Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypoplastic left heart physiology is defined as inability of the left heart to sustain adequate cardiac output following birth because of underdevelopment of one or more left heart structures despite surgical or medical intervention. Box 49-1 emphasizes four important implications of this definition.
The term left heart refers to the morphologic composite or unit that includes left atrium, mitral valve, left ventricle, aortic valve, and aorta. Each of these plays an important role in determining left heart function.
Definition of hypoplastic left heart is physiologic, not morphologic, despite morphologic abnormalities being the underlying cause of left heart inadequacy. A morphologic definition of the hypoplastic left heart is not possible because underdevelopment of a variable number of specific left heart structures, either alone or in various combinations, may be responsible for physiologic inadequacy of the left heart. Some morphologic constellations (e.g., aortic and mitral atresia in combination) essentially always result in hypoplastic left heart physiology, whereas others (e.g., aortic or mitral stenosis) may or may not. Nevertheless, typical morphologic abnormalities that result in hypoplastic left heart physiology can be identified. These include a constellation of atresia or marked hypoplasia of the mitral valve, left ventricle, and aortic valve, in association with hypoplasia of the ascending aorta and aortic arch, aortic coarctation, patent ductus arteriosus, and atrial septal defect. It is important, however, to reemphasize that this constellation does not define hypoplastic left heart physiology; hypoplastic left heart physiology can be present without including all morphologic abnormalities mentioned in this typical example.
Hypoplastic left heart physiology is present within a relatively narrow band of the broad continuum of hypoplastic lesions of the left heart. This continuum ranges from isolated simple lesions (e.g., discrete coarctation) at one end of the spectrum to complex multilevel lesions (e.g., combination of aortic atresia, mitral atresia, and absent left ventricle) at the other end. The point along this gradually increasing continuum of left heart abnormalities where hypoplastic left heart physiology is encountered is impossible to pinpoint morphologically. Hypoplastic left heart physiology may be the result of a severe abnormality of a single left heart structure (e.g., mitral atresia) or a combination of several milder abnormalities (e.g., mitral stenosis, left ventricular hypoplasia, and aortic stenosis).
Inclusion of the phrase despite surgical or medical intervention in its definition is critical to the concept of hypoplastic left heart physiology, because abnormalities such as isolated critical aortic stenosis or isolated severe aortic coarctation may meet the criteria of this physiologic definition before but not after intervention to correct the abnormality. As a result, the lesions cited in the previous sentence are not considered examples of hypoplastic left heart physiology. An important implication of the term hypoplastic left heart physiology is that the left heart is incapable of sustaining systemic cardiac output, thereby limiting therapeutic options to (1) reconstructions that use a single (right) ventricular pumping chamber (Norwood procedure, superior cavopulmonary connection, Fontan procedure) or (2) heart transplantation.
We have chosen to use the term hypoplastic left heart physiology rather than the more common and entrenched term hypoplastic left heart syndrome, because it more accurately describes the entity. In fact, both terms have important limitations when applied in the clinical setting. In practice, the exact point along the aforementioned continuum where an inadequate left heart is encountered cannot be defined with certainty. This limitation is not intrinsic to the definition of hypoplastic left heart physiology itself, but rather is due to two important factors: (1) tests designed to define morphologic and physiologic characteristics are limited in their ability to accurately predict overall left heart function, and (2) new developments and reconstructive techniques may allow previously unsalvageable left hearts to function adequately.
The concept of borderline hypoplastic left heart physiology is useful in this context because it emphasizes that it is not possible to define a specific point along the morphologic and physiologic continuum where the left heart becomes unquestionably unsalvageable. Borderline hypoplastic left heart physiology, therefore, refers to a zone along this continuum in which it is currently not possible to predict with certainty whether the left heart can be salvaged with surgical reconstructive methods. This concept is of practical importance because it helps characterize the clinical dilemma faced by surgeons who must make a dichotomous decision (reconstructive surgery to salvage the left heart vs. the Norwood procedure or transplantation) in the context of a continuum of morphologic and physiologic left heart compromise. At either pole of this continuum, decision making is straightforward. However, within the zone of borderline hypoplastic left heart physiology, the appropriate management decision requires exquisite attention to many subtle details.
The various malformations that result in hypoplastic left heart physiology together represent a special example of univentricular atrioventricular connection (see Chapter 41, Chapter 56 ). Because of the special clinical and surgical importance of this group of malformations, this subject is discussed separately from other forms of univentricular heart.
