Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acid-base disorders can have major clinical and diagnostic implications. If they generate extreme acidemia or alkalemia, the abnormal pH itself may result in pathophysiologic consequences. For example, the tertiary structure of proteins is altered by extreme pH conditions, potentially affecting the activity of enzymes and ion transport systems. Consequently, every metabolic pathway may be impacted by acidemia or alkalemia. In addition, extreme acidemia can depress cardiac function, impair the vascular response to catecholamines, and cause arteriolar vasodilation and venoconstriction, with resultant systemic hypotension and pulmonary edema. Insulin resistance, reduced hepatic lactate uptake, and accelerated protein catabolism are other effects of acidemia. Alkalemia can generate cardiac arrhythmias, produce neuromuscular irritability, and contribute to tissue hypoxemia. In alkalemic patients, cerebral and myocardial blood flow falls and respiratory depression occurs. Potassium disorders, a common accompaniment of acid-base perturbations, also contribute to the morbidity.
Although mild and moderate acid-base disorders may not directly affect physiologic function, the identification of such disorders may be an important diagnostic clue to the existence of serious medical conditions. Whenever an acid-base disorder is identified, the underlying cause should be sought. This diagnostic imperative often overrides the importance of any therapeutic intervention directed at the pH itself. The situation is analogous to the discovery of fever or hypothermia. Although very high or very low temperatures can themselves be dangerous and require aggressive therapy directed at restoration of a more normal temperature, often more important is the effort to identify and treat the underlying cause of the abnormal temperature. Similarly, the recognition of an acid-base disorder must generate a search for its clinical cause or causes, and recognition of a mixed acid-base disorder should trigger an investigation to determine the etiology of each component.
The acid-base status of the extracellular fluid (ECF) is carefully regulated to maintain the arterial pH in a narrow range between 7.36 and 7.44 (hydrogen ion concentration [H + ] 44 to 36 nEq/L). The pH is stabilized by multiple buffer systems in the ECF, cells, and bone. The CO 2 tension (pCO 2 ), primarily under neurorespiratory control, and the serum bicarbonate concentration ([HCO 3 − ]), primarily under kidney/metabolic regulation, are the most important variables in this complex system of buffers.
Currently, three different methodologic approaches are widely used to describe normal acid-base status and simple and mixed acid-base disorders.
The physiologic method uses measurements of arterial pH, pCO 2 , and [HCO 3 − ], together with an analysis of the anion gap (AG) and a set of compensation rules.
The base excess (BE) method uses measurements of arterial pH and pCO 2 and calculation of the BE and the AG.
The physicochemical method uses measurements of arterial pH and pCO 2 together with the calculated apparent (SIDa) and effective (SIDe) “Strong Ion Difference,” the “Strong Ion Gap” (SIG = SIDa − SIDe), and the total concentration of plasma weak acids (Atot).
Each of these approaches can be effectively used to characterize acid-base disorders, each has its vocal proponents and detractors, and each has certain unique characteristics that may be particularly helpful under certain conditions. We believe the physiologic method is the most straightforward and the easiest model to understand and use. It is generally acceptable in most clinical circumstances and will be the method we use in this chapter.
The physiologic method to elucidate acid-base disorders uses the following information:
Recognition of diagnostic clues provided by the patient’s history and physical examination
Analysis of the serum [HCO 3 − ], arterial pH, and pCO 2 (Although a blood gas analysis is not always necessary to make a diagnosis, it is generally required for complicated cases.)
Knowledge of the predicted compensatory response to simple acid-base disorders
Calculation of the AG, with consideration of the expected “baseline” AG for each patient
Analysis of the degree of change (Δ) in AG and the degree of Δ in [HCO 3 – ] to see if the magnitude of these respective changes is reciprocal. This has been dubbed the Δ[AG]/Δ[HCO 3 – ]
or Delta/Delta.
