Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The practice of surgical pathology has relied traditionally on evaluation of the gross and microscopic features of tissue specimens, integrated with the clinical history and other laboratory data, to reach a definitive diagnosis, assign prognosis, and assist the clinician and ultimately the patient to select the most appropriate form of therapy. Histologic examination of tissue specimens has, for many decades, occupied the central role in the determination of malignancy, the classification of neoplasms, the grading of tumors, and (in most circumstances) the staging of disease. This approach to diagnosis has long proved its value through correlation with response to therapy and clinical outcomes. Furthermore, the procedures are well established, the acquisition of diagnostic data can be rapid—especially if frozen sections are used—and the entire process is relatively inexpensive.
Yet there are undoubted limitations to morphology. The American Cancer Society cancer statistics for 2017 estimated that approximately 1.7 million new patients would be diagnosed with cancer in the United States, with about 600,000 deaths resulting from cancer (~35% mortality). Although expert practitioners of surgical pathology can do much to aid in optimal patient management, histologic examination of tissue sections, as performed in the past, will likely continue to fall short as a means of stratifying patients for optimal tumor-specific treatment in many cancers. This has been demonstrated in adenocarcinoma of the lung, in which a subset (~20%) of tumors shows dramatic favorable clinical response to therapies targeting the epidermal growth factor receptor (EGFR), but this subset is morphologically and immunologically indistinguishable from the majority of adenocarcinomas, which fail to respond to these therapies. Alternative biomarkers are required to identify patients for anti-EGFR therapy and other targeted therapies, and regulatory agencies increasingly make drug approval contingent on demonstrating the oncogenic drug target in each patient. As examples, in 2011, the US Food and Drug Administration (FDA) approved the use of two targeted cancer therapies only for patients with molecular biomarkers that predict treatment response—crizotinib for lung carcinomas with ALK gene rearrangements and vemurafenib for melanomas with a V600E point mutation in the BRAF gene. This trend has increased dramatically in the years since, with multiple molecularly targeted therapies approved for treatment in cancers when a molecular biomarker is demonstrated ( Table 31.1 ).
| Target | Biomarker | Therapeutic Agent | Cancer Type |
|---|---|---|---|
| EGFR | EGFR mutations | Afatinib, erlotinib, gefitinib, osimertinib | Lung adenocarcinoma |
| EGFR | EGFR T790M | Osimertinib | Relapsed lung cancer |
| EGFR | KRAS, NRAS, BRAF wild type | Panitumumab, cetuximab | Colon cancer |
| ERBB2 | ERBB2 amplification | Trastuzumab, lapatinib, pertuzumab | Breast, gastric, esophageal cancer |
| ALK | ALK fusion | Crizotinib, alectinib, brigatinib, ceritinib | Lung adenocarcinoma |
| ABL1 | BCR-ABL1 fusion | Imatinib, nilotinib, bosutinib, dasatinib, ponatinib | CML, ALL |
| BRAF | BRAF V600 mutation | Vemurafenib, dabrafenib | Melanoma, lung cancer |
| IDH1 | IDH1 or IDH2 mutation | Enasidenib | AML |
| KIT | KIT mutation | Imatinib, sunitinib, regorafenib | GIST |
| JAK2 | JAK2 mutation | Ruxolitinib | Myelofibrosis |
| MEK | BRAF mutation | Trametinib | Lung cancer, melanoma |
| RET | RET mutation, fusion | Vandetinib | Thyroid cancer |
| ROS1 | ROS1 fusion | Crizotinib | Lung cancer |
Besides the frequent lack of morphologic features to distinguish subsets of neoplasms that have different patterns of clinical behavior, histologic examination of tissues is subject to other limitations. Some are technical and involve artifacts or problems caused by poor preservation, fixation, or staining of specimens. Other difficulties arise from the subjective nature of the analysis, which is heavily influenced by the practitioner's experience, bias, and training. With difficult, poorly differentiated, or rare neoplasms, interobserver and intraobserver variability can be high. Even with common entities in which diagnosis should be relatively straightforward, opinions may vary in the absence of adherence to standardized criteria. It is in the desire to overcome the limitations of histologic examination that newer, tissue-based methods have been introduced, with the overall goal of classifying neoplasms into biologically homogeneous groups.
Three waves of innovation have had an impact on surgical pathology over the last several decades. The electron microscope, which first revealed the existence of a series of subcellular organelles, was rapidly embraced by surgical pathologists to incorporate ultrastructural features of differentiation into the diagnostic workup of difficult neoplasms. Subsequently, immunohistochemistry quickly gained a role in routine surgical pathology, facilitating distinction between tumors with overlapping histologic features and enabling finer reclassification of some cancers based on expression of specific proteins and carbohydrates. Molecular genetic analysis of tissue specimens is the latest innovation to affect surgical pathology. Some of the techniques in this area are already applicable to diagnostic surgical pathology, whereas others offer the potential to lead to discovery of novel markers and possibly new diagnostic procedures. This chapter outlines current molecular biologic markers and techniques that can be used to evaluate tissue specimens and summarizes research directions in this field that are likely to affect surgical pathology in the years to come.
The generic target in molecular genetic analyses of tissue specimens is nucleic acid—either deoxyribonucleic acid (DNA) or ribonucleic acid (RNA). Commonly, the DNA in a given cancer contains various mutations, which may be expressed as mutant RNA or proteins, although some DNA mutations in cancer lead to loss of genetic material and, concomitantly, loss of expression of the corresponding RNA and protein. Cancer is a genetic disease, in that the causal mechanisms of cancer include alterations of DNA by acquired mutations in the affected cells (i.e., somatic mutation) that disrupt normal cellular homeostasis and bestow on the cancer cells attributes that enhance survival, such as increased mitotic rate or decreased apoptosis. These mutations can accumulate within cancer cells, which often lose the ability to repair DNA damage, such that many cancers contain multiple mutations scattered among different critical genes rather than mutation in a single gene, which is the hallmark of inherited mendelian genetic disorders such as cystic fibrosis and sickle cell anemia.