The first description of aortic atresia was apparently by Canton in 1850. Although Abbott had recognized aortic and mitral atresia, Brockman in 1950 emphasized that in about 50% of cases of mitral atresia, there was coexisting aortic atresia and severe underdevelopment of the left side of the heart. In 1952, Lev further emphasized the group of congenital heart malformations associated with underdevelopment of the left-sided cardiac chambers and a small ascending aortic arch, articulating for the first time the concept that multiple left-sided structures tended to occur together. In 1958, Noonan and Nadas brought together the morphologic features of combined aortic and mitral atresia and introduced the phrase “hypoplastic left heart syndrome.” In 1976, Roberts and colleagues further organized the knowledge about this subject by emphasizing that in the presence of a large ventricular septal defect (VSD), aortic atresia can coexist with normal development of the left ventricle and mitral valve.
The history of attempted reconstructive procedures for hypoplastic left heart physiology dates back to 1970 when Cayler and colleagues described an anastomosis between right pulmonary artery and ascending aorta with placement of bilateral pulmonary artery bands. Other variations in neonatal reconstructive procedures designed to allow survival without the use of prostaglandins to maintain ductal patency were described by Doty and colleagues in 1977, Norwood and colleagues in 1980, Levitsky and colleagues in 1980, Behrendt and colleagues in 1981, and others.
Even though some of these reports noted short-term successes, there is no documentation of long-term survival. In 1983, Norwood and colleagues described for the first time neonatal palliative surgery leading to a subsequent successful Fontan procedure. Following this report and subsequent reports that systematically documented long-term survival of patients with hypoplastic left heart physiology, it has become widely accepted that many of the technical details of the procedure as described by Norwood are critical to achieving long-term survival. These technical details ensure long-term aortic growth potential, minimize pulmonary valve distortion, address distal arch obstruction, preserve pulmonary artery patency, balance pulmonary and systemic blood flows, and ensure adequate atrial-level mixing. Many of the procedures described before implementation of the Norwood procedure failed to address one or more of these issues critical for long-term survival.
Allograft heart transplantation for hypoplastic left heart physiology dates back to 1985, when Bailey performed the first successful cases as primary therapy in neonates. Since then, Bailey and colleagues and a limited number of other groups have considered transplantation as one option for treatment, along with reconstructive surgery.
The hybrid procedure for hypoplastic left heart physiology was first developed in 1993 in response to poor outcomes following the Norwood procedure. The hybrid procedure combines surgical placement of bilateral branch pulmonary artery bands, placement of a stent in the ductus arteriosus, and catheter-based atrial septostomy, avoiding cardiopulmonary bypass (CPB). Initially, it was not widely embraced because of poor interim outcomes; however, more recently it has been used by some programs as an alternative to the Norwood procedure in high-risk patients, and variations of the hybrid procedure have been used as a bridge to transplantation.
Currently, it is unclear whether the etiology of hypoplastic left heart physiology is similar in all cases. There are genetic factors involved, although these are multiple, complex, and poorly understood at the present time. Available evidence suggests that a number of primary morphologic etiologies may lead to the end result of hypoplastic left heart physiology. Primary morphologic abnormalities at the aortic valve level, mitral valve level, left ventricular myocardial level, or atrial septal level (intact atrial septum) could all in theory lead ultimately to hypoplasia of the entire left side of the heart as gestation progresses.
Fetal echocardiography has yielded much information regarding progression of hypoplastic left heart physiology. In some cases, critical aortic stenosis with documented forward flow on early fetal echocardiograms progresses to aortic atresia before birth. In such cases, the left ventricle shows evidence of progressive dysfunction and hypoplasia as stenosis proceeds to atresia. Echocardiographic evidence of fetal left ventricular dilated cardiomyopathy progressing to hypoplastic left heart physiology has been reported. Controversy remains as to whether a closed foramen ovale in utero is a cause or a result of hypoplastic left heart physiology.
In hypoplastic left heart physiology, the heart is enlarged to about twice normal weight for age. Its shape is determined by the large right and small left heart chambers ( Fig. 49-1, A ). Beyond this, morphologic details vary widely. Four morphologic subtypes of hypoplastic left heart physiology can be defined based on status of the left heart valves:
Aortic and mitral atresia
Aortic atresia with mitral stenosis
Aortic stenosis with mitral atresia
Aortic and mitral stenosis

Of these, aortic stenosis with mitral atresia is the least common subtype, representing approximately 5% of cases; aortic and mitral atresia is the most common, representing approximately two thirds of cases. Within these subtypes, the status of the atrial septum, size of the left ventricular cavity and muscle mass, ascending aorta and aortic arch, and ductus arteriosus are also important.
In aortic atresia, the aortic valve is totally absent. Diminutive aortic sinuses of Valsalva are frequently present, giving origin to relatively normally positioned right and left coronary arteries that have a normal distribution pattern. The ascending aorta is narrow, sometimes as small as 1.5 mm in diameter. The portion of the aorta between the atretic valve and brachiocephalic artery serves only as a conduit for coronary blood flow ( Fig. 49-2 ).

