Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Morbidity and mortality from atherosclerosis, the pathologic process underlying acute myocardial infarction, sudden death, stroke, and limb loss, represent an enormous burden on society and health care systems (see Chapter 144, Chapter 145, Chapter 146 ). Even though death rates from heart attack and stroke have dropped precipitously over the past 48 years (68% and 77%, respectively), cardiovascular disease (CVD) is still the number one cause of death in the United States, accounting for more than 30% of all deaths—approximately 2200 per day (or one every 40 seconds). Unfortunately, as the developing world adopts a more “Western” lifestyle marked by a high-fat, calorie-rich diet and limited physical activity, these statistics are becoming the norm worldwide. The annual financial burden of cardiovascular disease in the United States in 2015 was estimated by the American Heart Association (AHA) to be $351 billion, including $138 billion due to lost productivity. These costs are predicted to double over the ensuing 15 years, as analyses of population survey data predict a 30% increase in the number of people with CVD during this time. Importantly, improved treatments for acute complications of atherosclerosis also come with the burden of increased chronic complications (e.g., heart failure).
The increasing prevalence of other metabolic disorders such as the dual world-wide epidemics of type II diabetes and obesity increase risk of adverse thrombotic events and are associated with increased mortality from CVD, both directly and indirectly. Furthermore, the global SARS-CoV-2 pandemic that began in early 2020 has produced a large population of individuals with post-infection complications, so-called “long-COVID-19.” Some of these complications are cardiovascular in nature, but the long-term impact on CVD prevalence remains unknown at this time. Thus, concerns have been raised that reduction in mortality due to cardiovascular disease will eventually stop or even reverse direction.
Much of our knowledge of the epidemiology of cardiovascular disease comes from large-scale, long-term population studies, the most important being the Framingham Heart Study sponsored by the National Heart, Lung, and Blood Institute (NHLBI). This ongoing prospective study began in 1948 with a cohort of ≈5200 men and women between the ages of 30 and 62 in Framingham, Massachusetts, and has since added two subsequent generations and several other cohorts. Through this study and others elsewhere in the United States and Europe, major risk factors for cardiovascular disease have been identified, including cigarette smoking, low-density lipoprotein (LDL) cholesterol, family history, systolic blood pressure, male sex, waist circumference, menopause, physical inactivity, body mass index, high-sensitivity C-reactive protein (hsCRP), and coronary