Gene mutations take several forms and include changes in number (i.e., deletions, duplications, amplifications), breakage and fusion to a portion of another gene normally located on another chromosome (i.e., translocation) or elsewhere on the same chromosome, and smaller changes in the sequence of nucleotide bases (i.e., point mutations, microinsertions, microdeletions) leading to alteration in RNA and protein structure (i.e., missense mutations) or a prematurely truncated and dysfunctional protein (i.e., nonsense mutation). Ultimately, these mutations affect fundamental cellular pathways, such as regulation of the cell cycle, apoptosis, or cell-cell and cell-matrix interactions.
Generally speaking, mutations that activate a protein in a pathway that is typically silenced or tightly regulated (i.e., an activator of the cell cycle) exert a dominant effect (mutation in one allele is sufficient) and are termed oncogenes. Similarly, mutations that inactivate a protein in a cellular pathway that is typically active (i.e., repair of DNA errors that occur during replication) exert a recessive effect, in that inactivation of both alleles is required, and are termed tumor suppressor genes. Different neoplasms have different combinations of these genetic alterations, such that they can often be used for diagnosis as discrete, objective indicators of the malignant phenotype.
The methods used to detect these markers vary considerably, depending on the type of mutation being sought. In general, alterations in gene number are best assessed by cytogenetic techniques (e.g., karyotype or fluorescence in situ hybridization [FISH]), alterations in DNA sequence are best assessed by molecular diagnostic techniques (e.g., polymerase chain reaction [PCR], sequence analysis), and structural rearrangements such as translocations may be detected by either, depending on the rearrangement. Furthermore, multiple molecular alterations can now readily be detected simultaneously in panel assays, with techniques such as array comparative genomic hybridization (array CGH) for copy number assessment and microarrays, next generation sequencing, or multiplexed single nucleotide extension assays for point mutations, microdeletions, and microinsertions.
In addition to mutations in DNA or RNA, epigenetic changes, in which the nucleotide sequence of DNA is unaffected, also contribute to the malignant transformation of cells. These changes include DNA methylation alterations, chromatin structure modifications in particular regions of the genome, and abnormal gene expression, often as secondary effects of mutations in other genes or dysregulation by microRNAs. Some of these epigenetic nucleic acid perturbations are receiving increased attention as markers in tumor diagnosis, prognosis, and therapy. For example, epigenetic silencing of the MGMT (O -methylguanine-DNA methyltranferase) DNA-repair gene by promotor methylation in glioblastomas is associated with longer survival in patients treated with temozolomide. A final form of molecular biologic marker is exogenous nucleic acid derived from infectious agents, mostly viruses implicated in the cause of cancer. There are as yet only a handful of such agents, but in particular settings nucleic acid sequences in the genomes of these agents also can be useful markers in the diagnosis of tumors (e.g., human papillomavirus [HPV]).
Chromosomal aberrations have been known to occur in tumors since the work of Boveri in the early part of the last century, but specific, recurrent forms of structural abnormalities in chromosomes were first reported in 1960, when the Philadelphia chromosome (an example of a chromosomal translocation, as discussed later) was described in the cells of chronic myelogenous leukemia (CML). Since that time, other types of specific chromosomal abnormalities have been found in tumors, including inversions, deletions, and amplifications. However, chromosomal translocations have been the most important chromosomal abnormalities diagnostically and are particularly frequent in hematopoietic tumors and soft tissue sarcomas. Traditionally translocations were found in few carcinomas, which may in part reflect the greater technical difficulties of cytogenetic analysis in these tumors. However, in vitro assays and next generation sequencing of solid tumors, including RNA sequencing, has identified distinct gene fusions across multiple solid tumors, including carcinomas of the lung, thyroid, kidney, and prostate, which can serve as diagnostic, prognostic, or therapeutic biomarkers. Chromosomal translocations result from recombination of nonhomologous DNA between different chromosome regions and are usually reciprocal and balanced or near balanced, meaning that the exchange of chromosomal material between two chromosomes occurs without an accompanying substantial loss of DNA.
Molecular analyses of chromosomal translocations show that the recombination-associated chromosomal breaks typically result in altered gene expression, by either dysregulated expression of a nearby gene or direct disruption of a gene at one or both breakpoints. An example of the latter is the Philadelphia chromosome, which is abbreviated as Ph and is designated t(9;22)(q34;q11) using formal cytogenetic nomenclature (the two chromosomes participating in the translocation are indicated in the first parentheses; the arms—q for long, p for short—and bands of each chromosome containing the breakpoint are indicated in the second parentheses). The breakpoints of this translocation lie within two genes, ABL1 on chromosome 9 and BCR on chromosome 22 ( Fig. 31.1 ).