At and beyond the brachiocephalic artery, the aortic arch gradually widens and is joined beyond the origin of the left subclavian artery by a large patent ductus arteriosus. The ductus carries blood from the right ventricle into the descending aorta and retrograde to the brachiocephalic and coronary arteries. A localized aortic coarctation exists in approximately 80% of cases and is usually juxtaductal in location (see Chapter 48 ). Prevalence of coarctation is highest in patients with the most severe hypoplasia of the ascending aorta. In some cases, there may be only mild infolding of the aortic media on the wall opposite the ductal insertion site, or there may be no aortic coarctation whatsoever.
When a patent but hypoplastic aortic valve is present, there may be a variable but still reduced amount of forward flow across the aortic valve (see Chapter 47 ). The ascending aorta and arch tend to be larger than in aortic atresia, with the diameter of the ascending aorta ranging from 2 to 6 mm. Aortic coarctation is common.
The left ventricle is severely hypoplastic in 95% of cases of aortic atresia. In this setting, the ventricular septum is intact. The mitral valve is either atretic (about one third of patients) or patent but severely hypoplastic (about two thirds of patients) (see Fig. 49-1, B and C ). When the mitral valve is patent in association with aortic atresia, there may be left ventricular–coronary connections ( Fig. 49-3 ) similar to those present in the right ventricle in cases of pulmonary atresia with intact ventricular septum (see “Right Ventricle” under Morphology in Chapter 40 ). It is postulated that these connections serve to decompress the left ventricular chamber. Localized thickening of coronary arteries occurs adjacent to these connections, and there is also a variable degree of endocardial thickening (endocardial fibroelastosis).

Rarely, there is focal calcification and scarring limited to the ventricular subendocardium. The hypoplastic left ventricle shows myocardial fiber disarray qualitatively similar to that present in hypertrophic obstructive cardiomyopathy (see “Left Ventricle” under Morphology in Section II of Chapter 47 ), and in the right ventricle in pulmonary atresia with intact ventricular septum (see “Right Ventricle” under Morphology in Chapter 40 ).
In approximately 5% of cases of aortic atresia, the left ventricular cavity is near normal size in association with a large VSD. In such cases, there may be mitral valve atresia or a normal mitral valve. In cases of a normal mitral valve, the malformation does not represent hypoplastic left heart physiology, because such patients can undergo two-ventricle repair (see Special Situations and Controversies ).
When aortic stenosis rather than atresia is present, the left ventricle tends to be larger than the typically minute, slitlike left ventricle of aortic atresia. Its size may vary widely from extremely hypoplastic with hypertrophic muscle and severe endocardial fibroelastosis to a dilated, thin-walled, poorly functioning chamber. The mitral valve is almost always hypoplastic and may be atretic when severe aortic stenosis is present.
The right ventricle is enlarged, with uniform hypertrophy and a marked increase in cavity size (to approximately three times normal). Both tricuspid and pulmonary valves are larger than normal, and tricuspid regurgitation of variable degree is common.
The pulmonary trunk is large and continues directly into the large patent ductus arteriosus. The right and left branches arise relatively posteriorly and at right angles from the short pulmonary trunk.
The left atrium is relatively small and thick walled, with its long axis directed transversely toward the right atrium. The atrial septum is also thick, making balloon atrial septostomy generally unsatisfactory. An atrial communication is usually present; in the great majority of cases, this communication is a stretched patent foramen ovale. The septum primum is thickened and stretched so that it herniates into the right atrium and allows left-to-right shunting (see Fig. 49-1, B ). There may be an aneurysm of the septum primum projecting to the right. The right atrium is larger than normal, with uniform hypertrophy of its walls. When the atrial septum is intact or severely restrictive in association with mitral or aortic atresia or both, there is pulmonary venous hypertension and a variable degree of decompression of pulmonary venous return through connections to the systemic venous system. Pulmonary venous hypertension usually begins in fetal life. This may have important implications for fetal lung development. Dilated pulmonary lymphatic channels form, and these can have an important effect on postnatal lung physiology and surgical outcome.
Associated anomalies are uncommon. Structural abnormalities of the tricuspid and pulmonary valves are rare. Bicuspid pulmonary valve has been described in 4% of specimens; cleft tricuspid valve, tricuspid valve dysplasia, and double orifice tricuspid valve have also been reported. Other unusual cardiac anomalies include intact atrial septum, total anomalous pulmonary venous connection, levoatrial cardinal vein, coronary sinus atresia, atretic pulmonary veins, complete atrioventricular septal defect, transposition of the great arteries, and interrupted aortic arch. Coronary artery abnormalities are rare except in patients with aortic atresia and mitral stenosis, in which they occur in approximately 50% of cases.
Other abnormalities unrelated to the cardiovascular system are found frequently with hypoplastic left heart physiology. Chromosomal abnormalities, genetic defects, and major extracardiac structural malformations, including central nervous system abnormalities, occur in 28% to 40% of patients.