The normal arterial blood pH range is between 7.36 and 7.44 ([H + ] between 44 and 36 nEq/L). Acidemia is defined as an arterial pH <7.36 ([H + ] >44 nEq/L) and may result from a primary elevation in pCO 2 , a fall in [HCO 3 – ], or both. Alkalemia is defined as an arterial pH >7.44 ([H + ] <36 nEq/L). Alkalemia may result from a primary increase in [HCO 3 – ], a fall in pCO 2 , or both.
The relationship among pH, pCO 2 , and HCO 3 – concentrations is described by the familiar Henderson-Hasselbalch equation:
Acidosis and alkalosis are pathophysiologic processes that, if unopposed by therapy or complicating disorders, would cause acidemia or alkalemia, respectively.
The simple acid-base disorders are divided into primary metabolic and primary respiratory disturbances. Each of these simple, or single, acid-base disorders generates a compensatory response that acts to return the blood pH back toward the normal range. By convention, the physiologic approach to acid-base analysis considers the compensatory response to a simple acid-base disorder to be an integral component of that disorder. Hence, there are four primary simple acid-base disturbances (six, if each respiratory disorder is divided into an acute and chronic phase):
Metabolic acidosis: The underlying pathophysiology reduces the serum bicarbonate concentration [HCO 3 – ]. Although we refer to serum bicarbonate here, it is often directly measured as total CO 2 , which includes bicarbonate (HCO 3 – ), carbonic acid (H 2 CO 3 ), and dissolved CO 2 . The last two components account for a very small fraction of the total (roughly 1.2 mEq/L at normal pCO 2 ). Therefore, for clinical purposes, total CO 2 is equated to serum bicarbonate concentration. Causes of metabolic acidosis include excess generation of metabolic acids, excessive exogenous acid intake, reduced kidney excretion of acid, excessive exogenous loss of HCO 3 – (usually in stool or urine), or combinations of these abnormalities. Metabolic acidosis reduces the arterial plasma pH and generates a hyperventilatory compensatory response, which reduces the arterial pCO 2 and blunts the degree of acidemia.
Metabolic alkalosis: The underlying pathophysiology tends to increase the [HCO 3 – ]. Causes include exogenous intake of HCO 3 – salts (or salts that can be converted to HCO 3 – ) and/or endogenous generation of HCO 3 – . Regardless of the origin of the HCO 3 – , the pathology must also include reduced or impaired kidney HCO 3 – excretion. Metabolic alkalosis increases the arterial plasma pH and generates a hypoventilatory compensatory response, which increases the arterial pCO 2 and blunts the degree of alkalemia.
Respiratory acidosis: The underlying pathophysiology increases the arterial pCO 2 . The compensatory response is an increase of the plasma [HCO 3 – ] due to rapid generation from buffers and, over a period of days, kidney HCO 3 – generation and retention.
Respiratory alkalosis: The underlying pathophysiology decreases the arterial pCO 2 . The compensatory response reduces the plasma [HCO 3 – ]. This occurs acutely as H + is released from buffers and chronically, over a period of days, as the kidneys excrete HCO 3 – and/or retain acid.
The magnitude of each compensatory response is proportional to the severity of the primary disturbance. Generally, respiratory responses to primary metabolic acid-base disorders occur rapidly (within an hour) and are fully developed within 12 to 36 hours. In contrast, the compensatory metabolic alterations triggered by the primary respiratory disorders are divided into two phases. A chemical buffering response occurs within minutes (acute), whereas the quantitatively more significant kidney response takes several days (chronic) to develop fully. Hence, each primary respiratory disorder is subdivided into an acute and a chronic disorder to differentiate the expected compensatory response.
The expected degree of compensation for each simple disorder has been determined by studying patients with isolated simple disorders and normal subjects with experimentally induced acid-base disorders. These data have been used to create various graphic acid-base nomograms, simple mathematical relationships, and a number of mnemonic methods for predicting expected compensation ranges. Fig. 12.1 and Table 12.1 provide some of these “compensation rules.” Appropriate compensation should generally be present in all patients with an acid-base disorder, and when it is not identified, a complex, or mixed, acid-base disorder must be considered.