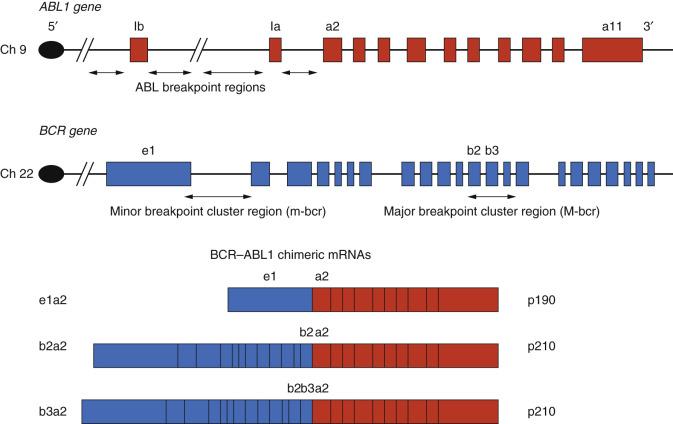
As a result of recombination at the breakpoints, the 5′ portion of the BCR gene, encoding the amino terminal part of the BCR protein, is joined to the 3′ portion of the ABL1 gene, encoding the carboxy terminal part of the ABL protein, creating a novel chimeric BCR-ABL1 gene. The breakpoints occur in both genes within introns (the noncoding segments of DNA that are interspersed between coding regions of the genes), and therefore the coding regions, or exons, of the gene remain intact and are transcribed and translated into a fusion protein. This fusion protein contains a functional ABL tyrosine kinase that can activate its usual downstream signaling pathways, but it does so outside of its normal regulation because the translocation places it under the control of the BCR promoter; the resulting dysregulation is oncogenic for hematopoietic cells.
Two breakpoint regions have been identified within the BCR gene that can be distinguished only by molecular diagnostic techniques (e.g., PCR) and not by cytogenetic methods such as FISH (see Fig. 31.1 ). The most common breakpoints occur in the 5.8-kilobase (kb) major breakpoint cluster region (M-bcr) of BCR and result in a 210-kiloDalton (kDa) fusion protein (p210), which has enhanced tyrosine kinase activity, has been shown to induce a disease resembling CML when expressed in mice, and can be specifically reduced by BCR-ABL1 fusion sequence–directed RNA interference (RNAi), leading to in vitro reduction in CML cell proliferation. Furthermore, a selective protein kinase inhibitor, imatinib mesylate, which suppresses the proliferation of cells expressing the p210 protein, has been shown to induce complete hematologic responses in patients with CML, and is approved as a first-line drug for treatment of CML. This was the first approved molecularly targeted drug that directly inhibits the specific oncoprotein signal in a cancer. Subsequently, secondary mutations that lead to resistance to imatinib have been shown to develop in treated patients with CML; accordingly, several second generation ABL kinase inhibitors have been developed to target these variants with greater efficacy. BCR-ABL1 translocation associated with the p210 protein is detected in all cases of CML, as well as in 10% of cases of adult acute lymphoblastic leukemia (ALL) and 5% of cases of adult acute myelogenous leukemia (AML).
The second, less common breakpoint region in the Ph lies more 5′ within BCR, in the first intron, and gives rise to a 190-kDa BCR-ABL1 fusion protein. This breakpoint is found in approximately 10% of adult ALL and 5% to 10% of childhood ALL. In the acute leukemias, the presence of a Ph , regardless of the BCR breakpoint site, confers a poor prognosis in patients treated by standard chemotherapy alone, prompting consideration of allogeneic hematopoietic stem cell transplantation. Thus the Ph and its novel gene products can be used as diagnostic markers in a myeloproliferative disorder and as a prognostic marker in acute leukemia. The chromosomal translocation, the recombinant BCR-ABL1 gene, its chimeric mRNA transcript, and the fusion protein product have all been used as targets for analysis in clinical assays of one form or another.
Production of chimeric, oncogenic proteins is a frequent consequence of chromosomal translocations in tumors; however, the products of genes near (or at) breakpoints are also altered by other mechanisms. For instance, the mRNA and protein products of a weakly expressed or unexpressed gene may be dramatically upregulated by translocating the gene adjacent to a constitutively active promoter region for a strongly expressed gene. In non-Hodgkin lymphomas, chromosomal translocations often involve an antigen receptor gene, such as the immunoglobulin heavy chain gene ( IGH ) on chromosome 14 band q32. This site, which normally undergoes intragenic recombination in developing B cells as a step toward synthesis of immunoglobulin (see later discussion), is thought to participate occasionally in aberrant recombination, whereby a portion of the immunoglobulin gene becomes joined to DNA in another chromosome. The site of recombination in the other chromosome may be near or within a gene whose transcription is then dysregulated by its proximity to the IGH enhancer, a regulatory sequence that stimulates transcription. Therefore translocational juxtapositions to the IGH enhancer result in overexpression of the proteins specified by the coding sequence of various oncogenes in B-cell lymphomas.
An example of this type of translocation is t(14;18)(q32;q21) of follicular lymphoma, in which the antiapoptotic gene BCL2 (B-cell leukemia/lymphoma 2) on chromosome 18 is constitutively expressed. The overexpression of BCL2 as a consequence of chromosomal translocation is, by itself, insufficient for neoplastic transformation, but probably contributes to prolonged survival of B cells through its antiapoptotic function. In a transgenic mouse into which dysregulated BCL2 has been inserted, overexpression of the gene results in follicular hyperplasia, but lymphoma does not develop unless another oncogene, MYC, is introduced as well or becomes activated through a spontaneous mutational event.
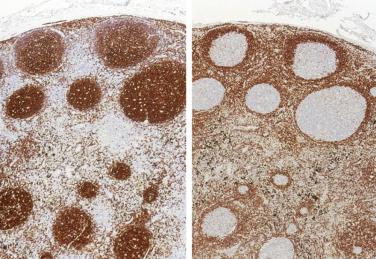
Evidence indicates that the t(14;18)(q32;q21) translocation and the resultant altered BCL2 gene expression is insufficient for neoplastic transformation in humans. Using sensitive PCR techniques, the t(14;18)(q32;q21) translocation can be detected at very low levels in occasional reactive lymph nodes, tonsils, or blood. Nonetheless, from a diagnostic perspective, the t(14;18)(q32;q21) translocation is a consistent genetic change correlated with the particular tumor phenotype of follicular lymphoma. The chromosomal translocation may be detected by a variety of techniques, including cytogenetics, FISH, Southern blot hybridization, and PCR. Because the translocation results in overexpression of the normal BCL2 coding sequence, immunohistochemical detection of increased protein can be used as an adjunct to histologic diagnosis. Normal reactive follicles in lymph nodes express low levels of BCL2 protein. The morphologic differential diagnosis of reactive follicular hyperplasia versus follicular lymphoma (see Chapter 21 ) can therefore be clarified by the demonstration of BCL2 expression in follicles in tissue sections ( Fig. 31.2 ).
