Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The gastrointestinal (GI) tract is a rich lymphoid organ by virtue of the fact that there are numerous immune cells in the lamina propria as well as abundant lymphoid follicles and Peyer’s patches distributed throughout, which is collectively referred to as mucosa-associated lymphoid tissue (MALT). Complex interactions between enterocytes, antigen-presenting cells, lymphocytes, and other elements of the immune system occur in this specialized microenvironment.
The GI mucosal immune system plays an important role in homeostasis. It is responsible for regulation of localized immune responses to the numerous antigenic substances that come into contact with the mucosa. The MALT orchestrates an immune response against pathogenic antigens (e.g., infectious microorganisms) while actively suppressing the immune response to nonpathogenic antigens (e.g., symbiotic microorganisms, food). Dysregulation of the molecular and cellular mechanisms that underlie these processes is associated with the development of multiple disease states.
Autoimmune disorders of the GI tract are commonly associated with extraintestinal manifestations, or they rarely manifest as primary gutcentric autoimmune disorders. Autoimmune disorders of the GI tract are rare if diseases such as idiopathic inflammatory bowel disease (IBD) and gluten-sensitive enteropathy (which are related to autoimmune mechanisms in some capacity) are excluded.
Several well-established autoimmune disorders, including gluten-sensitive enteropathy, autoimmune gastritis, and idiopathic IBD, are discussed in other chapters of this book. In this chapter, two conditions that have been extensively studied during the past few years are covered in detail: autoimmune enteropathy (AIE) in its various forms and immunoglobulin G4 (IgG4)-related disease.
AIE is an uncommon disorder with myriad clinical manifestations. It results from immune-mediated injury to the tubular gut and therefore normally responds to various degrees of immunosuppressive therapy. Although characteristic histopathological damage can be recognized, the diagnosis is best made collectively by both the pathologist and the treating physician. This requires a thoughtful review of all available clinical, endoscopic, and histological features. AIE is often misdiagnosed as celiac disease, thus the lack of response to gluten withdrawal often serves as the first clinical diagnostic clue of an inaccurate diagnosis.
AIE was first described in 1982 by Unsworth and colleagues, who reported a 15-month-old child with diarrhea, flattening of small-intestinal villi, and the presence of a circulating autoantibody to gut epithelium. After recognizing six additional pediatric cases, Unsworth and Walker-Smith proposed a four-point definition of AIE: protracted diarrhea and villous atrophy; no response to a gluten-free diet or total parenteral nutrition; circulating autoantibodies to gut epithelium or associated autoimmune diseases (suggesting a predisposition to autoimmunity); and exclusion of severe immunodeficiency. In 1986, Mirakian reported a series of 14 children with protracted diarrhea and evidence of autoimmunity, a condition that was described as an autoimmune variant of idiopathic protracted diarrhea of infancy.
AIE occurs in adults, but it more commonly affects infants during the first 6 months of life. The estimated incidence is less than 1 case per 100,000 infants, and it affects both sexes equally. Although traditionally considered a disease of the small intestine, the entire gut, pancreas, and liver may be affected.
Two syndromic forms of AIE are recognized: immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome (APECED), also known as autoimmune polyendocrine syndrome type 1 (APS-1). Both are monogenetic disorders caused by loss-of-function mutations in a gene that regulates T-cell development and function. The defective gene results in T-cell overactivity.
IPEX syndrome is an X-linked recessive disorder characterized by polyendocrinopathy, various autoimmune conditions, and severe, prolonged diarrhea. IPEX syndrome is caused by germline loss-of-function mutations in the forkhead box P3 gene ( FOXP3, located at Xp11.23-q13.3). FOXP3 is required for normal development and function of regulatory T cells, a small subset of CD4+ helper T cells of critical importance in the regulation of self-tolerance and immune homeostasis. Loss of FOXP3 activity leads to immune overactivity in response to antigen stimulation and cellular injury through CD4+ effector T cells.
IPEX syndrome classically manifests in early infancy with AIE, insulin-dependent diabetes mellitus, and cutaneous manifestations. , Dermatological involvement may manifest as eczema, atopic dermatitis, psoriasis, alopecia, and pemphigoid nodularis. , IPEX syndrome may have a wide array of other manifestations of autoimmunity ( Box 6.1 ). , Patients also may have lymphadenopathy, splenomegaly, and thymic involution. The prognosis is usually grim, with most patients dying within the first year of life. However, milder cases with onset later in life (≤24 years of age) have also been described. ,
Thrombocytopenia
Neutropenia
Hypothyroidism
Thyroiditis
Type 1 diabetes
Lymphadenopathy
Coombs-positive hemolytic anemia
Atopic dermatitis
Pemphigoid nodularis
Arthritis
Myositis
Tubulointerstitial nephritis
Membranous glomerulonephritis
APECED syndrome (i.e., APS-1) is a rare condition that is more prevalent among Finns, Sardinians, and Iranian Jews. This autosomal recessive disease is characterized by a triad of mucocutaneous candidiasis, autoimmune hypoparathyroidism, and Addison’s disease, although a wide spectrum of autoimmune abnormalities may be seen.
