Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vascular surgeons care for a diverse set of clinical manifestations related to atherosclerosis, from transient cerebral ischemic attacks, strokes, aortoiliac occlusions, aortic aneurysms, mesenteric ischemia, to lower extremity arterial occlusive disease. However, despite the wide range of manifestations, culprit lesions are more alike than different. Accumulation of large amounts of cholesterol ester in the arterial wall and formation of complex advanced plaque are common to all lesions. Although the formation of atherosclerotic lesions is insidious and spans decades, the lesions can reach a clinical dénouement within minutes and manifest as catastrophic myocardial infarction (MI), stroke, or limb ischemia. Atherosclerosis-related cardiovascular disease (CVD) is the leading cause of death and disability globally: ischemic heart disease and stroke are the world’s first and third causes of death, representing 84.5% of cardiovascular deaths and 28.2% of all-cause mortality. However, age-standardized CVD mortality rates have decreased globally in the last two decades, and improved survival has translated into growing disease prevalence and a staggering financial burden. It is estimated that 121.5 million Americans have CVD, with an estimated annual cost of treatment exceeding $350 billion. Globally, 202 million people have peripheral arterial disease (PAD), which disproportionately affects individuals living in low- to middle-income countries. , Particularly troubling is the high prevalence of CVD risk factors in children and young adults. , A sedentary lifestyle, abdominal obesity, and poor diet contribute to dyslipidemia and high blood pressure. Autopsy studies in children and young adults demonstrate a link between these risk factors and early lesions.
This chapter outlines existing theories about the pathophysiology of atherosclerosis and the relationship to traditional and emerging risk factors. It also describes our current conceptual understanding of the fundamental biology of atherosclerotic plaque.
Lesions of the arterial wall have been divided into eight types (type I to type VIII) based on their histopathologic features. However, while the classification system is useful for comparing pathologic specimens, it has limited clinical practicality.
Autopsy studies from the Bogalusa Heart Study and the Pathobiological Determinants of Atherosclerosis in Youth study demonstrate that atherosclerotic lesions form in early childhood and increase with age. , In an intravascular ultrasound (IVUS) study of heart donors, 17% of individuals younger than 20 years had evidence of atherosclerosis. Notably, both the Bogalusa Heart Study and the Pathobiological Determinants of Atherosclerosis in Youth study highlight that the number and severity of early lesions are directly related to known CVD risk factors, thus suggesting that the presence of traditional CVD risk factors (such as familial hypercholesterolemia, hypertension, severe obesity, type 2 diabetes mellitus) and certain medical conditions (such as type 1 diabetes mellitus, chronic kidney disease, chronic inflammatory conditions, and underlying structural or functional heart disease) in childhood warrant early risk stratification, close surveillance, preferential use of pharmacotherapy to reduce CVD risk in selected individuals, and heart-healthy therapeutic lifestyle behaviors.
Diffuse intimal thickening has been identified in the atherosclerotic-prone areas of coronary arteries as early as 36 weeks of gestation. Although the fatty streak – dominated by lipid-filled macrophages – is itself benign, it is the precursor of the more clinically relevant late lesion. The fatty streak is the first lesion visible to the naked eye. Its yellow color is attributed to lipid in the form of cholesterol and cholesterol esters within macrophages and smooth muscle cells (SMC).
Advanced lesions, or fibrous plaque, are characterized histologically by the amount of extracellular lipid and fibrous connective tissue. They are whitish in gross appearance and are elevated so that they protrude into the lumen. These fibroatheromas are prone to provoking clinical sequelae by erosion of the surface endothelial cells, rupture of the fibrous cap, erosion of a calcium nodule, or intraplaque hemorrhage ( Fig. 4.1 ). , Plaques differ in consistency and may be relatively soft and friable or densely sclerotic and calcific. Likewise, some have well-formed fibrous caps, whereas others are covered by a narrow zone of loose connective tissue or by endothelium alone.