Chromosomal translocations affect genes involved in diverse biochemical pathways, but there appear to be patterns in the types of genes involved in chromosomal translocations associated with certain tumors. Genes that encode transcription factors are particularly frequent among the translocations found in acute leukemias and sarcomas. The heterodimeric hematopoietic transcription factor, core-binding factor (CBF), composed of RUNX1 and CBFB subunits, is required for hematopoiesis and is the most common known target of recombination in translocations of acute leukemias. CBF is a transcriptional regulator of hematopoiesis, and many translocations create chimeric proteins that appear to function as transdominant negative inhibitors of CBF function and thereby affect the growth and differentiation pathways of hematopoietic cells. Translocations involving these genes occur in 30% of AML and 25% of ALL. RUNX1 is disrupted in acute myeloid leukemia with t(8;21)(q22;q22.1); RUNX1-RUNX1T1 and in pediatric B-lymphoblastic leukemia/lymphoma with t(12;21)(p13;q22.1; ETV6-RUNX1 ); the gene encoding the non-DNA binding subunit CBFB is disrupted in acute myeloid leukemia with inv(16)(p13.1q22) or t(16;16)(p13.1;q22; CBFB-MYH11 ).
Chromosomal translocations in sarcomas also involve genes encoding transcription factors (see Chapter 24 ). The EWSR1 gene encodes an RNA-binding molecule that, when fused to a heterologous DNA-binding domain, stimulates gene transcription. EWSR1 was isolated by molecular cloning of the breakpoints in t(11;22)(q24;q12) of Ewing sarcoma/peripheral primitive neuroectodermal tumor (PNET), the first sarcoma translocation to be characterized molecularly. EWSR1 is on the long arm of chromosome 22, whereas the site of recombination in chromosome 11 is in Friend leukemia virus integration site 1 (FLI1) . The FLI1 gene belongs to the erythroblastosis virus transforming sequence ( ETS ) family of related genes, all of which contain sequence-specific DNA-binding domains. The fusion EWSR1-FLI1 oncoprotein joins the N-terminal transactivation domain from EWSR1 with the C-terminal DNA-binding domain from FLI1 and is a potent transcriptional activator. Inclusion of these domains is apparently important for oncogenicity of the protein because, despite the variability in breakpoints among individual Ewing sarcoma/PNET tumors, all forms of EWSR1-FLI1 include the entire N-terminal region of EWSR1 (encoded in the first seven exons) and the entire DNA-binding domain of FLI1 . In approximately 10% of Ewing sarcoma/PNET, variant translocations, such as t(21;22)(q22;q12) and t(7;22)(p22;q12), are seen. In these, EWSR1 is joined to other members of the ETS family of proteins (e.g., ERG in chromosome 21, ETV1 in chromosome 7), and the chimeric proteins are structurally similar to EWSR1-FLI1.
EWSR1 gene rearrangements are seen in other sarcomas as well, such as clear cell sarcoma, intraabdominal desmoplastic small round cell tumor, and extraskeletal myxoid chondrosarcoma (among others). In each of these fusion proteins, the EWSR1 N-terminal region is postulated to provide transcriptional activation and other regulatory functions, whereas the second gene provides the sequence-specific C-terminal DNA-binding domains. The lineage specificity of these proteins among different types of sarcoma appears to be specified by the DNA-binding domains. In addition, the ETS transcription factors ERG and ETV1 have been identified in recurrent translocations in prostate adenocarcinoma, suggesting that similar mechanisms may contribute to oncogenesis in some epithelial neoplasms.
The use of chromosomal translocations as diagnostic markers has led to an improved classification of various malignancies. Mantle cell lymphoma was universally recognized as a distinct clinicopathologic entity only in the 1990s. Identification of the characteristic t(11;14)(q13;q32) led to refinement of the diagnostic criteria for this entity, which was previously thought to be low grade by morphologic criteria and is now understood to have a poor prognosis with an average survival of only 3 to 5 years (see Chapter 21 ). The t(11;14) translocation joins DNA in chromosome 11 at band q13 to DNA within the IGH gene in chromosome 14 band q32. Transcription of the cell-cycle regulatory protein cyclin D1, encoded by the CCND1 gene near the site of recombination in chromosome 11, is deregulated owing to juxtaposition with the IGH enhancer; cyclin D1 protein is consequently overexpressed in these lymphomas. The overexpression of cyclin D1 can be detected immunohistochemically and used diagnostically to distinguish mantle cell lymphoma from other B-cell lymphomas.
A further example of a diagnostically important chromosomal translocation is t(15;17)(q22;q21) of acute promyelocytic leukemia. This translocation, which joins two genes, PML and RARA, has special therapeutic implications because presence of the translocation is a specific marker for sensitivity to treatment with all- trans -retinoic acid, which induces terminal differentiation of the immature myelocytes making up the malignant cells in this neoplasm.