The syndrome is caused by loss-of-function mutations in the autoimmune regulator (AIRE) gene (located at 21q22.3). The AIRE gene encodes a transcription factor that regulates expression of self-antigens in the thymus, and it is critical for deletion of self-reactive T cells. , Defects in AIRE lead to the development of T cells that recognize self-antigen, resulting in autoimmune diseases.
Affected patients are commonly diagnosed soon after birth with mucocutaneous candidiasis affecting the tongue, esophagus, and nails. With time, chronic candidiasis predisposes to oral and esophageal squamous cell carcinoma. Autoimmune hypoparathyroidism and adrenocortical failure (Addison’s disease) typically develops in the first decade of the patient’s life. Enteropathy is diagnosed in approximately 20% of patients. A wide array of other autoimmune diseases may develop ( Box 6.2 ). , Various ectodermal abnormalities, including keratoconjunctivitis, vitiligo, alopecia, dental enamel hypoplasia, pitted nail dystrophy, and tympanic membrane calcification, may also develop. Splenic atrophy or asplenia may occur. Unlike IPEX syndrome, the onset of APECED syndrome is less fulminant, and the prognosis is significantly more favorable.
Candida infection
Addison’s disease
Keratoconjunctivitis
Vitiligo
Alopecia
Tubulointerstitial nephritis
Hemolytic anemia
Asplenia
Type 1 diabetes
Autoimmune hepatitis
Gonadal dysfunction
Hypopituitarism
Hypoparathyroidism
Autoimmune thyroiditis
Autoimmune gastritis
The clinical hallmark of AIE is refractory diarrhea, which may be accompanied by malabsorption and weight loss. In infants, low body weight and slow growth may be seen. The disease affects men and women within a broad age range. In the largest series of adult AIE cases reported, most patients were white, both sexes were equally affected, and the median age at diagnosis was 55 years.
This is an uncommon disease in adults, and most patients are initially misdiagnosed with celiac disease. A history of autoimmune disease is obtained in up to 87% of patients. Associated autoimmune conditions include hypothyroidism, rheumatoid arthritis, myasthenia gravis, idiopathic thrombocytopenic purpura, autoimmune gastritis, and polyneuropathy. Another notable association, particularly in children, is the relationship with other immunodeficiency syndromes. Three cases have been associated with thymoma. ,
Because of the association of AIE with other autoimmune diseases, serological testing for autoantibodies is commonly positive. , , , Autoantibody testing was positive in up to 67% of patients in one series. The antibodies, which are directed against a variety of antigens in patients’ sera, include ANA, anti-LKM, anti-SMA, anti-parietal cell, and many others ( Box 6.3 ). The relevance of gut epithelial cell antibodies is discussed later.
Antinuclear
Anti–liver-kidney microsomal
Anti–smooth muscle
Anti–gastric parietal cell
Anti–pancreatic islet cell
Antiinsulin
Antiendoplasmic reticulum
Antireticulin
Antigliadin
Antiadrenal cell
Antithyroglobulin
Anti–55-kDa protein in the jejunum
Antivillin
Anti–AIE-75
Other laboratory findings include normal B-lymphocyte and T-lymphocyte counts and complement levels. Some patients may have immunoglobulin A (IgA) deficiency, , but a broader immunoglobulin deficiency, especially if the albumin level is relatively normal, suggests common variable immunodeficiency (CVID), which can cause an enteropathy similar to celiac disease or AIE.