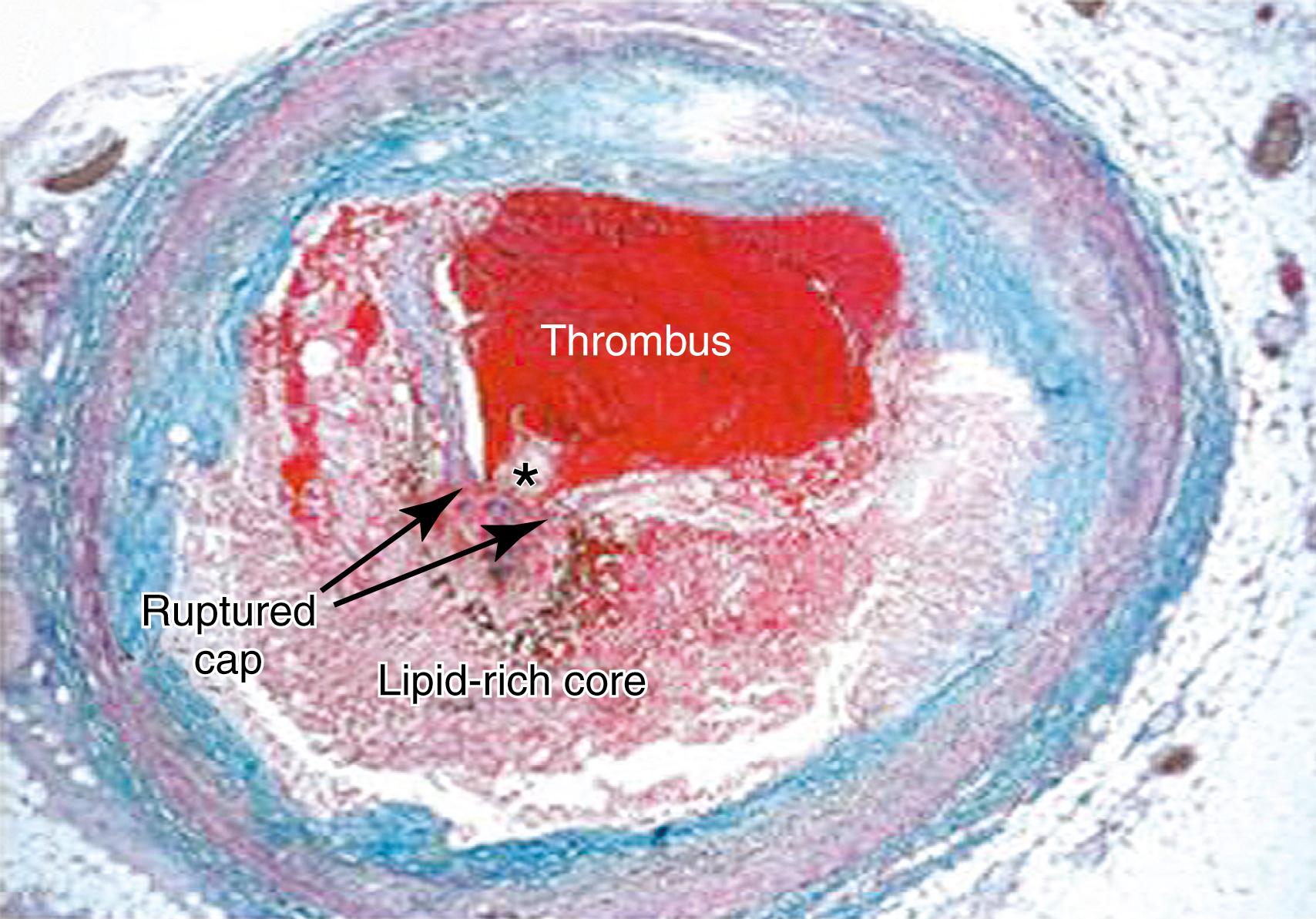
The necrotic core usually occupies the deeper central regions of the plaque and contains amorphous lipid and cholesterol crystals. The term atheroma is derived from the Greek word athere , meaning “porridge-like gruel.” SMC and inflammatory cells are located adjacent to the necrotic core and at the shoulders of the plaque, where it is most susceptible to rupture.
The fibrous caps contain varied levels of SMC adjacent to the collagen and basement membrane. These cells have reduced proliferative ability and may be regarded as senescent. However, the fibrous cap of ruptured plaque is often infiltrated with foam cells, which are largely of macrophage origin and thus indicative of active inflammation and vulnerability to rupture. A priori identification of these so-called thin-cap fibroatheromas is an active area of cardiovascular imaging research.
Each of the well-regarded theories of atherosclerosis discussed in this section attempts to explain the underlying pathogenesis of atherosclerotic plaque. Each theory has undergone a steady evolution since the time that it was initially proposed, a consequence of advancements in our scientific inquiry from histologic descriptions to discernment of powerful molecular mechanisms.