artery calcification scores. High-density lipoprotein (HDL) was identified as a protective factor. Based on these data, risk scores have been developed to guide physicians and patients, and public health campaigns developed targeting hypertension, elevated cholesterol, and lifestyle modifications. These have clearly contributed to the reductions in cardiovascular mortality, although they cannot account for the entire decrement. Cardiovascular risk can also be altered by dietary modifications. For example, Mediterranean diets and foods rich in fish oils like the omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid have been shown to reduce cardiovascular mortality and improve cardiovascular outcomes. Furthermore, racial and ethnic disparities are well described in cardiovascular risk, with Blacks having higher rates than all other groups.
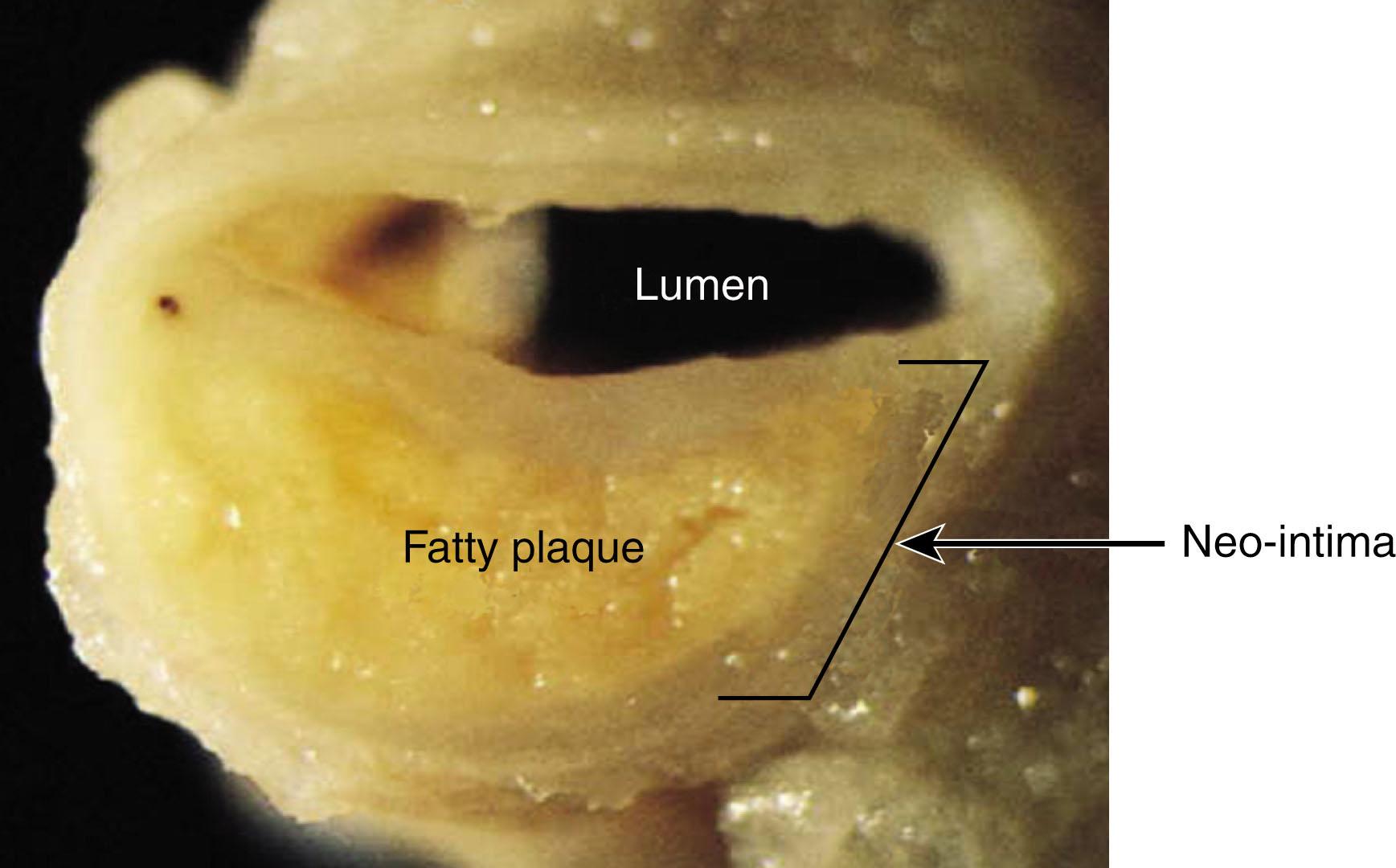
Atherosclerosis is caused by the progressive formation of arterial plaques, which are characterized by accumulation of lipids, in particular cholesterol and its derivatives, and inflammatory cells in the tunica intima of large- and medium-sized arteries ( Fig. 142.1 ). Although occlusion of blood flow by plaque encroachment into the lumen can cause ischemia of downstream tissues, most of the severe clinical events associated with atherosclerosis derive from acute or subacute thrombosis at a site of an unstable, inflamed, or ruptured plaque or at a site of recent mechanical or pharmacologic intervention, a process termed atherothrombosis. The past 45 years have seen a remarkable increase in our understanding of the basic pathophysiologic mechanisms that govern plaque formation, plaque progression and acute arterial thrombosis. Translation of this knowledge into diagnostic, preventive, and therapeutic strategies has been impressive, but there is still much unmet need for improvement. This chapter will summarize the current prevailing models of atherogenesis and atherothrombosis, highlighting some key knowledge gaps and potential new therapeutic targets.
Epidemiologic and clinical trial data, along with numerous animal model studies, show unequivocally that elevated levels of plasma lipids, especially LDL cholesterol, are essential for plaque development. Atherosclerotic lesions can be induced in “athero-resistant” animals, including mice and rabbits, by manipulating their diets and/or genomes to cause hypercholesterolemia. The development of mouse genetic models nearly 30 years ago has led to mechanistic insights into the underlying pathobiology of plaque formation. In humans, raising LDL cholesterol levels increases the risk for atherosclerosis proportionally, while lowering levels either by lifestyle change or pharmacologic intervention reduces risk. In general, lowering LDL cholesterol levels by 10% reduces risk for cardiovascular events by 25% and cardiovascular death by 10%.