The small-intestinal endoscopic features, which include duodenal scalloping, erythema, erosions/ulcerations, fissuring, and a mosaic pattern, are generally nonspecific. , Similarly, there may be endoscopic abnormalities of the colon, including loss of vascular pattern, mucosal friability, contact bleeding, erythema, and ulceration. , Severe macroscopic colitis (including pancolitis) has been reported in patients with IPEX syndrome. There may be signs and symptoms of extraintestinal involvement. Abdominal CT may reveal mesenteric lymphadenopathy. ,
Serological detection of antibodies directed against the intestinal brush border, and/or detection of cytoplasm of enterocytes and goblet cells, has constituted the mainstay of serological testing for autoimmune enteropathy. In their original series, Unsworth and Walker-Smith reported antienterocyte antibodies in 5 of 6 patients, and antienterocyte antibodies were detected in all 14 patients reported in two overlapping British series. , Antienterocyte antibodies are identified in 50% to 80% of patients, , , and anti–goblet cell antibodies are seen in approximately 30% of patients. However, although these tests aid in the diagnosis of AIE, it has been argued, often persuasively, that these antibodies should not be included as diagnostic criteria for AIE because their direct role in epithelial injury has not been documented. Of greater concern, however, is that these antibodies are not specific (as mentioned earlier) or entirely sensitive for AIE. Antienterocyte antibodies have been noted in HIV infection, and anti–goblet cell antibodies can be detected in patients with IBD, celiac disease, , autoimmune hepatitis, chronic hepatitis C virus (HCV) infection, insulin-dependent and non–insulin-dependent diabetes, and in healthy controls. , , , The presence of these antibodies may merely reflect epitope spreading and thus represent an epiphenomenon related to destruction of intestinal absorptive and goblet cells. Furthermore, there is no apparent correlation between antibody titer and histological severity.
As a practical matter, the presence of antienterocyte and anti–goblet cell antibodies should only be evaluated in conjunction with the clinical information and the histological findings. Their absence should not necessarily exclude the diagnosis of AIE.
Although the precise molecular mechanisms underlying AIE remain unknown, the robust relationship with other immunodeficiency disorders alludes to an alteration of gut immunity as the main mechanism behind this disorder. The intestinal mucosa is a site of complex interactions between the immune system and the environment, including dietary antigens and gut microflora.
The expression of HLA class II molecules, a key component of T-cell activation, is usually restricted to cells of the immune system, where they mediate antigen presentation and induction of the immune response. Although epithelial cells generally do not express HLA class II molecules, mature enterocytes at the tips of small-intestinal villi are an exception in that they constitutively express these molecules under normal physiological conditions and present antigens to autologous CD4+ helper T cells. Antigen presentation by enterocytes could, theoretically, suppress T-cell activity, which presumably allows for tolerance to dietary antigens encountered by small-intestinal epithelium, a phenomenon referred to as oral tolerance .
In contrast, enterocytes of AIE patients exhibit aberrant expression of HLA class II molecules, with inappropriate expression of HLA-DR in crypt enterocytes. , It has been hypothesized that aberrant HLA class II expression by crypt enterocytes induces CD4+ T-cell overactivity and subsequent T-cell–mediated injury of the intestinal epithelium through direct cytotoxicity or cytokine secretion. , , Increased numbers of CD25+ T cells have been documented in small-intestinal biopsies of AIE patients, , , and CD25+ T cells regressed with therapy in at least one case. Enterocyte injury by overactive T cells is emerging as a possible unifying mechanism to explain AIE.
The small intestine appears to be the primary organ involved in AIE and the focus of most histological descriptions. However, it is becoming increasingly clear that the disease is rarely restricted to the small bowel because involvement of the stomach and colon are also commonly reported.
The pathological features of AIE vary, but some patterns of injury may be considered characteristic, particularly in the duodenum, ileum, and jejunum. , , , , Small-intestinal AIE can be largely grouped into three general histological patterns: (1) chronic active enteritis, (2) celiac disease–like pattern, and (3) graft-versus-host disease (GVHD)-like pattern. Biopsies that show multiple histological patterns are not uncommon. Although these patterns do not correlate with a specific disease phenotype, they are conceptually useful for the pathologist in generating a differential diagnosis.
The chronic active enteritis pattern is the most common pattern of injury. It consists of a lymphoplasmacytic inflammatory infiltrate with moderate to severe villous blunting and crypt hyperplasia ( Fig. 6.1 ). Neutrophilic inflammation may be prominent and sometimes occurs with crypt abscesses. Apoptosis may be present. Surface erosions and gastric foveolar metaplasia may be present. Eosinophils are typically seen but are not prominent.