Cholesterol has been one of the most studied molecules in biomedical research. The modern era of cholesterol research began in St. Petersburg, Russia, at the turn of the 20th century, when Nikolaj Anitschkow produced vascular lesions in rabbits by feeding them purified cholesterol dissolved in sunflower oil. , These lesions closely resembled those seen in human atherosclerosis. He hypothesized that they were caused by elevated serum cholesterol and also noted a distinctive pattern consisting of the location of the lesions near arterial branch points and concluded that this was probably determined by hemodynamic factors. Nevertheless, this work was criticized because the levels of cholesterol produced were too high and could not be experimentally reproduced in more conventional animal models such as rats and dogs. ,
Cholesterol is insoluble in water, and the early work done in Russia provided no clues as to how it was transported to the arterial wall and formed plaques. The discovery that cholesterol was associated with proteins that allowed it to be transported in the aqueous environment led to investigations into lipoproteins. The first investigation in which the lipoprotein content in whole serum was accurately quantified involved analytic ultracentrifugation and was led by John Gofman of the Donner Laboratory at University of California at Berkeley. Lipoprotein fractions were isolated and characterized by their densities and flotation characteristics. Of note, this group noted that it was not simply the total cholesterol that was important but the species of lipoprotein contained within the cholesterol.
Cholesterol is transported in the aqueous environment by esterification of the sterol of long-chain fatty acids and packaging of these esters with the hydrophobic cores of plasma lipoproteins. With its polar hydroxyl group esterified, cholesterol remains sequestered within this core, which is essentially an oil droplet composed of cholesteryl esters and triglycerides, solubilized by a surface monolayer of phospholipid and unesterified cholesterol and stabilized by protein. In persons who are fasting, lipids circulate in plasma as lipoprotein particles that are defined on the basis of their density as very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). The protein of the VLDL, IDL, and LDL molecule is apoB-100, which has β-electrophoretic mobility.
The major milestone in the lipoprotein field was the discovery of the defective gene associated with familial hypercholesterolemia by Joseph L. Goldstein and Michael S. Brown at The University of Texas Southwestern. In fibroblasts cultured from normal human subjects, the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-controlling enzyme in cholesterol biosynthesis, is regulated by the content of LDL (but not HDL) in the culture medium. Goldstein and Brown found that HMG-CoA was not sensitive to normal feedback regulation by LDL in cultured skin fibroblasts from homozygous familial hypercholesterolemia patients. , They then determined that the defect was related to deficient binding of LDL to the cells of patients with familial hypercholesterolemia and that suppression of HMG-CoA activity was related to the amount of bound LDL. Therefore, these patients had overproduction of cholesterol because they lacked the appropriate receptor. They demonstrated that cellular uptake of LDL absolutely requires the LDL receptor, without which the LDL cholesterol concentration builds up to 800 to 1000 mg/dL (20.7–25.9 mmol/L). This important work culminated in a Nobel Prize in Physiology or Medicine for Goldstein and Brown in 1985.
Epidemiologic evidence from numerous studies, including the Japanese migration studies , and the Framingham Heart Study, demonstrated the association between cholesterol and incident CVD. Other studies showed that diets rich in unsaturated fats resulted in lowered cholesterol and reduced cardiovascular events.
Widespread acceptance of the causative role of cholesterol began with publication of the Coronary Primary Prevention Trial, sponsored by the National Institutes of Health. , This trial demonstrated that lowering cholesterol with the bile acid-binding resin cholestyramine reduced cardiovascular events. A decade later, this trial was reinforced with powerful data that emerged from the statin era. Although the primacy of the “cholesterol hypothesis” has been called into question, there is no doubt that lipids play a critical role in the pathogenesis of atherosclerosis. Indeed, recent clinical evidence with the PCSK9 inhibitors (inducing marked reduction of LDL) have suggested a significant benefit in patients with PAD with a 42% reduction in major adverse limb events over 2 years.
The basis of this hypothesis builds on earlier work by Jack Duguid, John Poole and Howard Florey, and John French and was formally advanced in 1973 by Russell Ross and John Glomset, who emphasized the importance of injury to the endothelium as a seminal event in the development of atherosclerosis lesions. Even though the definition of the term injury has been modified over the last three decades, all response-to-injury hypotheses have emphasized the primacy of the endothelium in stimulating the cascade of events leading to lesion formation.