![Fig. 12.1, The acid-base map. Shaded areas represent the 95% confidence limits for zones of compensation for the simple acid-base disorders. Numbered diagonal lines represent isopleths of plasma bicarbonate concentration ([HCO 3 − ]). Laboratory values that fall within a colored zone are consistent with the simple acid-base disorder, as shown. If the values fall outside a colored zone, a mixed acid-base disorder is likely. ALK , Alkalosis; N , normal range; RESP , respiratory. (Modified and updated from Goldberg M, Green SB, Moss ML, Marhacli MS, Garfinkel D. Computer-based instruction and diagnosis of acid-base disorders. JAMA . 1973;223:269–275.) Fig. 12.1, The acid-base map. Shaded areas represent the 95% confidence limits for zones of compensation for the simple acid-base disorders. Numbered diagonal lines represent isopleths of plasma bicarbonate concentration ([HCO 3 − ]). Laboratory values that fall within a colored zone are consistent with the simple acid-base disorder, as shown. If the values fall outside a colored zone, a mixed acid-base disorder is likely. ALK , Alkalosis; N , normal range; RESP , respiratory. (Modified and updated from Goldberg M, Green SB, Moss ML, Marhacli MS, Garfinkel D. Computer-based instruction and diagnosis of acid-base disorders. JAMA . 1973;223:269–275.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ApproachtoAcidBaseDisorders/0_3s20B9780323791229000121.jpg)
| Primary Disorder | pH | Initial Chemical Change | Compensatory Response | Expected Compensation |
| Metabolic acidosis | Low | ↓ [HCO 3 − ] | ↓ p CO 2 | P CO 2 = (1.5 × [HCO 3 − ]) + 8 ± 2 P CO 2 = [HCO 3 − ] + 15 P CO 2 = decimal digits of pH |
| Metabolic alkalosis a | High | ↑ [HCO 3 − ] | ↑ P CO 2 | P CO 2 variably increased P CO 2 = (0.9 × [HCO 3 − ]) + 9 PCO 2 = (0.7 × [HCO 3 − ]) + 20 |
| Respiratory Acidosis | ||||
| Acute | Low | ↑ P CO 2 | ↑ [HCO 3 − ] | [HCO 3 − ] increases 1 mEq/L for every 10 mm Hg increase in P CO 2 |
| Chronic | Low | ↑ P CO 2 | Further ↑ [HCO 3 − ] | [HCO 3 − ] increases 3 to 4 mEq/L for every 10 mm Hg increase in P CO 2 |
| Respiratory Alkalosis | ||||
| Acute | High | ↓ P CO 2 | ↓ [HCO 3 − ] | [HCO 3 − ] decreases 2 mEq/L for every 10 mm Hg decrease in P CO 2 |
| Chronic | High | ↓ P CO 2 | Further ↓ [HCO 3 − ] | [HCO 3 − ] decreases 5 mEq/L for every 10 mm Hg decrease in P CO 2 |
a Compensation formulas for metabolic alkalosis have wide confidence limits because the P CO 2 of individuals with this disorder varies greatly at any given [HCO 3 − ]. [HCO 3 − ], Serum bicarbonate concentration; PCO 2 , arterial partial pressure of carbon dioxide.
In general, with one exception, compensatory responses return the pH toward the normal range but do not completely normalize the pH. The exception is chronic respiratory alkalosis, wherein compensation results in a pH that is normal. With all other disorders, some degree of acidemia or alkalemia remains, even after full compensation. Compensatory responses result in the pCO 2 moving in the same direction as the primary [HCO 3 – ] change in case of metabolic acid-base disorder and the [HCO 3 – ] moving in the same direction as the primary pCO 2 change in case of respiratory acid-base disorder (see Table 12.1 ). If the pCO 2 and [HCO 3 – ] are deranged in the opposite directions (i.e., the pCO 2 or [HCO 3 – ] is increased and the other variable is decreased), then a mixed disturbance must exist.