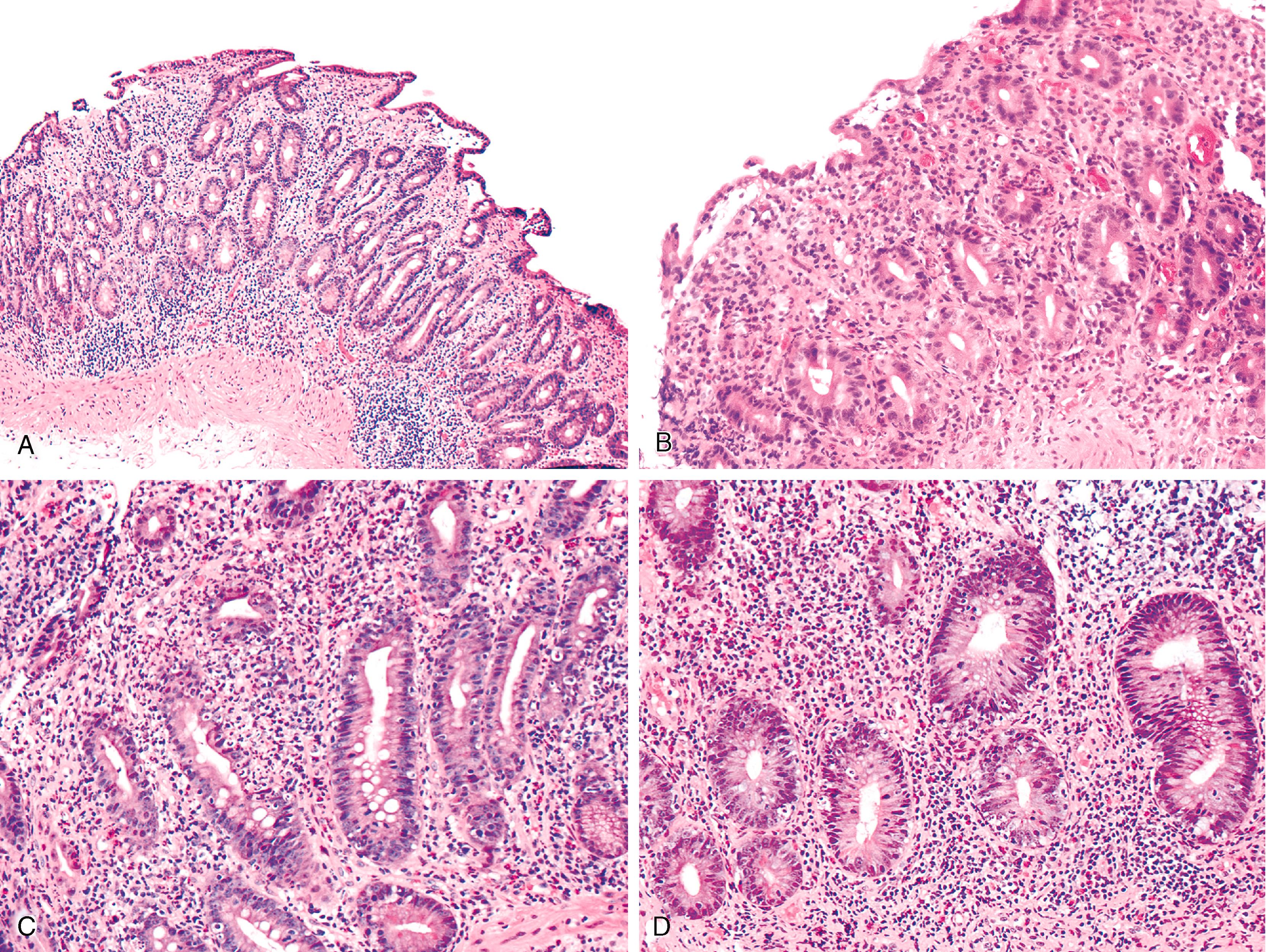
The celiac disease pattern features increased intraepithelial lymphocytes, and villous blunting, and may be histologically indistinguishable from celiac disease ( Fig. 6.2 ). The GVHD pattern features increased apoptosis in crypt epithelium with a relative absence of inflammation, similar to acute GVHD. Apoptosis may be a striking feature in terms of the number of cells affected, and partial or total glandular destruction may be present. Other nonspecific reactive or regenerative epithelial changes include mucin depletion, crypt architectural irregularity, and increased mitotic activity.