Endothelial injury may result from mechanical disruption, exposure to toxic or infectious agents, or endogenous inflammatory signals. Injury to the endothelium allows adhesion of platelets and an influx of LDL and other serum factors into the subendothelial space. Platelets release their alpha granules and stimulate migration of SMC into the intima, where they proliferate and form a thickened neointima responsible for narrowing of the arterial lumen. Restoration of a healthy endothelial cell layer abates the process. During work on this hypothesis, Ross et al. performed experiments leading to the discovery of platelet-derived growth factor (PDGF). ,
Other investigators have countered that it is not injury to the endothelium that is the initiating event but rather retention of inflammatory lipids in the subendothelial space that renders a particular area susceptible to atherosclerosis. The observation that LDL accumulates in the intima within two hours after a bolus infusion and that the accumulation occurs before the formation of fatty streaks , led to the conclusion that retention of LDL is the initiating event of atherosclerosis. The so-called “response-to-retention” theory purports that retained apoB-containing lipoproteins stimulate a macrophage- and T cell-dominated inflammatory response in the arterial wall. ,
This hypothesis suggests that each lesion of atherosclerosis is derived from a single SMC that serves as a precursor for the clonal expansion of proliferating SMC. The hypothesis put forth by Earl and John Benditt used the concept that in every female cell there is only one active X chromosome and the progeny of that cell will express the same X chromosome as the parent cell. Glucose-6-phosphate dehydrogenase (G6PD) has two isoforms that can be separated by electrophoresis. Its gene is located on the X chromosome and can therefore be used to identify the progeny of a parent cell. This approach was used to determine that uterine leiomyomas are composed of cells with the same active X chromosome, whereas adjacent normal myometrium is composed of a mixture of cells containing both G6PD isoforms, implying that both X chromosomes are active.
The Benditts examined a series of atherosclerotic plaques from four black females and compared them with adjacent normal areas of arterial wall. They determined that SMC from the plaque contained only one G6PD isoform, whereas adjacent control areas contained a mixture of isoforms. This allowed them to conclude that each lesion is a clonal outgrowth derived from a single precursor SMC located in the intima. It is noteworthy that SMC within individual neonatal intimal thickenings are monoclonal in origin, whereas cells from the subjacent media are polyclonal.
Others challenged the hypothesis by noting that identification of a single enzyme phenotype does not necessarily imply clonal origin. , Regardless, the work was importantly heuristic and hinted toward the role that modern molecular biology would play in unraveling the genetic basis of atherosclerosis.
The original version of the response-to-injury hypothesis of atherosclerosis proposed that endothelial denudation was the first step in atherosclerosis. Subsequent versions of the hypothesis proposed that an endothelium chronically bathed in serum with high concentrations of LDL or exposed to other cardiovascular risk factors would render susceptible areas of the endothelium dysfunctional or activated. Indeed, an intact endothelium may be a necessary factor for lesion progression, and it is now clear that developing atheromas are covered by an intact endothelium throughout most stages of lesion progression. , , In humans, only the most advanced ulcerated lesions are focally devoid of endothelium. The injury results in increased adhesiveness and an increase in permeability of the endothelium to inflammatory cells ( Fig. 4.2 ).