In recognition of the diagnostic utility of such chromosomal translocations and other chromosomal aberrations, the World Health Organization (WHO) classification scheme for hematolymphoid neoplasms categorizes many of the AML and ALL subtypes primarily by the specific cytogenetic abnormalities, which are associated with morphologic and/or distinctive clinical features ( Table 31.2 ). Similar classification schemes in which cytogenetic abnormalities play a prominent part exist in solid tumors, such as renal cell carcinoma, soft tissue tumors, and salivary gland carcinomas. Furthermore, in addition to their role in the classification of tumors, translocations and other cytogenetic abnormalities can be used to assess prognosis or response to therapy in many tumors, as discussed later.
| Chromosomal Translocation | Genes Involved | Risk Group |
|---|---|---|
| ACUTE MYELOGENOUS LEUKEMIA | ||
| t(8;21)(q22;q22.1) | RUNX1-RUN1T1 | Favorable |
| inv16(p13.1;q22) | CBFB-MYH11 | Favorable |
| t(15;17)(q22;q11-12) | PML-RARA | Favorable |
| t(9;11)(p2.3;q23.3) | KMT2A-MLLT3 | Unfavorable |
| B-LYMPHOBLASTIC LEUKEMIA | ||
| t(12;21)(p1213.2;q22.1) | ETV6-RUNX1 | Favorable |
| t(9;22)(q34.1;q11.2) | BCR-ABL1 | Unfavorable |
| t(1;19)(q23;p13.3) | TCF3-PBX1 | Favorable |
| t(v;11q23.3) | KMT2A rearranged | Unfavorable |
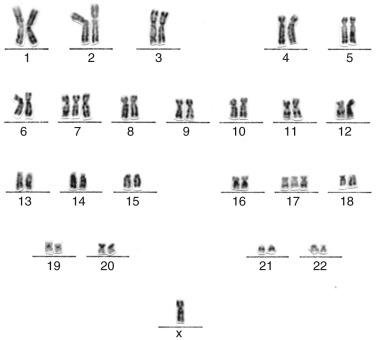
Techniques are available for detection of chromosomal translocations in neoplasms. Cytogenetic (karyotype) analysis (the conventional method of chromosome analysis, which is more morphologic than strictly molecular) provides a survey of all chromosomal changes but requires fresh tissue, cell culture, specialized laboratories, and technologists and professionals expert in recognizing deviations in normal chromosome banding patterns. To perform cytogenetic analysis, suspensions of cells from the disaggregated tumor specimen are cultured for several days, and the dividing cells are arrested in metaphase by the addition of an inhibitor of the mitotic spindle, such as colchicine or vinblastine. The condensed metaphase chromosomes are stained with Giemsa to reveal characteristic patterns of bands (G-banding) that are used to identify individual chromosomes and any structural or numeric aberrations among them.
Karyotype analysis is labor intensive and expensive, requires highly trained specialists, and is best used when analysis of many potential abnormalities is needed for diagnostic or prognostic purposes, such as the subclassification of AMLs. Karyotype analysis also has limitations in the resolution of cryptic translocations and small deletions (<5 megabases). However, the greatest disadvantage is that this technique depends on successful in vitro proliferation of the neoplastic cells to obtain metaphase chromosome preparations. Cytogenetics is often unsuccessful when neoplastic cells fail to grow well in culture; this may result from poor viability because of previous treatment, inherent necrosis, or overgrowth by nonneoplastic stromal elements.
In situ hybridization (ISH) techniques can be used to detect specific chromosomal translocations, but these methods typically do not survey the entire genetic constitution of the cell, as does conventional cytogenetic analysis. In FISH, fluorescently tagged DNA probes are hybridized to relevant regions of chromosomes. The probes can be applied to metaphase chromosomes ( Fig. 31.3 ) to confirm karyotype analysis in challenging situations, such as complicated interchange of more than two chromosomes, or to search for small exchanges of chromosomal material that fall below the resolving power of conventional cytogenetic analysis.
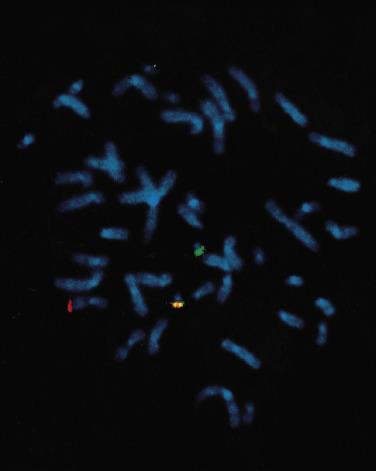
However, the ability to perform analysis directly on intact, nondividing (interphase) cells makes FISH especially valuable. Nuclei can be obtained from touch preparations, aspirate smears, cytologic preparations, disaggregated nuclei from paraffin sections, frozen tumor, or paraffin sections. By selecting DNA probes that hybridize to loci adjacent to breakpoint regions, the pattern of DNA signal in the nuclei (separation of signals flanking a breakpoint in the normal chromosome, fusion of signals hybridizing near the two breakpoints in normal chromosomes) indicates the presence of the chromosomal translocation. FISH can also detect chromosomal deletions, polysomies, and amplifications of specific genetic loci. FISH is less expensive than standard cytogenetic analysis, does not require fresh tissue, and is relatively fast. However, it does require a fluorescence microscope and special computer software to optimize the fluorescent signals. In addition, conventional FISH can be used only to identify specific chromosomal changes and does not provide a broad cytogenetic overview of the neoplasm.