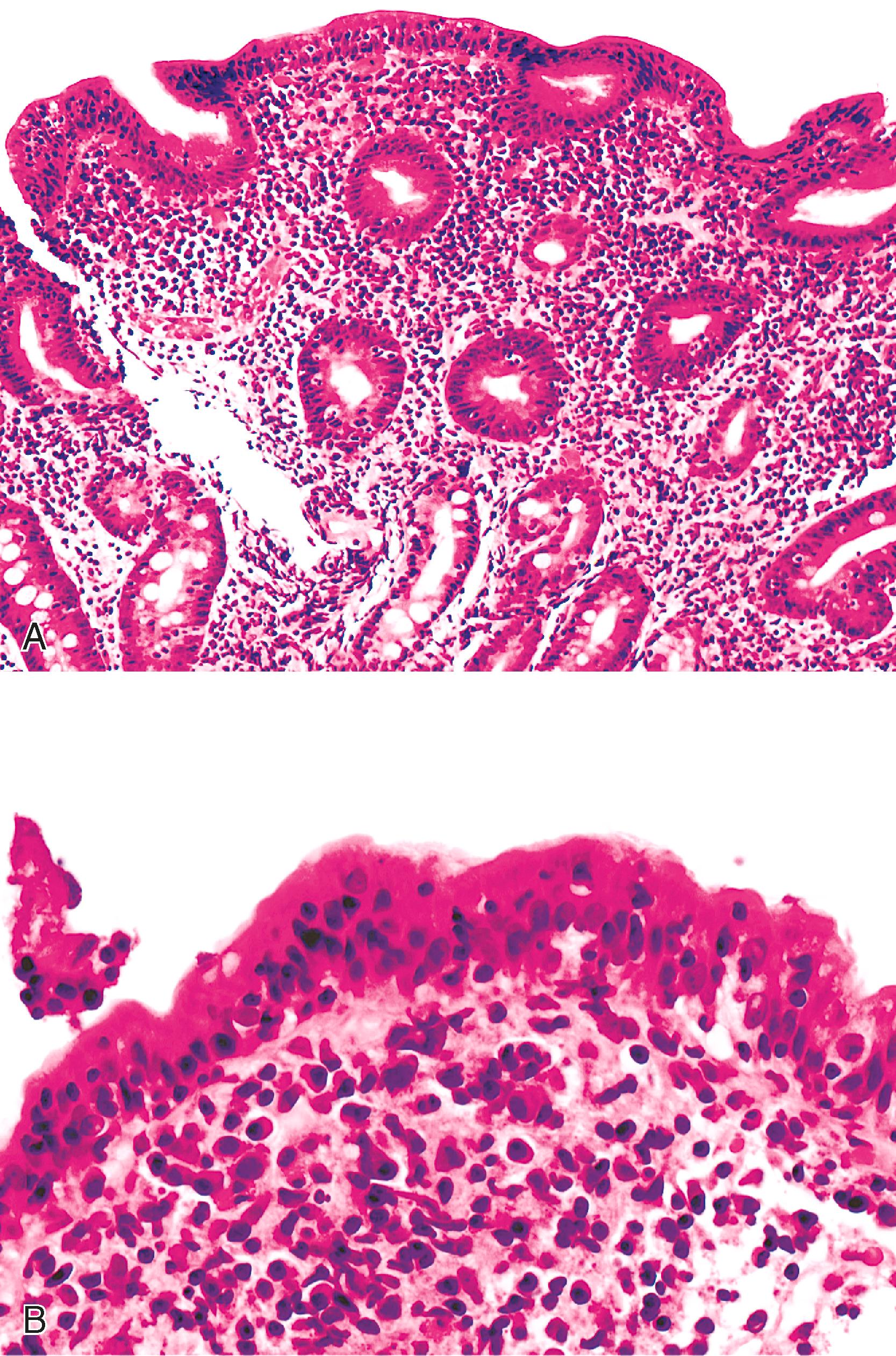
In addition to the findings noted earlier, small-bowel biopsies may also show loss of multiple cell lineages, including goblet cells, endocrine cells, and Paneth cells (see Fig. 6.2 ). , , , In one study, loss of neuroendocrine cells in the intestinal crypts was observed in 78% of small-bowel and/or colon biopsies in AIE patients; occasional loss (9%) was observed in CVID patients, while neuroendocrine cell density was intact in patients with IBD and IgA deficiency. In this study, loss of goblet cells occurred in 89% of cases, whereas loss of Paneth cells occurred in 78%. In some cases, the absence of goblet cells in biopsies coincided with detection of anti–goblet cell antibodies. An absence of plasma cells in the lamina propria may be an indication of concurrent CVID.
In our experience, the features most helpful in suggesting a diagnosis of AIE are villous blunting, lamina propria expansion by mixed but predominantly mononuclear inflammation, neutrophilic activity, patchy increase in intraepithelial lymphocytes, and loss of one of more cell lineages, most often endocrine and goblet cells.