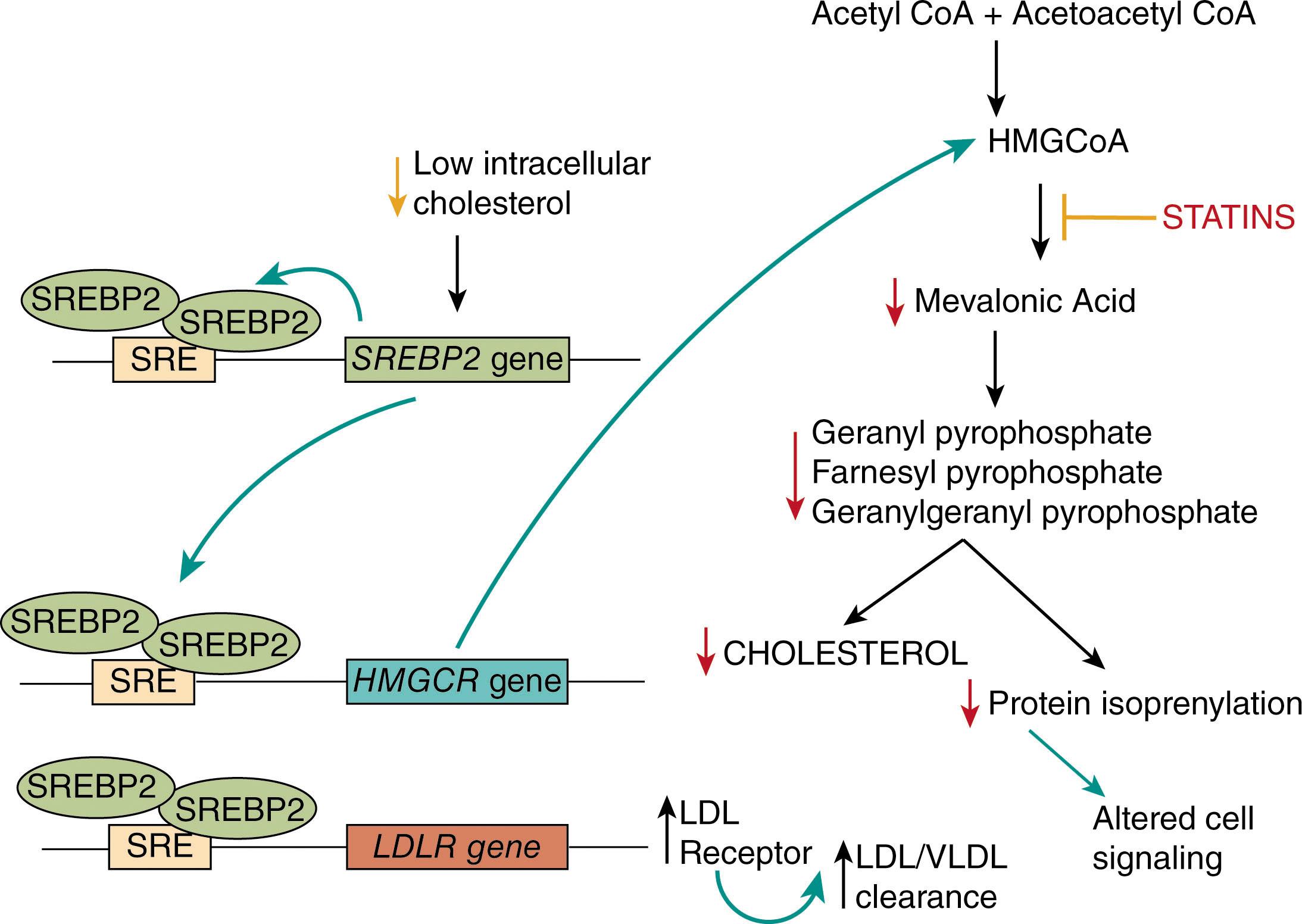
Normally, cholesterol levels are tightly controlled in response to diet and cellular needs by a complex transcriptional pathway mediated by sterol response element-binding protein 2 (SREBP2). At low levels of cellular cholesterol, SREBP2 is activated and binds to specific DNA sequences in target genes known as sterol response elements to activate their transcription. The two key target genes are HMGCR and LDLR , which encode 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase (the rate-limiting enzyme in cholesterol biosynthesis) and the LDL receptor and their activation increases cholesterol production and cellular LDL uptake, respectively. At high levels of cellular cholesterol, SREBP2 is inactive, cholesterol biosynthesis is turned off, and LDL receptor expression is downregulated. As shown in Fig. 142.2 , statin drugs decrease cellular cholesterol biosynthesis by inhibiting HMGCoA reductase and thereby inducing an increase in LDL receptor expression and driving down plasma LDL cholesterol levels.
Multiple randomized clinical trials have demonstrated efficacy of statin class drugs in reducing risk for cardiovascular events in high-risk individuals. Initial studies focused on secondary prevention in patients with a history of a previous atherosclerotic event, but later trials demonstrated efficacy as part of a primary prevention strategy in subjects with LDL cholesterol greater than 190 mg/dL or in subjects with “normal” LDL cholesterol (70 to 190 mg/dL) in the setting of a high-risk score. These studies, along with those showing benefit of lowering LDL cholesterol to levels well below “normal” (e.g., to 70 mg/dL in high-risk individuals), are consistent with the concept that “normal” levels of LDL cholesterol, as defined by population means in the Western world, are not reflective of a true normal biology.