The ion profile of normal serum is depicted in Fig. 12.2 A. In any solution, the total cation charge concentration must be equal to the total anion charge concentration (all measured in units of electrical charge concentration, i.e., mEq/L). Now consider only the three serum electrolytes that are at the highest concentration: Na + , Cl − , and HCO 3 – . The cation charge concentration [Na + ] normally exceeds the sum of the anion charge concentrations [Cl − ] and [HCO 3 – ]. If the sum of the two anions is subtracted from [Na + ], an “AG” is noted (see Fig. 12.2 B):
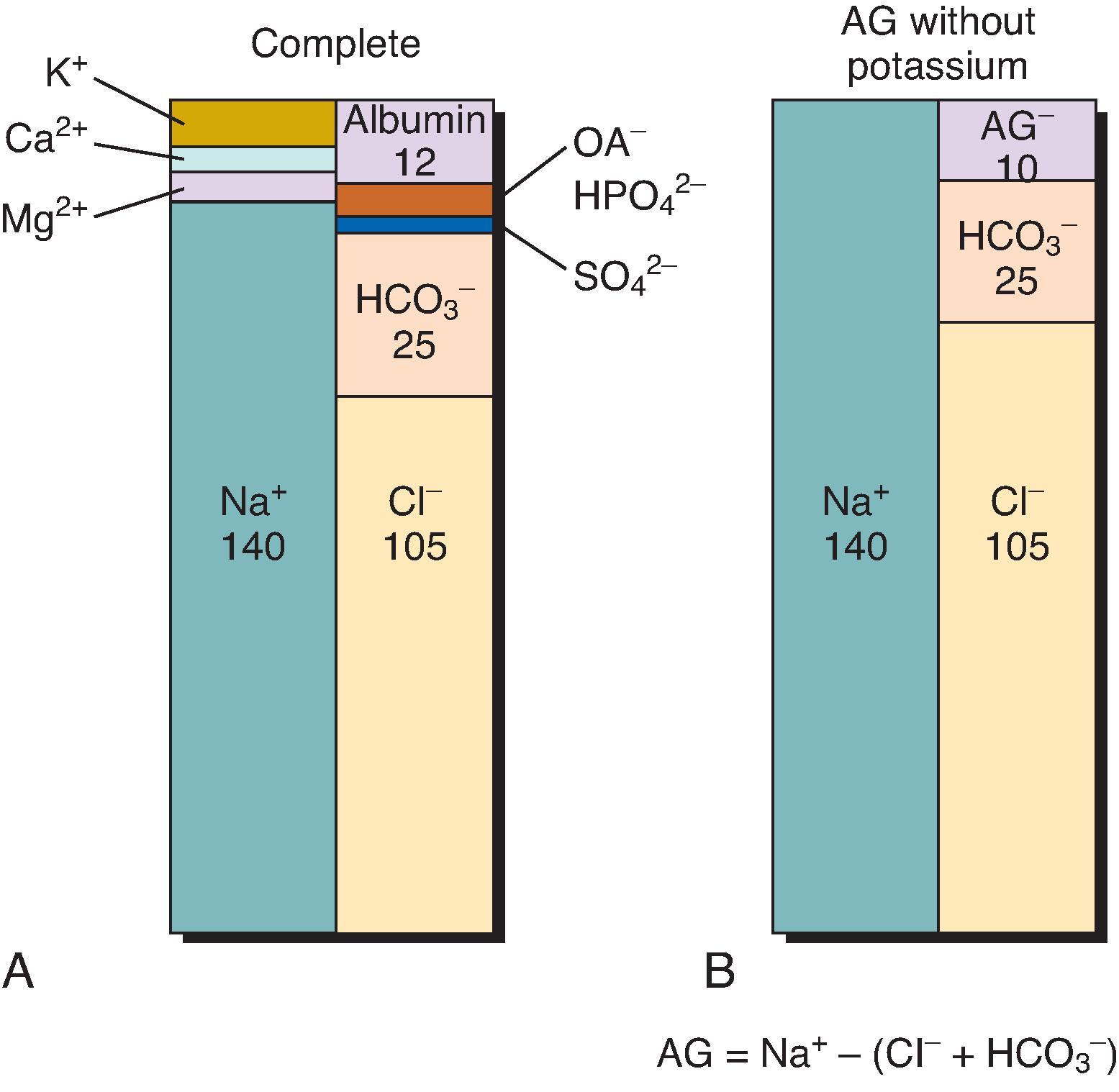
This AG is, of course, a function of the decision to consider only the three major serum electrolytes and not other ions that normally exist in serum. Nevertheless, the AG, as defined in this fashion, is a very useful diagnostic tool.