Colonic involvement has been reported in a relatively large number of cases. , , , It affects most patients with AIE and IPEX syndrome. Histological features include lymphoplasmacytic expansion of the lamina propria; neutrophilic cryptitis, either with or without crypt abscesses; and increased apoptosis in crypt epithelium ( Fig. 6.3 ). , , Increased apoptosis may be striking, and the predominant feature may be reminiscent of acute GVHD ( Fig. 6.4 ).
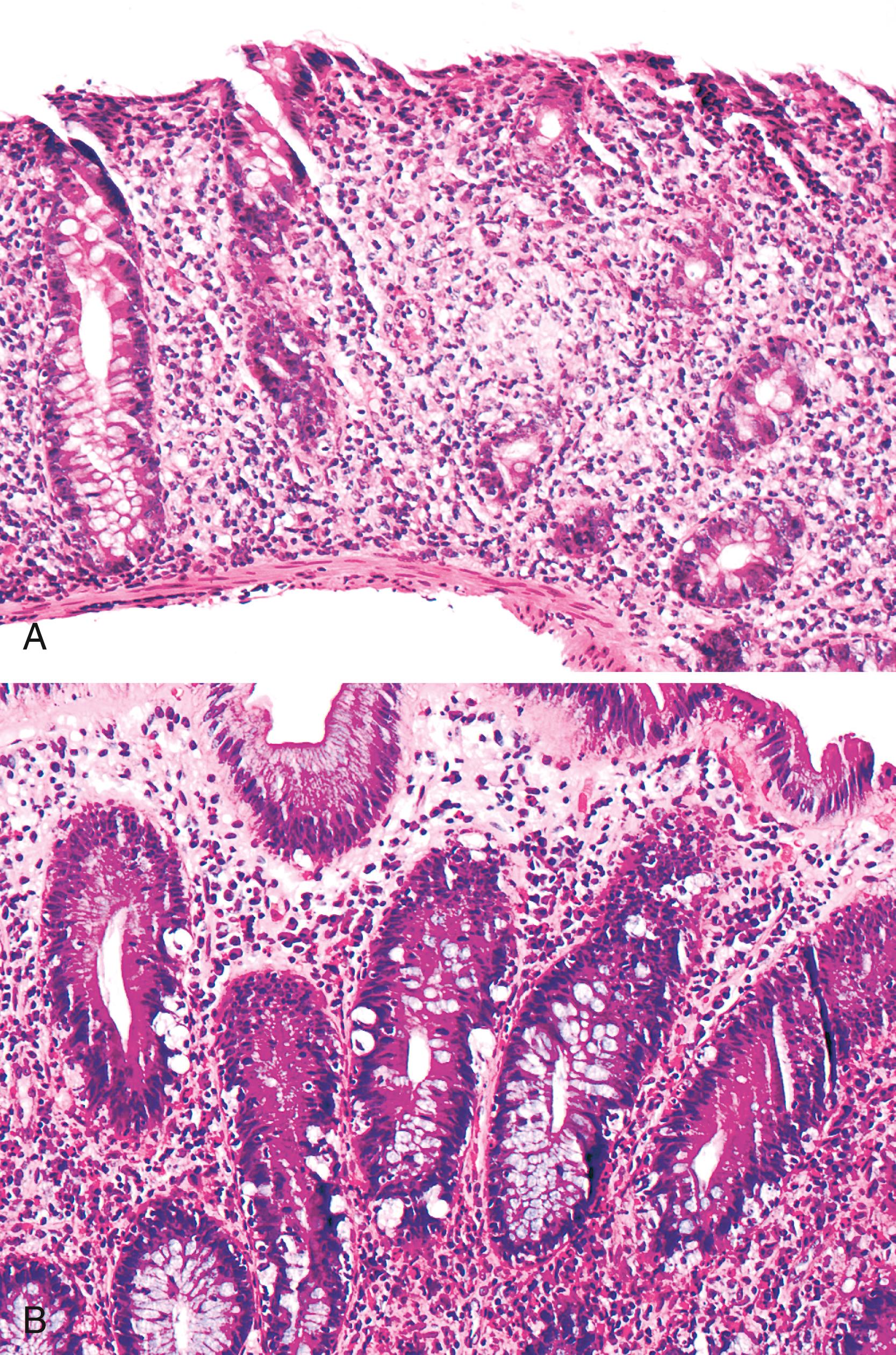
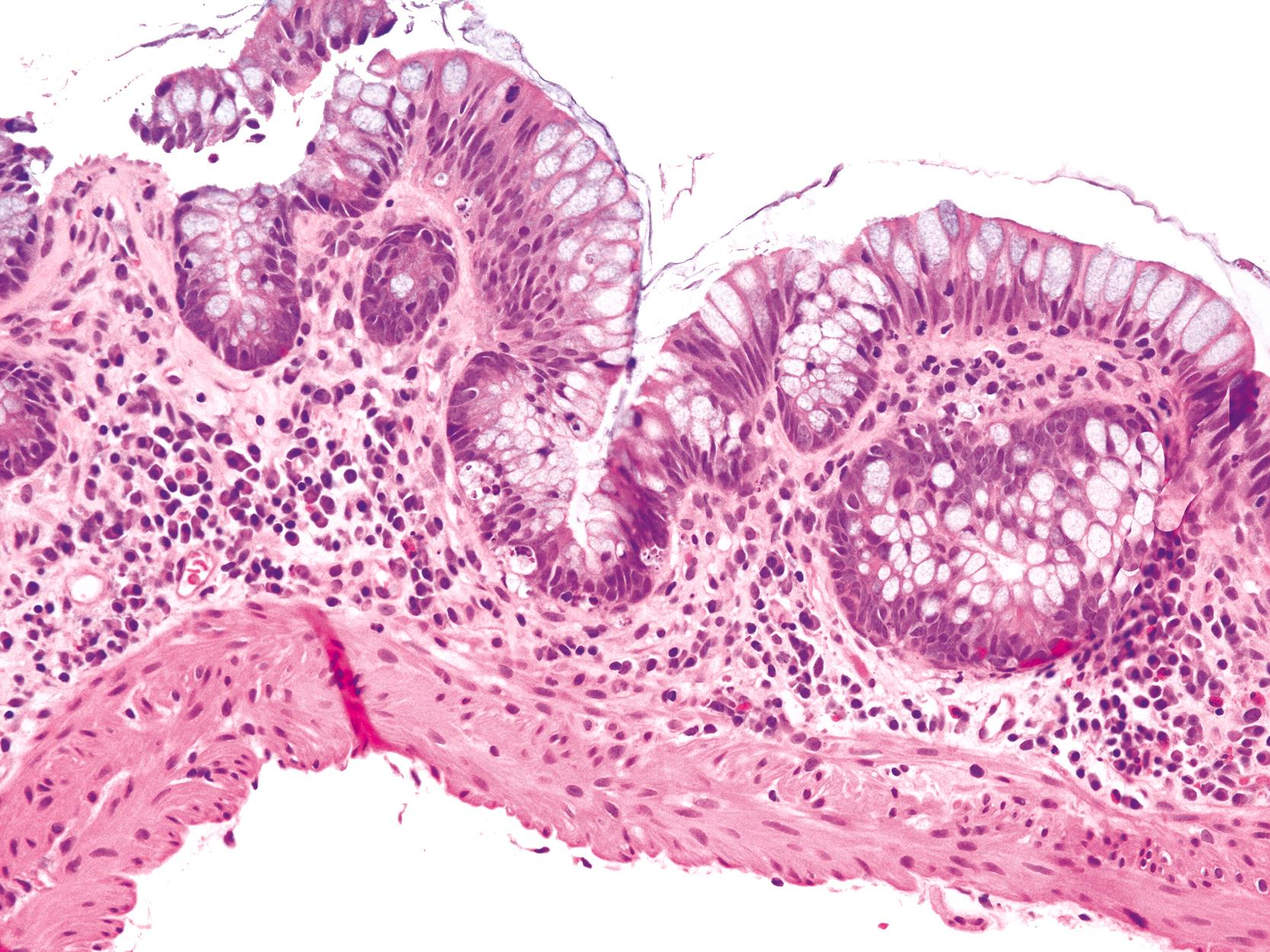
Colonic biopsies may exhibit features that are not immediately identifiable as AIE, such as severe colitis with crypt destruction, pyloric metaplasia, and/or crypt atrophy. , A lymphocyte-predominant pattern of inflammation that is indistinguishable from lymphocytic colitis may be present as well. One case with thickening of the subepithelial collagen layer in the duodenal mucosa and in the entire colorectal mucosa, which was indistinguishable from collagenous colitis, has also been reported. In our experience, colonic involvement may also be reminiscent of IBD, with crypt architectural irregularities, multinucleated epithelioid giant cells, and Paneth cell metaplasia. However, the full-blown histological features of active chronic colitis of the IBD type are not seen.