Genetic studies of rare individuals with extremely low LDL cholesterol levels identified a null mutation in a gene known as PCSK9 that encodes a serine protease involved in downregulating LDL receptor expression. Individuals with this mutation seem to be otherwise normal, despite nearly absent LDL, suggesting that targeting this enzyme could represent an effective strategy for cholesterol-lowering therapeutics. Indeed, humanized monoclonal antibodies inhibiting PCSK9 function were quickly tested through clinical trials (OSLER and ODYSSEY) and approved by the FDA in 2015 as second line therapy for hypercholesterolemia. The trials showed that these agents are well-tolerated and lower LDL cholesterol by approximately 60%, decrease cardiovascular events by half, and long-term efficacy and safety were demonstrated in at least one study. Emerging information on expression patterns of PCSK9 and regulation of other cellular receptors by this enzyme points to potential for off-target effects, and, thus, the need for long term follow-up of individuals on this therapeutic modality.
Niemann-Pick C1-like1 protein is expressed on the surface of enterocytes and hepatocytes and facilitates cholesterol absorption. Ezetimibe, a well-tolerated FDA-approved drug, inhibits this protein and lowers LDL cholesterol by approximately 20%. Early enthusiasm for this drug waned when studies failed to show improved cardiovascular outcomes, raising doubts about the relationship between biochemical endpoints and clinical efficacy, but a more recent trial of Ezetimibe in combination with a statin showed a 10% reduction in cardiovascular events at 7 years associated with a mean decrease in LDL cholesterol of 16 mg/dL compared with statin alone. The most recent clinical guidelines published by AHA and the American College of Cardiology (2018) recommend considering non-statin drugs, including Ezetimibe and PCSK9 inhibitors, in higher risk individuals whose LDL cholesterol levels remain above 70 mg/dL after maximizing statin therapy.
Peripheral cells have the capacity to eliminate excess cholesterol through a process known as reverse cholesterol transport (RCT) . In this pathway, summarized in Fig. 142.3 , post-lysosomal trafficking of intracellular cholesterol to the plasma membrane, mediated in part by the actions of acyl-CoA cholesterol acyltransferase (ACAT) and Niemann-Pick type C (NPC) protein, allows the cell surface adenosine triphosphate (ATP)–binding cassette (ABC) proteins ABCA1 and ABCG1 to transport excess cholesterol to apolipoprotein A (apoA)-containing lipoproteins, either nascent HDL (via ABCA1) or mature HDL (via ABCG1). HDL then “delivers” the cholesterol back to the liver, where it is selectively taken up by hepatocytes through a protein known as scavenger receptor B1 (SRB1) and ultimately secreted into bile and excreted in feces. The role of HDL in RCT probably accounts for its association with lowered risk for cardiovascular disease, but HDL particles also contain anti-inflammatory and antioxidant proteins that may further contribute to lowering atherosclerosis risk.
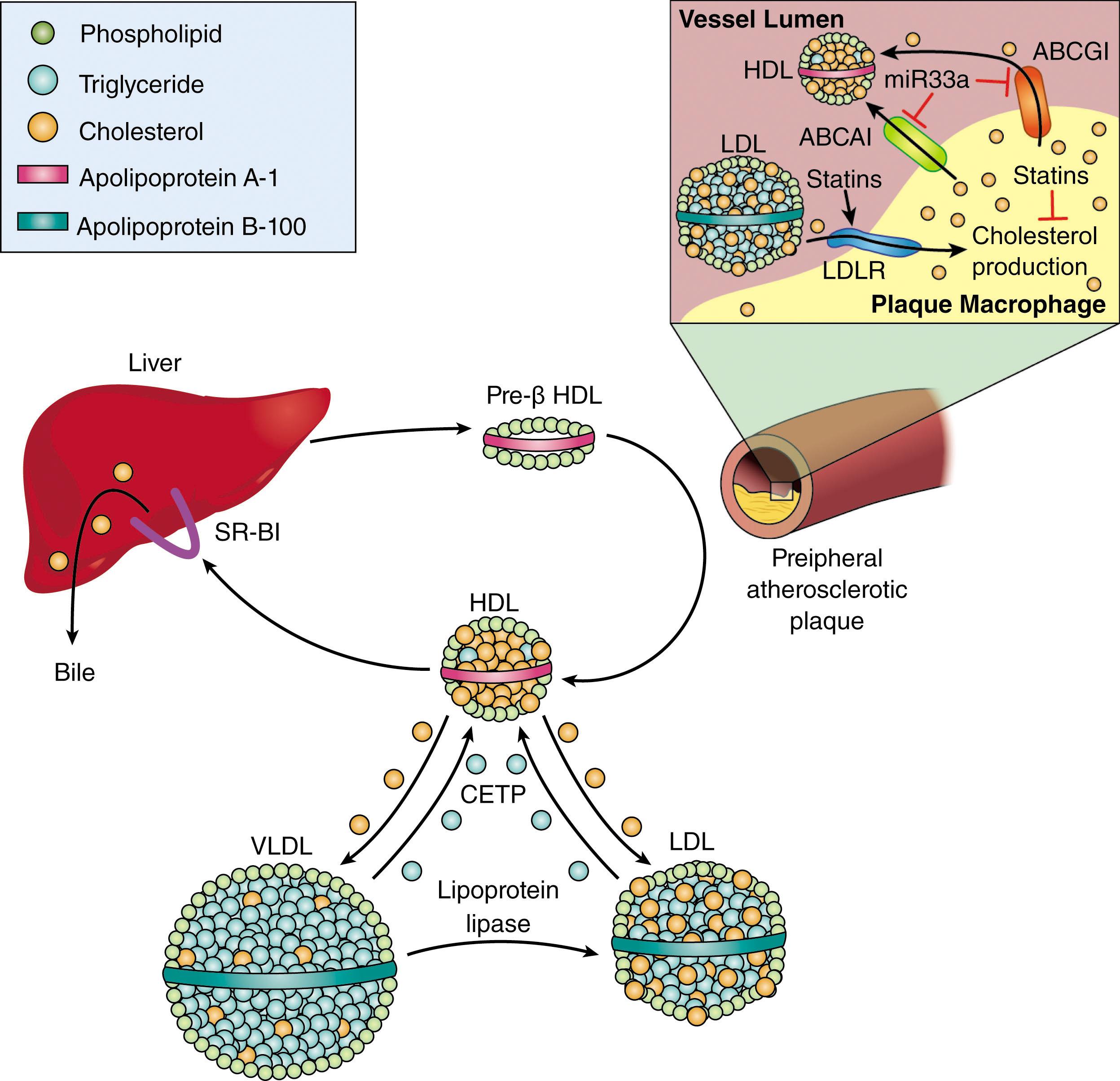
