Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors are grateful to their patients for their time and effort in laboratory testing. We also thank Drs. Jose Fayad, Robert Shannon, and William Hitselberger, who contributed to the previous edition of this chapter.
![]() Please access Videos to view the corresponding videos for this chapter.
Please access Videos to view the corresponding videos for this chapter.
Loss of auditory nerve integrity, as often occurs after the removal of vestibular schwannomas (VS) in neurofibromatosis type 2 (NF2), has historically left patients completely deafened. Sign language, lip reading, and vibrotactile aids have provided some communication assistance but could not restore useful auditory sensations. The development of the auditory brainstem implant (ABI), and auditory midbrain implant (AMI), provided a means of bypassing the cochlea and auditory nerve to stimulate the more central auditory pathways directly, thereby giving sound sensations to otherwise deaf patients.
This chapter discusses the updates of clinical and surgical aspects of ABI, and AMI electrode array placement and perceptual performance. The techniques are derived from experience in the implantation of more than 300 patients with various devices since 1979 at the House Clinic and elsewhere. More than 1250 patients have received implants worldwide. U.S. Food and Drug Administration (FDA) approval was obtained in October 2000 for the multichannel ABI manufactured by Cochlear Limited. Typically, ABI recipients are implanted with a 21-electrode ABI. ABIs also have been produced by other implant manufacturers, including Med-El.
General technical and theoretical considerations of central auditory implantation and stimulation have been reviewed elsewhere. ,
Originally, patients received the ABI under a protocol monitored by the FDA. The criteria for implantation are listed in Table 53.1 . The device was originally designed for patients with NF2 manifesting bilateral VS, although others with compromised auditory nerves were considered to be eventual ABI candidates. At least 90% of patients with NF2 exhibit bilateral eighth nerve neuromas. An unpublished review of patients with NF2 seen at the House Clinic revealed that two-thirds had bilateral internal auditory canal cerebellopontine angle (CPA) tumors alone or with one other tumor as the only central nervous system manifestation of their disease. The patients were young (average age, 28 years). With improvements in medical care and surgical techniques, the lifespan of most of these patients has been significantly prolonged. The restoration of even rudimentary auditory function can enhance their quality of life and ability to function in a hearing world. Our results have shown that the multichannel ABI has the potential of offering even greater benefits.
| Evidence of bilateral CN VII–VIII tumors |
| Involvement of the internal auditory canal or cerebellopontine angle |
| Language competency |
| Age ≥12 years |
| Psychological suitability |
| Willingness to comply with research follow-up protocol |
| Realistic expectations |
The current NF2 protocol allows implantation at the time of first- or second-side acoustic neuroma removal or in patients whose tumors have previously been removed. Implantation during removal of the first tumor has allowed experience with the device and may enhance performance when the patient loses all hearing. Moreover, implantation on the first side gives the patient two chances to obtain an optimally functioning system if the procedure in the first side is not successful, which, even with experienced surgeons, may occur in up to 8% of patients.
The management of bilateral acoustic neuromas should be highly individualized. Hearing preservation remains an ideal goal in the management of these tumors in patients with NF2, and early identification and treatment have permitted this in a number of patients. An intact auditory system is highly preferable to an artificial means of restoring hearing. Therefore, preserving as much of the patient’s own hearing as possible is paramount. Patients meeting the criteria listed in Table 53.2 may be considered and observed accordingly. The availability of the ABI provides an alternative to a desperate attempt to preserve nonserviceable hearing when large tumors are removed and hearing conservation is unlikely.