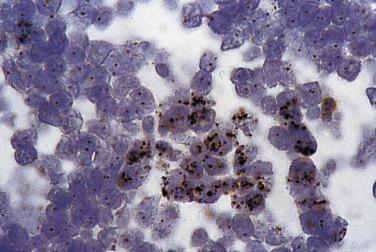
Locus-specific ISH also can be applied to histologic sections rather than to intact nuclei extracted from paraffin-embedded tissues. The advantage of using histologic sections is that the architectural and cytologic features of the neoplasm are intact and can be correlated with chromosomal changes. The hybridization signals are more difficult to interpret, however, owing to the slicing and overlapping of nuclei. Locus-specific ISH in chromosomes and chromosomal loci on either intact nuclei or histologic sections can be performed with enzymatic detection methods and brightfield microscopy, rather than fluorescence (chromogenic in situ hybridization [CISH]). Although less sensitive than fluorescence, CISH does not require the fluorescence microscope, imaging equipment, and software needed for FISH. It has been applied most readily to the assessment of gene amplification, such as HER2 / NEU (ERBB2) in breast carcinoma ( Fig. 31.4 ).
FISH occupies an intermediate position between conventional cytogenetic analysis and molecular diagnostic techniques (discussed later), with regard to resolution; that is, FISH can detect chromosomal rearrangements that are missed by conventional cytogenetics but may not detect small changes in DNA (particularly <10 kb of sequence) that can be picked up only by molecular techniques. Conversely, FISH is probably the technique of choice in detecting translocation breakpoints that are heterogeneous and distributed widely over large stretches of DNA, such as the translocations involving the MYC gene in Burkitt lymphoma.
Chromosomal translocations also may be detected by Southern blot hybridization, provided the breakpoints in one of the participating chromosomes are clustered within approximately a 15-kb to 20-kb region of DNA. However, in most clinical settings this obsolete laborious technique has been supplanted by more rapid techniques, such as FISH and PCR methods.
The sensitivity, versatility, and speed of PCR have had a significant impact on the detection of chromosomal translocations, as they have had on the entire field of molecular pathology. The technique routinely allows regions of DNA up to about 1000 base pairs (bp) of DNA to be exponentially amplified in vitro. However, the DNA sequences immediately flanking the region to be amplified typically must be known. To replicate the target sequence from the tested sample (template) DNA, PCR uses purified thermostable bacterial DNA polymerases and takes advantage of two properties of these polymerases: (1) They cannot initiate synthesis of DNA de novo but can only extend the 3′ end of a preexisting strand of DNA annealed to a complementary longer strand of DNA, and (2) they can withstand the high temperatures needed to separate the two strands of a DNA double helix. High temperature is used to dissociate (denature) the two strands of the template DNA. Low temperature is then used to facilitate the hybridization (annealing) of a short single-stranded DNA molecule (primer) that is complementary to the template sequence, just upstream of the area of interest; a different primer anneals to each of the two strands of the template DNA. An intermediate temperature activates the DNA polymerase, which recognizes the 3′ end of the primer and begins to synthesize (extend) a new sequence complementary to each of the template strands; guanosine nucleotides (Gs) are added to the growing primer strand opposite cytidine nucleotides (Cs), adenosine nucleotides (As) are added opposite thymidine (Ts), Cs are added opposite Gs, and Ts are added opposite As. The polymerase, working in parallel on both of the original template strands, will thereby produce two complementary copies of the original double-stranded template ( Fig. 31.5 ). By changing the temperature in a thermocycler, the three stages of the process—denaturation, annealing, extension—can be orchestrated in a chain reaction leading to exponential amplification of the target sequence. In principle, the DNA being amplified doubles during every cycle, so that after approximately 20 cycles a million DNA fragments have accumulated for each template molecule present at the start of the reaction. Although actual PCR amplifications are rarely this efficient, sufficient numbers of fragment products are usually generated to detect them as a band in an electrophoretic gel under ultraviolet light after staining of the DNA with the fluorescent dye ethidium bromide.
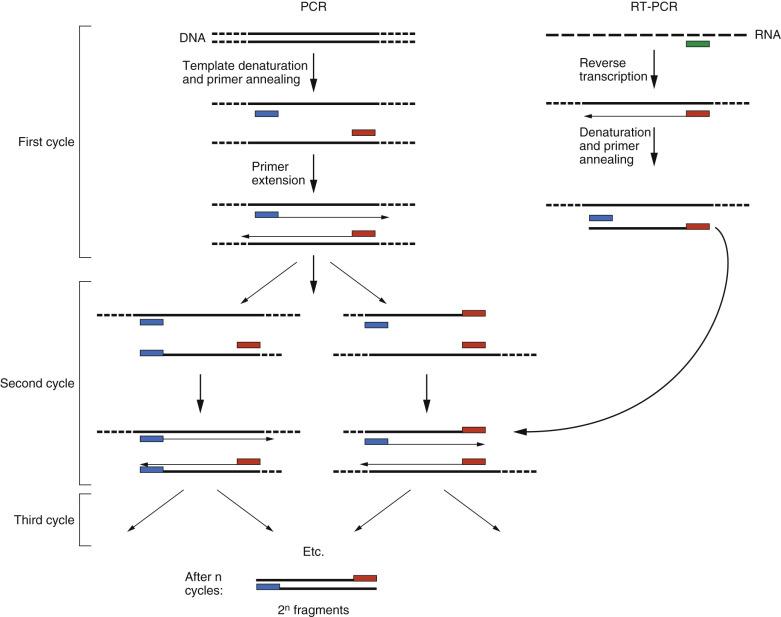
PCR can detect chromosomal translocations in which the breakpoints in both participating chromosomes cluster reproducibly in well-defined, narrow regions of DNA. A pair of primers is constructed so that one will anneal specifically to the DNA in each gene, just outside each breakpoint region, and a PCR product will be generated across the site of recombination. However, in normal DNA the primers anneal to DNA in separate chromosomes or to widely separated regions on a single chromosome, and no PCR product can result. In some translocations, the breakpoints may be scattered over large regions (hundreds of kilobases) of DNA, as in the translocations involving MYC in Burkitt lymphoma, which is too great a distance for PCR to amplify successfully.