Pharmacologic and lifestyle approaches to enhance RCT have received significant attention as potential anti-atherosclerosis strategies. HDL levels can be raised by physical exercise, as well as by moderate alcohol ingestion, and these are both associated with lower cardiovascular risk. The only FDA-approved pharmacologic agent capable of raising HDL is niacin, but high doses are required, and these are often poorly tolerated because of side effects such as facial flushing. A combination of high-dose niacin with a prostaglandin D 2 receptor antagonist to block facial flushing and an extended-release form of niacin have been developed, but although both raise HDL levels, neither decreased cardiovascular risk.
The circulating plasma enzyme, cholesteryl ester transfer protein (CETP), functions to transfer cholesteryl esters from HDL to very low-density lipoprotein (VLDL) and LDL (see Fig. 142.3 ) in exchange for triglycerides. Inhibiting CETP raises HDL levels (and lowers LDL) significantly in humans, but multiple large phase III randomized clinical trials with pharmacologic CETP inhibitors failed to show efficacy despite raising HDL levels and at least one agent was associated with excess mortality.
Failure of CETP inhibitors and niacin led to doubts about the value of targeting HDL for anti-atherosclerosis therapy. An emerging concept, however, is that HDL particles are sensitive to oxidative modification in the pro-atherogenic milieu, and that these modifications can render HDL particles incompetent to function in RCT. Simply raising HDL in this milieu, therefore, might not impact RCT. In this regard, novel assays to assess HDL function—so-called cholesterol efflux capacity—have been developed and show potential as biomarkers for RCT in future drug development efforts. One such potential new target is microRNA-33a (miR-33a) which is encoded by a DNA sequence embedded in the SREBP2 gene and was found to repress several key genes involved in cholesterol homeostasis (see Fig. 142.3 ), including ABCA1 and ABCG1, as well as genes involved in fatty acid oxidation and glucose metabolism. Blocking miR-33a in mice increased HDL levels and inhibited atherosclerosis, suggesting that this could be a good target for therapeutic development. Other strategies under development to enhance RCT include direct infusion of cholesterol acceptors from ABCA1 or G1, including recombinant apoA or small apoA mimetic amphipathic peptides.
Multiple epidemiologic and genetic studies have shown positive associations between circulating triglyceride levels and coronary artery disease risk (see Chapter 145 ). Genetic variants in the gene encoding lipoprotein lipase, a key regulator of triglyceride metabolism, have also been associated with circulating triglyceride levels and with coronary artery disease risk, as have genetic variants in the APOC3 and APOA5 genes, which encode apolipoproteins in triglyceride-rich lipoproteins that critically regulate lipoprotein lipase in a reciprocal manner. Common variants in the ANGPTL3 gene, another regulator of lipoprotein lipase activity, have also been identified. Therapeutic interventions targeting the lipoprotein lipase pathway, including APOC3 antisense oligonucleotides or monoclonal antibody therapy against ANGPTL3 are under development.