| Second tumor in an only hearing ear |
| Any tumor in a hearing ear that measures >2 cm in the largest diameter (hearing preservation unlikely with removal) |
| Short life expectancy because of other tumors, medical problems, or advanced age |
| Serviceable hearing with a tumor that shows no significant growth by sequential magnetic resonance scans and stable hearing by serial audiograms |
The anticipated applications of the ABI and similar devices have included bilateral temporal bone fractures, cochlear ossification, cochlear nerve avulsion, and anatomical birth anomalies. In addition, the ABI may be beneficial in patients with demyelinating diseases affecting the eighth cranial nerve but sparing at least one cochlear nucleus. Congenitally deaf children who are deemed not to be candidates for cochlear implantation (CI) or who have failed to progress with CI because of severe cochlear malformation or cochlear nerve aplasia have been implanted with success , and this is an area of active investigation and the subject of a clinical trial at our institution (see Chapter 31 ). ,
The goal of implantation is to place a safe and stable device that provides the patient with some degree of environmental sound awareness and recognition and also improves communication in conjunction with lip reading without side effects. A minority of patients may also obtain some open-set speech understanding with sound alone. Prospective patients are apprised of the goals, limitations, and risks of the ABI during two or three preoperative evaluation and counseling sessions. It is important to impress on the potential candidate that, although the ABI is similar to a CI, it has provided generally lower levels of performance with more gradual improvement over time. The candidate should also understand that results vary considerably from patient to patient. The implant candidate’s expectations are carefully evaluated and informed consent is obtained. The importance of an experienced multidisciplinary implant team including the neurotologist, neurosurgeon, audiologist, neuro-auditory physiologist, and neuroradiologist cannot be overemphasized.
Several factors contribute to a successful result from implantation. Experience of team members can greatly influence outcomes. Chief among these factors are the correct identification of the implantation site and the achievement of a stable placement of the electrode array. This, of course, is essential to obtain auditory sensations from stimulation and optimize performance. The overall results of tumor removal and postoperative recovery also play a role. For example, factors such as eye dryness related to postoperative facial nerve function may affect lip reading ability and communication using the ABI. General health, social activity level, and presence of a support group also can affect ABI use and benefit. Patient expectations are important and may be influenced by publicity about CIs. Assessing expectations for the ABI and ensuring informed consent before implantation are highly important but may be complicated in candidates overwhelmed by a plethora of preoperative concerns. A thorough and frank appraisal before surgery of the potential benefits, limitations, and requirements for adjusting to the device will help increase the likelihood of a satisfied user in the long run. At this time, the ABI requires a certain level of acceptance, motivation, and commitment from the recipient to maximize benefit; therefore, the device may not be for everyone.
The ABI hardware has evolved through a number of modifications since the original ball electrode was inserted by Drs. William Hitselberger and William House in 1979. , Significant design changes have involved transitioning from a percutaneous connector to a transcutaneous coil link to the implant, converting from ribbon electrodes to 0.7-mm diameter disk electrodes, and fabrication of a semiflexible silicone electrode carrier (2.5 × 8.5 mm) with a specialized mesh backing to stabilize placement. The first 25 ABI recipients were fitted with a single-channel sound processor and a two- or three-electrode array. From 1992 until about 2000, the electrode array used in most patients employed eight platinum disks in a perforated silicone and mesh carrier connected to an implantable receiver-stimulator. From about 2000 to 2016, the 21-electrode Nucleus ABI24 ( Fig. 53.1 ) has been used and the majority of patients implanted to date use this device. An experimental device, penetrating Electrode Auditory Brainstem Implant (PABI), was also designed with the hope of improving speech recognition among recipients. The device was the subject of a clinical trial and resulted in lower device charge requirements but did not achieve higher speech discrimination.