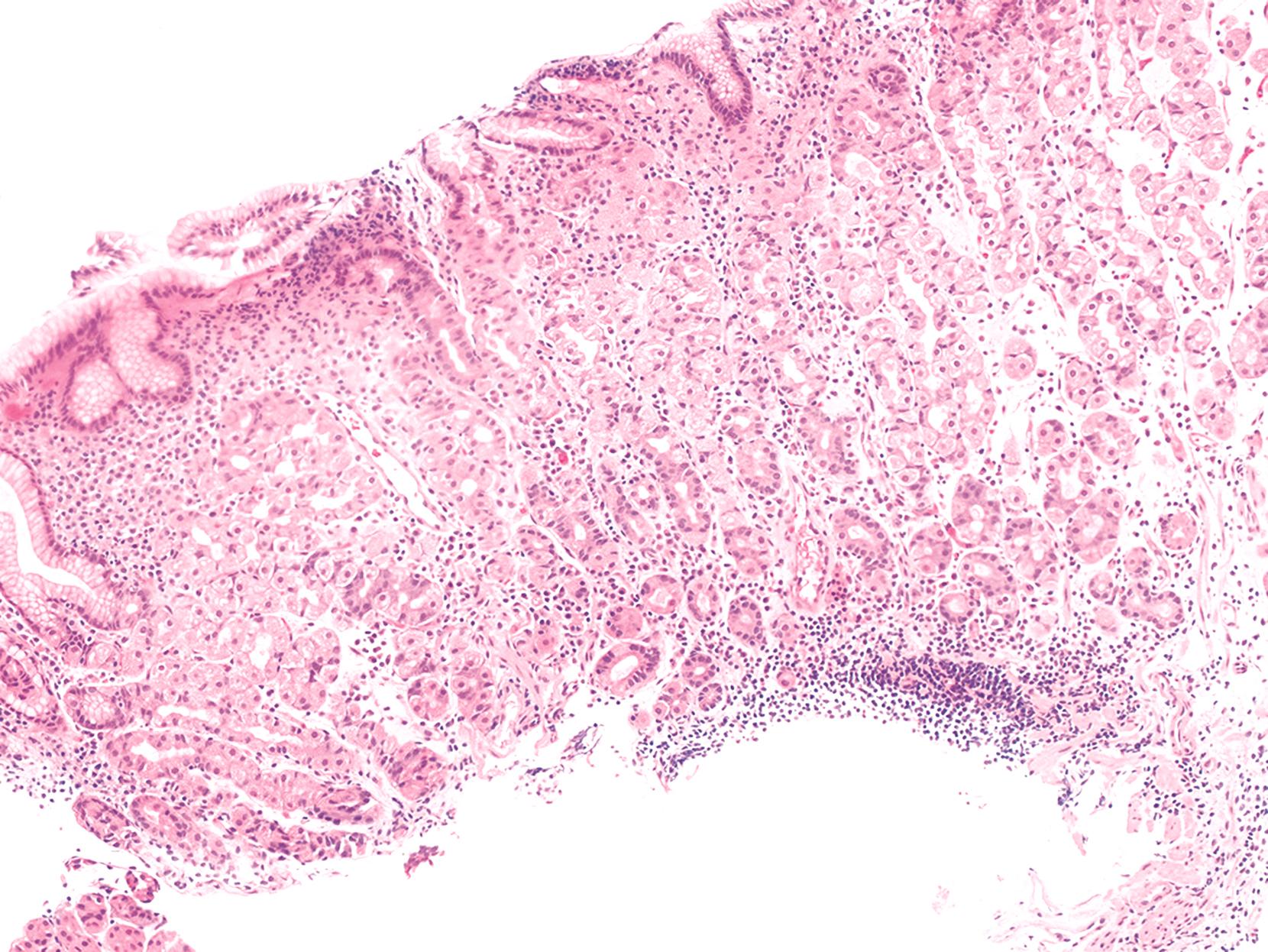
Involvement of the stomach may occur in the form of moderate to severe gastritis with expansion of the lamina propria, neutrophils, and apoptotic activity ( Fig. 6.5 ). , , , , Active gastritis with glandular destruction has been reported. A lymphocytic gastritis-like pattern has also been reported. Some patients exhibit a peculiar form of atrophic gastritis that resembles autoimmune atrophic gastritis but involves the entire stomach and lacks neuroendocrine cell hyperplasia, distinguishing this disease from autoimmune gastritis. In our experience, the stomach may also exhibit an acute GVHD-like appearance, with near-normal mucosa with increased apoptosis in glandular epithelium, with or without glandular atrophy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here