Atherosclerotic plaque develops in a geographically discontinuous manner very slowly and continuously evolves over many decades. Autopsy studies of motor vehicle accident victims showed early “pre-atherosclerotic” lesions present in the aorta of otherwise healthy young children. These small superficial lesions consist predominantly of lipid-laden macrophages, so-called foam cells , and are termed fatty streaks . These are considered precursors of atherosclerotic plaque.
The initial event in formation of the fatty streak and atherosclerotic plaque is most likely transudation of apolipoprotein B–containing lipoproteins across the endothelial cell monolayer into the arterial wall ( Fig. 142.4 ). A transcytosis mechanism mediated by scavenger receptors (SRs) expressed on the luminal cell surface of endothelial cells may also play a role. These lipoproteins are predominantly LDL although remnant lipoproteins derived from chylomicrons produced by the intestinal epithelium are also found. The primary sites of lipoprotein entry are at arterial branch points or curvatures, explaining the discontinuous nature of atherosclerotic lesions. These locations are where normal laminar blood flow (and T-cell activity) is disturbed. Normal laminar flow activates a specific genetic “protection” program in endothelial cells that enhances barrier function and promotes an anti-inflammatory, antioxidant, and antiatherogenic phenotype. Loss of these shear-dependent signals disrupts the normal endothelial architecture and increases permeability, allowing unregulated entry of lipoproteins into the tunica intima. Within the intima, the lipoproteins become “trapped” by specific interactions with normal components of the vessel wall, such as glycosaminoglycans, and undergo structural modifications, including aggregation, oxidation, and in the setting of hyperglycemia, glycation.
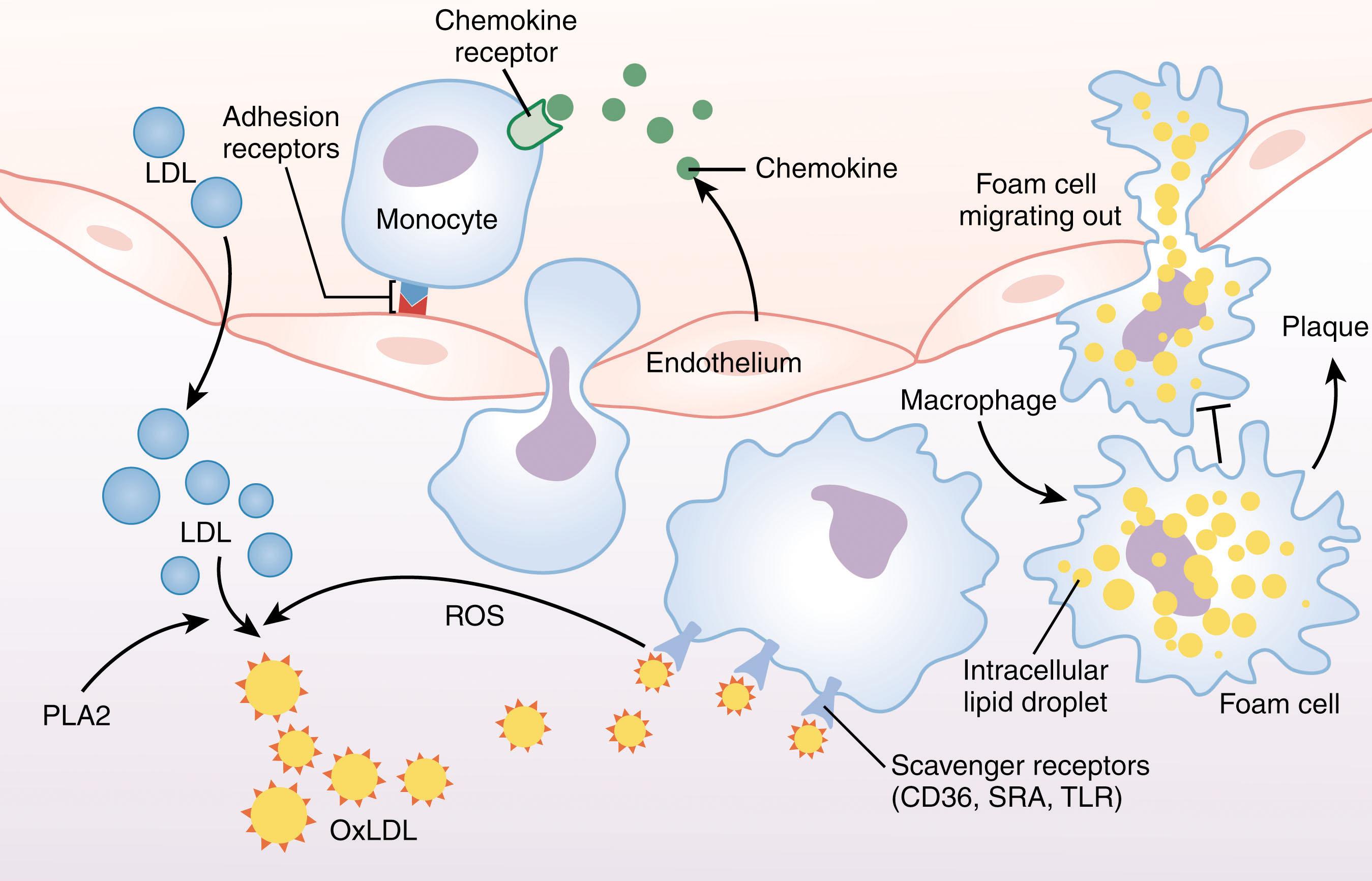
Just as data show an unequivocal role for LDL cholesterol in atherogenesis, equally compelling studies in humans and animal models show that mononuclear phagocytes are necessary for plaque formation and progression, and that atherosclerosis is associated with a chronic inflammatory state in the vessel wall and in the systemic circulation. Targeted genetic mutations that block monocyte development from hematopoietic precursors or that prevent monocyte trafficking into tissues dramatically protect mice from experimental atherosclerosis. The earliest and most prominent cell types in human and mouse atherosclerotic lesions are monocytes and macrophages. Initial monocyte entry into the arterial intima is in response to poorly understood cues related to endothelial cell dysfunction. Environmental and genetic influences, such as hypertension, angiotensin II production, cigarette smoke, diabetes, metabolic