The normal value of the AG varies among laboratories as a result of the wide variety of analyte measurement technologies and unique normal ranges for each instrument. Typically, the normal AG range is considered to be 8 to 12 mEq/L. The normal AG is primarily composed of anionic albumin and, to a lesser degree, other proteins, sulfate, phosphate, urate, and various organic acid anions such as lactate. In general, if the concentration of these “unmeasured” anions increases, the AG increases. Conversely, the AG falls when the concentration of unmeasured anions is reduced. For example, hypoalbuminemia is a common cause of a reduced AG, with the AG falling about 2.5 mEq/L for each 1 g/dL reduction of albumin below the normal range.
The disorders that produce metabolic acidosis can be subdivided on the basis of an increased or normal AG. An examination of the AG equation reveals that the only way the [HCO 3 – ] can fall while the AG remains normal is for the [Cl − ] to increase relative to the [Na + ]. Consequently, all “non-AG” metabolic acidoses must be hyperchloremic metabolic acidoses. This is shown graphically in Fig. 12.3 .
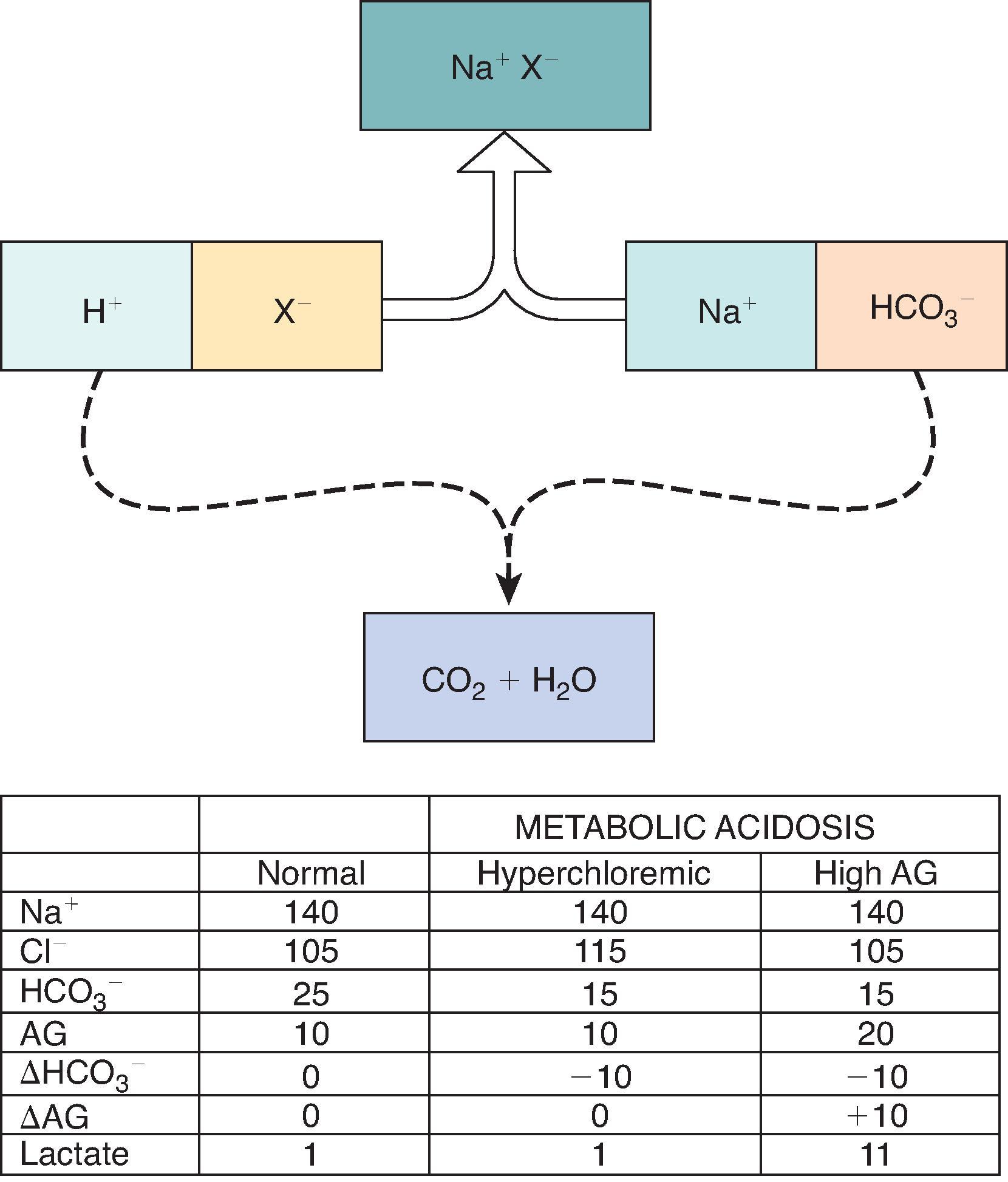
Most often, an elevated AG indicates the presence of a metabolic acidosis. Several mnemonics have been published as guides to the most common clinically relevant etiologies of high AG metabolic acidosis. We suggest the mnemonic “GOLDMARK” for this purpose (Glycols [ethylene, propylene, and diethylene], Oxoproline [acetaminophen], L-Lactate, D-Lactate, Methanol, Aspirin, Renal failure, Ketoacidosis). However, a high AG can sometimes occur in the absence of metabolic acidosis. Exceptions include:
Dehydration, as the loss of water in excess of salts, increases the concentration of all electrolytes, including albumin and other unmeasured ions, thereby increasing the AG.
Rapid infusion, and transient accumulation, of metabolizable sodium salts such as lactate, acetate, citrate, and so on. To the extent these salts are metabolized, they generate NaHCO 3 , and the AG does not increase; if metabolic conversion is delayed, the AG increases.