Presentation is in the newborn period, with mild cyanosis, respiratory distress, and tachycardia. If supportive measures are not undertaken, there can be rapid deterioration, heart failure, and death due to a combination of pulmonary overcirculation and systemic obstruction from ductal closure. Ductal closure is almost inevitable, but its timing varies from hours to weeks. This event is followed by rapid circulatory collapse.
On examination, there is a hyperactive right ventricular precordial impulse and a moderate-intensity midsystolic murmur along the left sternal border. The second heart sound is accentuated and single. Heart failure is associated with rales and liver enlargement. In many instances, peripheral pulses and perfusion are poor and blood pressure is low.
The chest radiograph shows moderate cardiomegaly and pulmonary plethora secondary to increased pulmonary blood flow. The electrocardiogram demonstrates right axis deviation and right ventricular hypertrophy and usually no left ventricular forces. However, left ventricular voltages can be present but do not necessarily signify an adequate left ventricular cavity.
Two-dimensional echocardiography is diagnostic and usually definitive. It demonstrates the large right ventricle, tricuspid valve, and ductus arteriosus and the small or absent left ventricle, aortic and mitral valves, and ascending aorta ( Fig. 49-4 ). Status of the atrial septum is also easily determined. Doppler color flow signals indicate retrograde flow in the aortic arch and ascending aorta in cases of aortic atresia. Antegrade flow in the ascending aorta in the setting of aortic atresia strongly suggests that left ventricle–to–coronary artery fistulae are present, with left ventricular blood flowing retrograde in the coronary arteries and into the ascending aorta. When the aortic valve is not atretic, forward flow from the left ventricle across the aortic valve is normal. The amount of flow varies widely and may reach as far as the aortic arch. If Doppler color flow indicates substantial forward flow to the level of the arch or bidirectional flow in the patent ductus arteriosus, the patient should be considered to have borderline hypoplastic left heart physiology (see Special Situations and Controversies ).

Cardiac catheterization is rarely indicated if it is clear by echocardiographic evaluation that the patient has unequivocal hypoplastic left heart physiology. However, in borderline hypoplastic left heart physiology, cardiac catheterization is often indicated to further characterize the physiology, especially mitral valve gradient and left ventricular end-diastolic pressure. The physiologic information obtained may help determine whether two-ventricle reconstruction is advisable. Catheterization is also indicated when a severely restrictive or intact atrial septum is present, resulting in pulmonary venous hypertension.
Rather than urgently bringing an hours-old infant to surgery under unstable conditions, the atrial septum can be opened by various interventional techniques (e.g., balloon dilatation, blade septostomy, atrial septal puncture with dilatation).
At this writing, computed tomography (CT) and magnetic resonance imaging (MRI) have a limited role in neonates with hypoplastic left heart physiology. CT angiography, however, may play an important role in follow-up, providing precise anatomic detail of aortic arch and pulmonary artery growth and development ( Fig. 49-5 ). MRI can provide quantitative analysis of neoaortic and tricuspid valve regurgitation, and may be helpful in assessing left ventricular size in neonates with borderline left heart physiology in whom two-ventricle reconstruction is being contemplated.
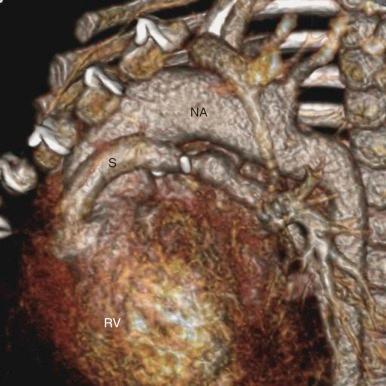
Various morphologic forms of hypoplastic left heart physiology constitute the fourth most common congenital cardiac defect. About 70% of cases are boys. Severe heart failure usually develops in the first week of life. Many neonates die within 1 to 2 weeks of birth; only 40% survive the neonatal period, and survival beyond 6 weeks of age is uncommon ( Fig. 49-6 ). Hypoplastic left heart physiology accounts for 25% of cardiac deaths during the first week of life and 15% of those in the first month of life.
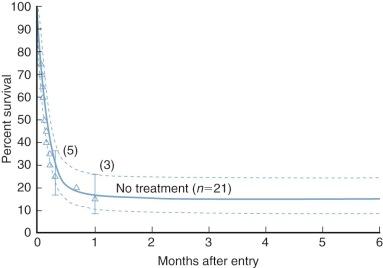
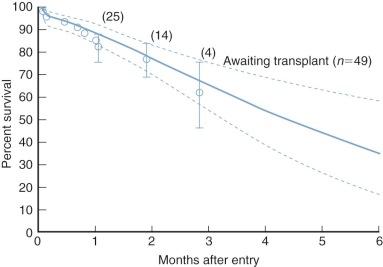
The ductus arteriosus typically begins to close shortly after birth. In some infants, ductal closure leads to restriction of systemic perfusion, metabolic acidosis, and circulatory collapse; and death. If the ductus continues to remain patent, a progressive increase in pulmonary circulation and a subsequent decrease in systemic circulation lead to pulmonary edema, coronary hypoperfusion, generalized systemic hypoperfusion, and ultimately death. Rarely, long-term survival will occur if the ductus remains patent and pulmonary vascular resistance (Rp) fails to fall in the perinatal and neonatal period.