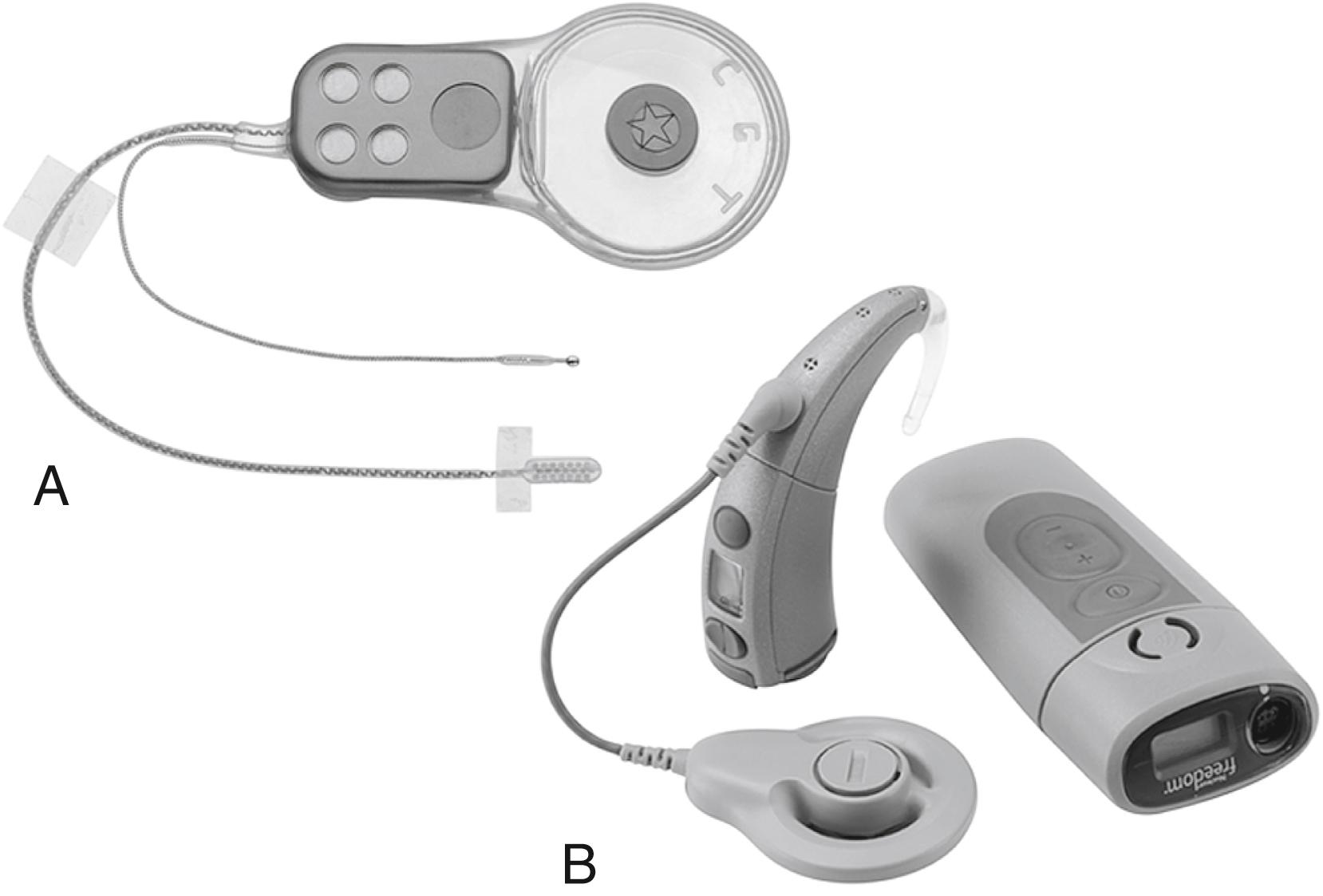
Current patients receive a 21-electrode array ( Fig. 53.1 ) interfaced with the Freedom postauricular sound processor and ear-level or body-worn controller (Cochlear Corporation). The device was further upgraded in June of 2016 to the ABI541. The Med-El Synchrony device, used outside of the United States, uses a smaller surface array with 12 electrodes. The device also has a four-channel test electrode that allows confirmation of electric auditory brainstem responses (EABRs) prior to ABI implantation.
The target of the ABI electrodes is the cochlear nucleus complex: the dorsal and ventral cochlear nuclei. In humans, the cerebellar peduncle that forms the base of the pons covers the auditory nuclei. This means that the nuclei are not visible to the surgeon and must be located from surface landmarks. Fig. 53.2 illustrates the major structures of the pontomedullary junction region with the translabyrinthine approach surgical field of view within the dashed lines. The terminus of the sleeve-like lateral recess forms the foramen of Luschka. Just inferior to the foramen is the root of the glossopharyngeal (ninth) nerve. Superior to the foramen lie the root entry and exit zones of the vestibulocochlear and facial nerves. This area is frequently distorted by the tumor.