Designing primers to detect all of the possible translocation products by PCR of DNA in this situation is typically not practical for clinical diagnostic laboratories. However, if the breakpoints are distributed within large introns and a chimeric RNA is expressed, a variation of the PCR technique, reverse transcriptase PCR (RT-PCR), may be used to detect these translocations. In RT-PCR, RNA serves as the template after being converted to complementary DNA (cDNA) with a primer-dependent retroviral RT enzyme; once the cDNA is formed, PCR may proceed as described earlier. Although the DNA products of translocations may vary widely across cases, the chimeric RNA transcribed from the fusion gene is more constant from case to case, which makes RT-PCR a viable option for molecular diagnosis of translocations. For example, in the Ph of CML, the breakpoints in the BCR gene are clustered within a few introns in the breakpoint cluster region, and the breakpoints in the ABL1 gene are located in the very large first intron. Only a few different chimeric RNAs are produced from these translocations, depending on where the breakpoint lies in BCR . Therefore RT-PCR with a few PCR primers, to cover the different possible positions of the BCR breakpoint, can be used to detect this translocation in CML mRNA. Unfortunately, RNA is less stable than DNA because of ubiquitous environmental RNases, and special techniques, supplies, and reagents are required to handle and work with RNA templates.
PCR and RT-PCR offer several advantages for detecting translocations in tissue specimens. Because of the exponential amplification of DNA during PCR, only small numbers of neoplastic cells are needed, and PCR can be performed when limited biopsy material is available. PCR and RT-PCR also can be performed on formalin-fixed, paraffin-embedded tissues, which have fragmented DNA and RNA. However, the greatest advantage of PCR is probably its extraordinary sensitivity. Because even one template molecule can lead to a detectible PCR product, these assays can detect as few as one neoplastic cell in several hundred thousand cells, limited only by the amount of total cellular nucleic acid that can be conveniently assayed in one reaction. In contrast, the lower limit of sensitivity of histologic analysis or cytogenetic analysis and FISH is approximately 1 in 50. Given the sensitivity of PCR-based tests for chromosomal translocation, patients are now followed after therapy for the presence of minimal residual disease at submicroscopic levels far below that which can be detected by conventional histologic analysis.
Furthermore, the advent of real-time PCR technologies whereby the kinetics of the PCR reaction are monitored over the course of the reaction has enabled quantitative analysis, providing superior clinical information for monitoring response to therapy. At the beginning of a PCR reaction, when all of the reagents are abundant, the kinetics of the reaction favor an exact doubling of the amplicons (exponential amplification), and the reaction is specific and precise. As the reagents are consumed, the reaction slows while the products begin to degrade. Conventional endpoint detection PCR analyzes the results of the reaction at the end or plateau phase. Real-time PCR technologies incorporate fluorescence into the reaction so that the exponential phase of the reaction can be assessed.
In hybridization probe real-time PCR, a probe is designed to anneal to the specific sequences of the target gene between the forward and reverse primers. The probe has a high-energy reporter dye at the 5′ end and a low-energy quencher dye at the 3′ end. In an intact probe, the reporter and quencher are in close proximity. When excited by a light source, the reporter dye emission is transferred to the quencher dye, and energy release is suppressed. The DNA polymerase has 5′ exonuclease activity that hydrolyzes the hybridization probe during extension, releasing the reporter dye from the quencher and enabling the fluorescent emission of the reporter dye. The fluorescence signal is captured by the instrument and recorded over time. The amount of reporter signal increase is proportional to the amount of product generated by a sample. The greater the amount of starting template at the beginning of the reaction, the sooner the reaction reaches exponential phase and the sooner fluorescence will be detected.
The hybridization probe system is an alternative real-time PCR technology that similarly relies on fluorescent resonance energy transfer. In this technology, two oligonucleotides, each labeled with a different fluorescent dye, are designed to anneal to the target immediately adjacent to one another. When excited by a light source, transfer of energy occurs from one dye to the other, resulting in the generation of light, which is monitored by the instrument. Both of these technologies have been used to detect tumor cells with chromosomal translocations and monitor tumor burden after therapy (see discussion of minimal residual disease).
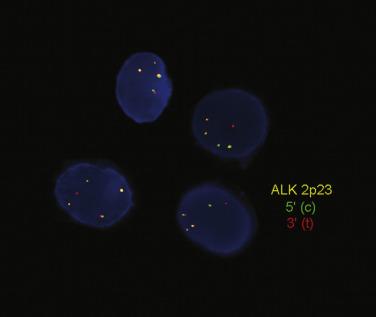
Although less frequent and generally less amenable to purely molecular diagnostic techniques, recurrent chromosomal abnormalities other than translocations contribute to the malignant phenotype of various tumors and can be useful diagnostic markers. Chromosomal inversions involve breakage within a segment of a chromosome, with a 180-degree rearrangement so that there is a reversal in the orientation of the chromosome segment. These segments may represent any portion of a chromosomal arm, such as the inversion on the short arm of chromosome 2 that leads to ALK rearrangements in lung adenocarcinoma, or they may be pericentric, with breakpoints on either side of the centromere, as in the inv16 of acute myelomonocytic leukemia with eosinophilia. Inversions are functionally similar to translocations in that alterations affect the structure or activity of genes near one or other breakpoint.
Chromosomal deletions can result from the loss of a whole chromosome, leaving only one homolog (monosomy); loss of whole chromosomal arms; or loss of interstitial segments of a chromosomal arm, as in the 5q− syndrome associated with myelodysplasia. Deletions often have variable breakpoints and usually result in the oncogenic consequences of losing a tumor suppressor gene within the deleted segment.