There are two basic surgical options— reconstruction and cardiac transplantation —for treating hypoplastic left heart physiology. Reconstructive surgery includes the Norwood procedure and its variants, and the hybrid procedure. In a recent survey of practices related to this condition, 86% of 52 institutions recommend as primary treatment the Norwood procedure or one of its variants, and 14% did not make a recommendation, but left the decision solely up to the parents. No institution recommended primary transplantation, the hybrid procedure, or comfort care.
The overall goal of reconstructive surgery is similar to that for any patient with single-ventricle physiology (see Chapter 41 )—that is, establishing in the neonatal period an effective mixed circulation in which pulmonary ( ![]() ) and systemic (
) and systemic ( ![]() ) blood flow are well balanced, followed by one or more operations performed later in infancy or early childhood after Rp has dropped to normal postnatal levels. The purpose of subsequent operations performed outside the neonatal period is to move away from the inefficiency of the completely mixed circulatory state. It should be emphasized that all definitive repairs in hypoplastic left heart physiology are palliative.
) blood flow are well balanced, followed by one or more operations performed later in infancy or early childhood after Rp has dropped to normal postnatal levels. The purpose of subsequent operations performed outside the neonatal period is to move away from the inefficiency of the completely mixed circulatory state. It should be emphasized that all definitive repairs in hypoplastic left heart physiology are palliative.
The exact form of definitive repair may vary from patient to patient based on the individual's physiologic status. Given the inherently limited reserve of the single right ventricle, it is generally agreed that a completely mixed circulatory state, even one that provides ideal balance between ![]() and
and ![]() , is not an acceptable definitive state for hypoplastic left heart physiology. Acceptable definitive repairs include the completed Fontan procedure, the Fontan procedure with fenestration, superior cavopulmonary anastomosis, and superior cavopulmonary anastomosis with additional limited systemic to pulmonary blood flow.
, is not an acceptable definitive state for hypoplastic left heart physiology. Acceptable definitive repairs include the completed Fontan procedure, the Fontan procedure with fenestration, superior cavopulmonary anastomosis, and superior cavopulmonary anastomosis with additional limited systemic to pulmonary blood flow.
Regardless of the definitive repair, principles of initial surgical management are generally agreed upon. Some variation of the Norwood procedure is considered optimal initial therapy. Its purpose is to provide (1) a completely unobstructed systemic arterial pathway from the right ventricle to all organs, (2) a restrictive connection between the systemic and pulmonary circulations such that ![]() and
and ![]() are adequately balanced, and (3) unobstructed flow of pulmonary venous return across the atrial septum to the right atrium.
are adequately balanced, and (3) unobstructed flow of pulmonary venous return across the atrial septum to the right atrium.
Perinatal preoperative management is critical to successful outcome. This may include prenatal transport of the mother and fetus to a cardiac center following fetal diagnosis. Circulatory collapse is usually the result of closure of the ductus arteriosus in the setting of undiagnosed hypoplastic left heart physiology. This may occur when the infant is still in hospital following birth, or after discharge home. If prenatal diagnosis is made and mother and fetus are transferred to an appropriate facility where the infant will undergo surgery, circulatory collapse is all but eliminated.
After diagnosis, the infant is resuscitated, and prostaglandin E 1 (PGE 1 ) therapy is initiated. Depending on details of the physiologic status of the infant and stability of the infant at initial diagnosis, subsequent preoperative management may vary from essentially no further intervention on the one hand, to maximal intervention on the other. The typical patient, however, shows signs of pulmonary overcirculation, and preoperative management is aimed at reversing or at least controlling this to preserve end-organ and myocardial function.
Supportive therapy in the perinatal and neonatal periods can substantially alter natural history. Judicious use of inotropic support, PGE 1 therapy, nutritional supplementation, and ventilation with 17% or 19% oxygen along with supplemental CO 2 administration may delay the typical physiologic decompensation for a number of weeks ( Fig. 49-7 ). When indicated, mechanical ventilation can add an extra measure of support. In many cases, the respiratory depression side effect of PGE 1 therapy may warrant mechanical ventilation.