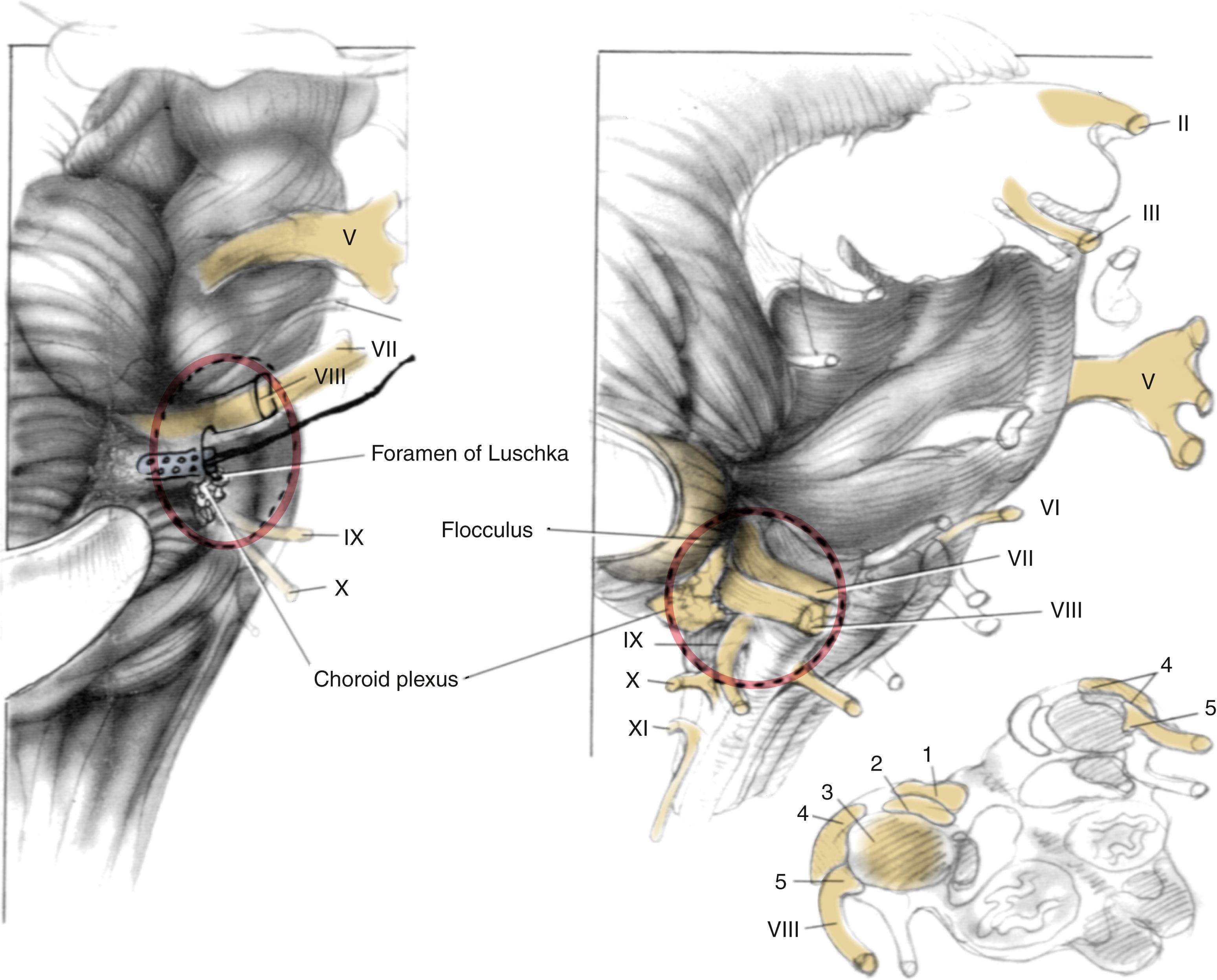
The cochlear nuclei come closest to the surface of the brainstem within the medial and superior aspect of the lateral recess. , The main target for stimulation is the ventral cochlear nucleus, which forms the main relay for cochlear nerve input and the greater part of the ascending auditory pathway. Placement of the electrodes within the recess has resulted in the fewest side effects, and the device preserves auditory stimulation even though some part of the electrode array lies adjacent to the dorsal cochlear nucleus. Moreover, the disadvantage of lack of exposure is partially offset by the positional stability provided to the electrode carrier by the limited space in the lateral recess.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here