Duplications of whole chromosomes (e.g., trisomy 12, found in about 15% of chronic lymphocytic leukemias) or intrachromosomal duplications of various regions (e.g., BRAF duplication in pilocytic astrocytomas) are also characteristic of certain tumors.
Isochromosomes represent combinations of duplication and deletion. In these chromosomes, one arm is lost and the other duplicated, resulting in trisomy for one arm accompanied by monosomy for the other, such as isochromosome 7q of hepatosplenic γδ T-cell lymphoma.
Ring chromosomes are combinations of deletions and translocations, in which breakage and rejoining has occurred between the arms of one chromosome, near the ends of the chromosome (or the telomeres). Supernumerary ring chromosomes characteristic of well-differentiated liposarcoma or atypical lipomatous tumors contain amplifications of a subset of genes on the long arm of chromosome 12, including CDK4 and MDM2 .
Amplifications, or tandem reiteration of chromosomal segments, may be found within chromosomes as homogeneously staining regions or as numerous, freely replicating extrachromosomal fragments (double minute chromosomes). Outstanding examples of clinically useful amplifications manifest either as homogeneously staining regions or double minute chromosomes, involving the MYCN gene in neuroblastoma and HER2/NEU (ERBB2) in infiltrating ductal breast cancer, are discussed later.
Many of the previously mentioned types of chromosomal abnormalities, or multiple examples of a particular type of abnormality, may be found together in individual tumors. Various sets of abnormalities, rather than any single one, are increasingly being recognized as characteristic of certain tumor subtypes. The Heidelberg classification of epithelial tumors of the kidney illustrates how cytogenetic analysis refined and reinforced the older morphologic classification scheme, a trend that continued into the 2016 WHO classification. For many years, renal neoplasms with granular cytoplasm were grouped as granular variants of clear cell carcinoma. Clinicopathologic studies, immunohistochemistry, and cytogenetic analysis have shown that this set of neoplasms is actually composed of oncocytomas, some chromophobe carcinomas, clear cell carcinomas, collecting duct carcinomas, papillary renal cell carcinomas, and epithelioid angiomyolipomas (see Chapter 12 ). Similarly, papillary renal neoplasms were grouped with common renal (clear) cell carcinoma despite their distinctive morphologic features. Cytogenetic studies have confirmed the concept that these are biologically distinct neoplasms by demonstrating consistent and different patterns of chromosomal abnormalities among them. More than 90% of clear cell carcinomas have deletions in chromosome 3p as well as other abnormalities, whereas papillary renal cell carcinomas have trisomies of chromosomes 7, 17, and 20, without 3p deletions ( Fig. 31.6 ). Chromophobe carcinomas are hypodiploid (less than the full complement of chromosomes), with loss of heterozygosity at chromosomes 1, 2, 6, 10, 13, 17, and 21. Additional subsets of MiT family translocation pediatric renal cell carcinoma have been defined on the basis of transcription factor ( TFE3 and TFEB ) gene fusions. Grouping renal cell tumors by cytogenetic categories has clarified diagnostic morphologic features for these tumors, and, having been clarified in this manner, stringent morphologic criteria identifiable in routine sections are usually sufficient to classify the tumors into biologically coherent groups.
Conventional cytogenetic analysis or FISH usually detects gross chromosomal abnormalities other than translocations, such as amplifications, deletions, and duplications. Application of FISH to amplifications is relatively straightforward and widely practiced. Detection of deletions and duplications requires more care because of the considerable potential for false-negative and false-positive artifacts arising from failure of the probe to gain access to the target sequence or from spurious background signals. This problem is partly overcome by including additional probes in the hybridization as internal controls (e.g., probes designed to recognize regions adjacent to sites of deletion or centromeric probes) ( Fig. 31.7 ). Modified versions of the FISH technique also can be used to detect these abnormalities, but these are of limited clinical utility and have largely been superceded.
CGH is a technique that evaluates genome-wide deletions and amplifications and has the advantage of being applicable to frozen and paraffin-embedded tumor tissue, as well as to fresh tissue. In CGH, DNA is extracted from a tumor sample and from a nontumor cell population. The two DNA samples are differentially labeled and cohybridized to normal metaphase chromosomes or, more commonly, microarrays. These assays are known as metaphase CGH and array CGH, respectively. Regions of chromosomal duplication or amplification in the tumor will be highlighted by a color shift toward that of the tumor DNA fluorescent tag. The opposite result is obtained for chromosomal deletions in the tumor—the color of the corresponding region in the metaphase chromosomes or microarray shifts toward the color of the tag in the probe prepared from the normal DNA. The technique can be applied to DNA samples from small numbers of cells obtained by tissue microdissection and unbiased whole-genome amplification by PCR. CGH is a useful discovery tool for genomic imbalances, but has limited application in translocations, inversions, or ploidy changes.
In array CGH, the resolution is determined by the array target and the array density. In bacterial artificial chromosome (BAC) arrays, clones are spotted onto glass slides; this technique can resolve DNA deletions or amplifications of regions spanning 100,000 or more nucleotides. Oligonucleotide arrays, in which oligonucleotides are spotted onto glass slides or directly synthesized in situ, can increase resolution dramatically. In addition to defining loss and gain of regions of chromosomes in tumors, array CGH has demonstrated much greater variations of gene copy number in the human genome than was expected. Copy number variation is likely to correlate with gene expression and may be associated with human phenotypic variation and susceptibility to disease. Oligonucleotide array CGH is already being used in diagnostic laboratories to enhance resolution and refine disease classification.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here