syndrome, and chronic periodontal infection may contribute to generalized endothelial dysfunction, but it is likely that the modified lipoproteins, particularly oxidized LDL (oxLDL) trapped in the vessel wall, play a major initiating role. OxLDL activates an endothelial cell receptor known as LOX1 to induce expression of monocyte adhesion molecules and this response is amplified in the presence of angiotensin II. Other components of the “dysfunctional” endothelial cell phenotype include von Willebrand factor release from Weibel-Palade bodies and altered homeostasis of nitric oxide and eicosanoid pathways (see Chapter 122 ).
Monocyte recruitment to the vessel wall (see Fig. 142.4 ) begins with their capture and rolling along the activated endothelium mediated by specific interaction of selectin family adhesion molecules (L-selectin on circulating monocytes and P-selectin on activated endothelial cells) with their counter receptors, including PSGL1. Subsequent signals induced by endothelial-derived chemokines, including CCL5/RANTES, CCL2/MCP1, IL-8, and CXCL1, lead to further recruitment of monocytes and facilitate firm adhesion of the rolling monocytes to the vessel wall. The latter is mediated by interaction of ICAM1 and VCAM on the activated endothelial surface with specific integrin family counter receptors on monocytes, β2 integrins for ICAM1 and α4β1 for VCAM. The adherent monocytes then go through diapedesis via disrupted endothelial junctions and enter the intima. Recent studies have suggested that a particular subset of circulating “inflammatory” monocytes, defined by high expression of the Ly6C antigen (in mice) or CD14 (in humans), are the predominant source of entering cells. The normal vasculature also contains a small number of resident macrophages, as well as “patrolling” monocytes. The latter are distinct from the Ly6C + /CD14 hi cells and exhibit a less inflammatory phenotype.
The fate of monocytes within the intima is probably determined in part by lineage commitment programs carried by the entering monocytes and in part by local environmental cues. Most, however, seem to polarize toward the so-called M1 inflammatory macrophage phenotype. Investigators have, however, detected dendritic cell-like phenotypes (expressing CD11c), M2-like cells that express arginase and eNOS and are highly phagocytic and anti-inflammatory, and proangiogenic VEGF-expressing macrophage phenotypes within plaque. In addition to monocytes, lymphocytes, particularly T cells, also enter the intima, where they contribute to plaque formation by secreting cytokines and other mediators. Of these infiltrating lymphocytes, interferon-γ producing Th1 cells and interleukin-17 producing Th17 cells have been shown to promote atherosclerosis, while the Treg cell subset is protective.