These maneuvers are aimed at achieving a balance of ![]() and
and ![]() and maintaining unobstructed and adequate systemic perfusion. Because flow into the pulmonary circuit is unobstructed, Rp at the microvascular level will determine
and maintaining unobstructed and adequate systemic perfusion. Because flow into the pulmonary circuit is unobstructed, Rp at the microvascular level will determine ![]() . Any maneuver that causes dilatation of the pulmonary microvasculature will result in excessive
. Any maneuver that causes dilatation of the pulmonary microvasculature will result in excessive ![]() . Specifically, avoiding supplemental inspired oxygen is critical to the overall strategy; 21% oxygen or even lower F io 2 helps maintain tone in the pulmonary microvasculature. If this maneuver is not adequate, controlled ventilation through an endotracheal tube achieves moderate elevation of Pa co 2 , causing acidosis, which further constricts pulmonary microvasculature. PGE l maintains ductal patency, ensuring unobstructed blood flow to the systemic circulation.
. Specifically, avoiding supplemental inspired oxygen is critical to the overall strategy; 21% oxygen or even lower F io 2 helps maintain tone in the pulmonary microvasculature. If this maneuver is not adequate, controlled ventilation through an endotracheal tube achieves moderate elevation of Pa co 2 , causing acidosis, which further constricts pulmonary microvasculature. PGE l maintains ductal patency, ensuring unobstructed blood flow to the systemic circulation.
Inotropic agents can be used to enhance cardiac output in the setting of moderate pulmonary overcirculation, but this strategy must be undertaken cautiously because these agents also affect systemic and pulmonary vascular resistances and may unpredictably alter ![]() . Epinephrine and high-dose dopamine, which profoundly increase systemic vascular resistance, should be avoided (see Section IV of Chapter 4 ). Low- to moderate-dose dopamine and dobutamine should be considered the first-line inotropic agents when supplemental cardiac output is considered necessary.
. Epinephrine and high-dose dopamine, which profoundly increase systemic vascular resistance, should be avoided (see Section IV of Chapter 4 ). Low- to moderate-dose dopamine and dobutamine should be considered the first-line inotropic agents when supplemental cardiac output is considered necessary.
All these maneuvers are used to create optimal preoperative cardiopulmonary status. Assessing cardiopulmonary status is somewhat indirect. Currently, ![]() and
and ![]() cannot be easily directly measured in the cardiac intensive care unit (ICU). Indirect measures of adequate systemic output include normal peripheral perfusion, adequate urine output, and absence of metabolic acidosis. Evidence of a reasonable balance of
cannot be easily directly measured in the cardiac intensive care unit (ICU). Indirect measures of adequate systemic output include normal peripheral perfusion, adequate urine output, and absence of metabolic acidosis. Evidence of a reasonable balance of ![]() and
and ![]() includes a Pa o 2 of about 40 mmHg (Torr) and a systemic diastolic blood pressure greater than 30 mmHg. Even these ideal values do not guarantee that the expected blood flow values in fact do exist. For example, Pa o 2 can be influenced by other factors: hemoglobin level, metabolic state, temperature, and presence of sepsis to name a few. Furthermore, the inevitable reduction in Rp that occurs over time commonly thwarts all efforts to maintain systemic output and balanced
includes a Pa o 2 of about 40 mmHg (Torr) and a systemic diastolic blood pressure greater than 30 mmHg. Even these ideal values do not guarantee that the expected blood flow values in fact do exist. For example, Pa o 2 can be influenced by other factors: hemoglobin level, metabolic state, temperature, and presence of sepsis to name a few. Furthermore, the inevitable reduction in Rp that occurs over time commonly thwarts all efforts to maintain systemic output and balanced ![]() and
and ![]() . If this occurs, operation should be scheduled immediately.
. If this occurs, operation should be scheduled immediately.
For the typical neonate diagnosed early after birth and in whom circulatory collapse has not occurred, the ideal time for surgical intervention is about age 2 to 5 days. In this window of time, the infant completes the profound physiologic changes from fetal life to independent life, yet consequences of a continuously increasing ![]() have not yet taken their toll. If circulatory collapse does occur and end-organ damage results, a longer time before operation is often necessary to allow end-organ recovery.
have not yet taken their toll. If circulatory collapse does occur and end-organ damage results, a longer time before operation is often necessary to allow end-organ recovery.
Although not always advisable, ideally, normal function of renal, hepatic, neurologic, gastrointestinal, and cardiopulmonary systems should be documented following resuscitation prior to proceeding with operation. It is not uncommon for organ systems to recover fairly rapidly but then plateau short of complete recovery. Further delay of operation at that point is usually detrimental.