Infusion of nonmetabolizable sodium salts, other than sodium chloride or bicarbonate. For example, anionic antibiotics such as carbenicillin and penicillin G may be infused as sodium (or potassium) salts and, to the extent that they accumulate, increase the AG.
Metabolic alkalosis causes a small increase in AG (usually less than 3 to 4 mEq/L) as a result of (1) increased concentrations of the anions of organic acids (mainly lactate), which accumulate because of metabolic stimulation of production, and (2) increased concentration of albumin, due to ECF volume contraction.
Laboratory error, or measurement artifact, of one or more analytes.
A mixed acid-base disturbance is the simultaneous existence of two or more simple acid-base disturbances. Mixed acid-base disorders may develop concurrently or sequentially. The disorders may be additive, with each process having a similar directional effect on pH. Alternatively, they may oppose each other, having offsetting effects on pH. Sometimes three simultaneous acid-base disorders, or a triple acid-base disturbance, can be identified.
Recognition of mixed acid-base disorders is important for several reasons. First, when these disorders are additive (i.e., coexisting metabolic and respiratory acidoses or coexisting metabolic and respiratory alkaloses), the pH excursions may become severe, with toxic consequences. When offsetting disorders coexist, the pH may be normal or near normal. Nonetheless, their identification serves as an important diagnostic clue to the underlying pathophysiology. Mixed disorders often suggest specific clinical derangements. For example, concurrent high AG metabolic acidosis and respiratory alkalosis are typical of salicylate poisoning, whereas patients with diabetic ketoacidosis often vomit and may present with concurrent high AG metabolic acidosis and metabolic alkalosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here