Reactive oxygen species (ROS) generated by the inflammatory milieu within the atherogenic vessel wall modify the trapped LDL by oxidizing both protein and lipid moieties (see Fig. 142.4 ), creating what are called collectively oxLDL and affecting the 2,3-diphosphoglycerate (2,3-DPG, also known as 2,3-bisphosphoglycerate or 2,3-BPG) levels. Modified LDL lose their affinity for the LDL receptor but gain affinity for a genetically unrelated family of receptors known as SRs, including SRA1 and CD36, which are present at high levels on the macrophage surface. These receptors are part of the innate immune system, and their recognition of specific structures within oxLDL presumably relates to their mimicry of similar structures found on pathogenic organisms. CD36, for example, also recognizes mycobacterial and Staphylococcus cell wall components, certain fungal structures, and falciparum malaria–infected erythrocytes. TLR family members, including TLR2, TLR4, and TLR6, can also recognize modified LDL and can partner with CD36 in these functions. Unlike the LDL receptor, which is downregulated in the setting of excess intracellular cholesterol, SR expression is not regulated in that manner, and, in fact, is increased by the presence of oxLDL. This, in part, results from internalization of oxidized fatty acids that serve as ligands for the PPAR family of transcription factors, particularly PPARγ, which is a major positive regulator of CD36 expression. Thus, over many months and years, the continued entry and oxidation of LDL in the intima coupled with upregulated expression of SRs lead to massive intracellular accumulation of cholesterol and formation of lipid-laden foam cells.
OxLDL-SR interactions initiate a cascade of events in macrophages, including internalization of the bound oxLDL, activation of proinflammatory pathways, and activation of transcriptional pathways. The CD36-oxLDL interaction, in addition to promoting ROS formation, also decreases expression of endogenous antioxidant pathways and inhibits macrophage migration. Furthermore, recognition of oxLDL by CD36 initiates metabolic repurposing from mitochondrial respiration to a glycolytic-like state. Although not mirroring the Warburg effect in cancer cells, CD36-mediated metabolic repurposing results in mitochondrial dysfunction, ROS production, and phenotypic polarization toward a proinflammatory state. These events result in a feed forward loop that increases leukocyte recruitment into the vessel wall, inhibits macrophage migration out of the vessel wall, and increases oxLDL formation. The net effect is formation and accumulation of lipid-laden foam cells and proinflammatory immune cells, which together form plaque (see Fig. 142.4 ).
Although abundant data from animal models and correlative human studies support the oxidative stress hypothesis, considerable controversy remains because interventional trials of antioxidant therapy in humans have generally failed to prevent the complications of atherosclerosis. These trials, however, are difficult to interpret because of lack of convincing evidence that the tested “antioxidant” therapies target the relevant vascular or circulating oxidant pathways. These oxidant systems include NADPH oxidase, xanthine oxidase, myeloperoxidase (MPO), uncoupled nitric oxide synthase, and dysfunctional mitochondrial electron transport chain. MPO is of particular interest because it generates a highly specific oxidized phospholipid ligand for CD36 from LDL and because circulating MPO levels correlate with risk for atherosclerosis and acute cardiovascular events.
Another potential problem with the antioxidant clinical trials is that the choice of antioxidants may have been flawed. Most used vitamin E formulations containing mainly α-tocopherol. Recent studies showed that tocopherols, in addition to having activity as antioxidants, have important cell-signaling functions mediated by specific cellular receptors. Therapy with formulations containing primarily α-tocopherol may downregulate endogenous γ-tocopherol levels, leading to imbalance in natural tocopherol signaling pathways. Furthermore, tocopherols are lipid-based structures that are themselves subject to oxidation, producing lipid peroxides that can promote further oxidative stress. Nevertheless, it is also possible that pathways of cholesterol uptake unrelated to LDL oxidation and SR expression, such as by micro- and macropinocytosis of “native” LDL or aggregated LDL, play a significant role in foam cell formation.
Important unresolved issues in the pathogenesis of early atherosclerotic lesions include understanding why macrophages do not exit the vessel wall and travel to regional nodes after ingesting modified LDL, as they would after ingesting exogenous pathogens, and why cholesterol efflux cannot keep up with LDL uptake to maintain homeostasis. OxLDL inhibition of macrophage migration (see Fig. 142.4 ) may explain the former, suggesting that blocking oxLDL-mediated signaling pathways might have therapeutic potential by facilitating macrophage exit from developing plaque. Indeed, in experimental animals, transplantation of atherosclerotic aortae from hypercholesterolemic animals into normal recipients induces migration of lipid-laden macrophages out of the vessel wall and plaque regression. The second issue might relate to differential gene regulation of SRs compared with cholesterol efflux transporters, and/or to intracellular cholesterol trafficking that might make it inaccessible to efflux transporters. In addition, as noted previously, HDL particles, like LDL, are sensitive to oxidative modification which may render them dysfunctional in RCT and, thus, lose their atheroprotective properties.
An intriguing recent development in understanding the cellular composition of plaque comes from lineage marker studies which show that a surprisingly large percentage of foam cells expressing macrophage markers are derived from a smooth muscle cell lineage. These studies suggest that local cues in plaque might interact with intimal or adventitial smooth muscle cells to redirect their genetic programs toward a macrophage-like phenotype. It is possible that such cells do not possess the full migratory capacity of macrophages, and this could contribute to the trapping of foam cell in plaque.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here