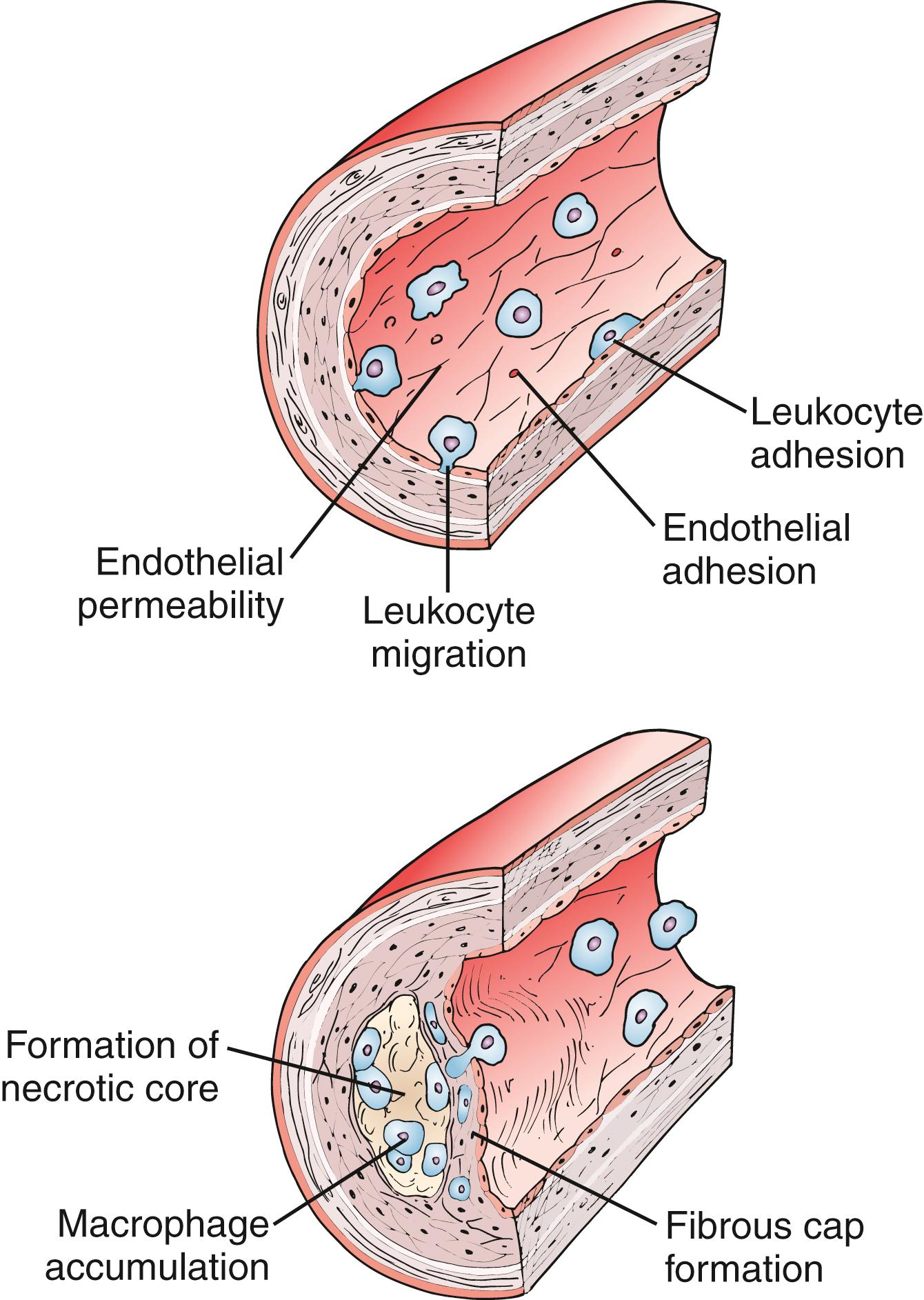
Atherosclerosis is now recognized as an inflammatory disease, and components of the innate and adaptive immune system are involved in every step of the atherosclerotic process. Much of our modern understanding comes from examination of human pathology specimens and transgenic animals. Genetic deletion of apolipoprotein E (ApoE − / − ) or the LDL receptor (Ldlr − / − ) , which produce mice with severe hypercholesterolemia and atherosclerotic lesions with features of mature human atheroma, have become cornerstones in atherosclerosis research laboratories. The importance of the LDL receptor in cholesterol regulation was noted by Goldstein and Brown when studying patients with familial hypercholesterolemia. Apolipoprotein E suppresses atherosclerosis, and ApoE −/− mice have very low levels of pre-β HDL and their plasma is poor at promoting the efflux of cholesterol from lipid-laden macrophages. Crossbreeding these mice with other strains carrying null mutations in immunologically relevant genes produces a robust research tool to dissect out the contribution of individual components of immune pathways in atherosclerosis. For example, some of the earliest approaches using compound mutant mice involved the global loss of the entire adaptive immune system. Rag1 − / − and Rag2 − / − mice lack the V(D)J recombinase required to form lymphocyte antigen receptor genes and hence have a complete loss of B and T cells. ApoE − / − mice fed a regular chow diet developed plasma cholesterol levels between 390 and 470 mg/dL. Double knockout ApoE − / − /Rag1 − / − mice had a 40% reduction in aortic atherosclerotic lesions compared with immunocompetent animals, thus demonstrating the role cellular immunity plays in the pathogenesis of atherosclerosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here