Although mild obstruction of flow across the atrial septum is typical at the time of Doppler color flow interrogation during diagnostic echocardiography, severe obstruction at the atrial septum may occur, resulting in a clinical presentation similar to that found with obstructive total anomalous pulmonary venous connection (see Chapter 31 ), with deep cyanosis, pulmonary edema, and eventual hemodynamic instability. This presentation evolves rapidly immediately after birth and must be addressed within hours. Such patients are best managed with percutaneous interventional techniques to create an adequate atrial septal opening, followed by several days of stabilization before proceeding with operation. Management as described earlier continues during transport to the operating room and during surgery until CPB is instituted. The operation can be performed using continuous CPB by way of antegrade cerebral perfusion or using hypothermic circulatory arrest, according to choice of the operating surgeon. Both techniques are described in text that follows.
Neurologic development following operations for hypoplastic left heart physiology is below normal. Although impaired neurologic development is multifactorial, there is little question that circulatory arrest is contributory. Antegrade cerebral perfusion provides the advantage of continuous blood flow and oxygenation to the brain; however, it is possible that this technique also introduces new risks. It is unlikely that techniques of reconstruction that avoid circulatory arrest will result in dramatic changes in short-term survival, because factors related to hypothermia, CPB itself, and myocardial ischemia are not avoided. Long-term benefits related to neurologic development may exist but are yet to be proven.
Although several techniques for accomplishing the Norwood procedure using continuous perfusion have been described, the one presented has been used routinely since 1997 by one of the authors (FLH), with some modifications.
After median sternotomy, the thymus is subtotally removed and the anterior pericardium opened widely. Aortic arch vessels are dissected well above the brachiocephalic vein. The small aorta is separated from the pulmonary trunk and right pulmonary artery, and the ductus arteriosus, aortic arch, and proximal descending thoracic aorta are dissected.
Marking 7-0 monofilament sutures are placed on adjacent portions of the pulmonary trunk and ascending aorta to indicate the point of eventual pulmonary trunk–to-aorta anastomosis. Positions of these marking sutures are chosen with great care because they will determine the correct orientation of, and incisions in, the aorta and pulmonary trunk necessary to create a functional anastomosis; alignment of this anastomosis is critical for unobstructed coronary blood flow in aortic atresia. The first marking suture is placed in the adventitia of the pulmonary trunk 1 to 2 mm above the sinutubular junction and circumferentially exactly where the small ascending aorta lies against it. The second suture on the aortic adventitia is placed so that its position coincides exactly with the pulmonary trunk suture.
A purse-string suture is placed on the brachiocephalic artery about 5 mm distal to the takeoff of the artery from the arch. It is often necessary to place it above the brachiocephalic vein. Purse-string sutures are placed on the superior and inferior venae cavae. A 5-0 monofilament suture is placed around the pulmonary artery end of the ductus arteriosus.
An 8F (or in patients weighing <3 kg, 6F) arterial cannula is inserted into the brachiocephalic artery, and angled 12F venous cannulae are placed into the venae cavae. Alternatively, a single venous cannula can be used, placed in the right atrial appendage ( Fig. 49-8, A ). Brachiocephalic artery cannulation must be performed accurately; however, experience shows that it can be routinely performed successfully even in patients weighing 3.0 kg or less. Cannulation is best performed by puncturing the artery without using a clamp, and inserting the tip of the cannula to a depth less than the width of the artery, typically 2 to 3 mm.

After venous cannulation and institution of CPB, the ductus arteriosus is immediately ligated (see Fig. 49-8, A ). Core temperature is reduced to 20°C. During cooling, the pulmonary trunk is transected just above the pulmonary valve in standard fashion ( Fig. 49-8, B ). The distal opening in the pulmonary trunk is then closed directly or patched with an oval piece of pulmonary allograft or other material (see Fig. 49-8, B ). A clamp is placed across the ascending aorta just proximal to the brachiocephalic artery once the target core temperature has been achieved. Cardioplegia is introduced into the ascending aorta. The ascending aorta is then opened to the level of the transected pulmonary trunk, and the side-to-side anastomosis between proximal pulmonary trunk and ascending aorta is accomplished with interrupted 6-0 or 7-0 monofilament sutures ( Fig. 49-8, C ).
In preparation for arch reconstruction, direct CPB flow is isolated to the brachiocephalic artery only. This is accomplished by clamping the base of the brachiocephalic, left carotid, and left subclavian arteries individually with delicate neurovascular clips, and placing a C-shaped clamp across the descending thoracic aorta approximately 1.5 to 2 cm below the ductal insertion ( Fig. 49-8, D ). Perfusion, now through the distal brachiocephalic artery only, is reduced to 30 to 40 mL · kg −1 · min −1 , allowing normal brain perfusion.
The original clamp placed across the ascending aorta is removed. The ductus is divided distal to the previously placed suture. The previous incision in the ascending aorta is then continued around the arch, then beyond the ductus, approximately 1.5 cm onto the descending aorta. Ductal tissue is trimmed, and allograft patch reconstruction of the aortic arch, ascending aorta, and pulmonary trunk is performed. The caval cannulae are snared. A right atriotomy is made, and the septum primum is completely removed to create a nonrestrictive interatrial communication. The right atriotomy is closed, and the neurovascular clips on the base of the brachiocephalic artery and the C-clamp on the descending aorta are removed. Total body perfusion is reestablished and increased to normal levels; rewarming is begun.
