44-1 , one notes that an aviator breathing pure O 2 in an unpressurized airplane can ascend to far higher altitudes than one breathing air. For example, the arterial saturation at 47,000 feet when one is breathing O 2 is about 50% and is equivalent to the arterial O 2 saturation at 23,000 feet when one is breathing air. In addition, because an unacclimatized person usually can remain conscious until the arterial O 2 saturation falls to 50%, the ceiling for an aviator for short exposure times in an unpressurized airplane when breathing air is about 23,000 feet and, when breathing pure O 2 , is about 47,000 feet, provided that the equipment supplying the O 2 operates perfectly.
Some of the important acute effects of hypoxia in the unacclimatized person breathing air, beginning at an altitude of about 12,000 feet, are drowsiness, lassitude, mental and muscle fatigue, sometimes headache, occasionally nausea, and sometimes euphoria. These effects progress to a stage of twitchings or seizures above 18,000 feet and, above 23,000 feet in the unacclimatized person, end in coma, followed shortly thereafter by death.
One of the most important effects of hypoxia is decreased mental proficiency, which decreases judgment, memory, and performance of discrete motor movements. For example, if an unacclimatized aviator stays at 15,000 feet for 1 hour, mental proficiency ordinarily falls to about 50% of normal and, after 18 hours at this level, it falls to about 20% of normal.
A person remaining at high altitudes for days, weeks, or years becomes more and more acclimatized to the low P o 2 , so it causes fewer deleterious effects on the body. After acclimatization, it becomes possible for the person to work harder without hypoxic effects or to ascend to still higher altitudes.
The principal means whereby acclimatization comes about are as follows: (1) a great increase in pulmonary ventilation; (2) increased numbers of red blood cells; (3) increased diffusing capacity of the lungs; (4) increased vascularity of the peripheral tissues; and (5) increased ability of the tissue cells to use O 2 , despite low P o 2 .
Immediate exposure to low P o 2 stimulates the arterial chemoreceptors, and this stimulation increases alveolar ventilation to a maximum of about 1.65 times normal. Therefore, compensation occurs within seconds for the high altitude, and this alone allows the person to rise several thousand feet higher than would be possible without the increased ventilation. If the person remains at a very high altitude for several days, the chemoreceptors increase ventilation still more, up to about five times normal.
The immediate increase in pulmonary ventilation on rising to a high altitude blows off large quantities of CO 2 , reducing the P co 2 and increasing the pH of the body fluids. These changes inhibit the brain stem respiratory center and thereby oppose the effect of low P o 2 to stimulate respiration via the peripheral arterial chemoreceptors in the carotid and aortic bodies . However, this inhibition fades away during the ensuing 2 to 5 days, allowing the respiratory center to respond with full force to the peripheral chemoreceptor stimulus from hypoxia, and ventilation increases to about five times normal.
The cause of this fading inhibition is believed to be mainly a reduction of bicarbonate ion concentration in the cerebrospinal fluid, as well as in the brain tissues. This reduction, in turn, decreases the pH in the fluids surrounding the chemosensitive neurons of the respiratory center, thus increasing the respiratory stimulatory activity of the center.
An important mechanism for the gradual decrease in bicarbonate concentration is compensation by the kidneys for the respiratory alkalosis, as discussed in Chapter 31 . The kidneys respond to decreased P co 2 by reducing hydrogen ion secretion and increasing bicarbonate excretion. This metabolic compensation for the respiratory alkalosis gradually reduces plasma and cerebrospinal fluid bicarbonate concentrations and pH toward normal and removes part of the inhibitory effect on respiration of a low hydrogen ion concentration. Thus, the respiratory centers are much more responsive to the peripheral chemoreceptor stimulus caused by the hypoxia after the kidneys compensate for the alkalosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here