The last step in the operation is creating a source of pulmonary blood flow. At the choice of the operating surgeon, this can be achieved using either a restrictive systemic arterial–to–pulmonary arterial shunt or a restrictive right ventricular–to–pulmonary arterial conduit. If a shunt is chosen, typically a polytetrafluoroethylene (PTFE) interposition graft is sewn into place from the junction of the brachiocephalic and right subclavian arteries to the proximal portion of the right pulmonary artery ( Fig. 49-8, E ). Both anastomoses are performed using end graft–to–side artery connections with running 7-0 monofilament or PTFE suture. Variation in positioning the PTFE shunt must be considered based on individual patient characteristics to achieve an appropriate balance of ![]() and
and ![]() .
.
In patients weighing less than 3 kg and in those demonstrating very low Rp preoperatively, it may be necessary to perform the systemic connection of the shunt anastomosis at a more distal site on the right subclavian artery. In most cases, a 3.5-mm-diameter PTFE tube graft is used for the shunt procedure; rarely is a larger diameter necessary. Often in patients weighing less than 3 kg, and most frequently in patients weighing less than 2.5 kg, a 3.0-mm-diameter graft should be considered, although risk of shunt thrombosis may increase with a small-diameter shunt. If the right ventricular–to–pulmonary arterial conduit is performed, a PTFE graft of appropriate diameter is chosen. Alternatively, a composite graft consisting of a proximally positioned PTFE tube and a distally positioned small (6- to 7-mm diameter) allograft pulmonary or aortic valve can be used ( Fig. 49-8, F and G ). Regardless of whether a simple conduit or composite conduit is chosen, the diameter of the PTFE tube determines the resistance to flow into the pulmonary arteries. Typically, a 4-mm-diameter graft is used for patients weighing less than 3 kg, a 5-mm-diameter graft for those between 3 and 4 kg, and a 6-mm-diameter graft for those greater than 4 kg.
An incision is made in the infundibular portion of the right ventricle, just below the pulmonary valve. A 5- to 6-mm-diameter circular full-thickness resection of infundibular muscle is then made. It is extremely important that the caliber of the resulting hole in the infundibulum is maintained transmurally to prevent premature stenosis at this level postoperatively. Using a running 7-0 monofilament suture, the graft is first sewn end to side to the pulmonary trunk, either to the pulmonary artery directly adjacent to the suture line that previously closed the distal pulmonary trunk, or to the center of the patch that was used to close the distal pulmonary trunk. The graft is then positioned to the left of the reconstructed aorta and tailored in length to reach the infundibulotomy. The proximal end of the graft is carefully beveled to the appropriate angle to ensure a smooth course around the large reconstructed aorta. The anastomosis is performed using a running 6-0 monofilament suture.
Once normothermia is achieved, the patient is separated from CPB and decannulated. Management following separation from CPB is described under “Post–Cardiopulmonary Bypass Management” later in this chapter.
The heart is exposed by median sternotomy, removal of most of the thymus gland, and opening of the pericardium. If the patient is unstable at this point because of increased ![]() , the right pulmonary artery can be exposed immediately and clamped to reduce overall
, the right pulmonary artery can be exposed immediately and clamped to reduce overall ![]() and maintain systemic circulation until CPB is established.
and maintain systemic circulation until CPB is established.
The patient is prepared for CPB by placing a purse-string suture on the pulmonary trunk just distal to the pulmonary valve. A second purse-string suture is placed around the tip of the right atrial appendage. At that point, if the patient is physiologically stable, the pulmonary trunk is separated from the ascending aorta using either scissors or electrocautery. The ductus arteriosus, aortic arch, and arch vessels are then mobilized using scissors or electrocautery all the way to the first set of intercostal vessels on the descending aorta. Temporary snares are placed around all brachiocephalic arteries.
If the patient becomes physiologically unstable, CPB can be initiated at any time and the great vessel dissection performed with its support. CPB is established using an arterial cannula in the pulmonary trunk and a single venous cannula in the right atrial appendage ( Fig. 49-9, A ). At initiation of CPB, the branch pulmonary arteries are temporarily occluded with clamps or snares to eliminate pulmonary blood flow.

After initiating CPB, while the pulmonary trunk and aorta are still distended with blood, 7-0 monofilament sutures are placed on adjacent portions of the pulmonary trunk and ascending aorta to mark the point of eventual pulmonary trunk–to-aorta anastomosis. Positions of these marking sutures are chosen with great care because they will determine the correct orientation of, and incisions in, the aorta and pulmonary trunk necessary for creating a functional anastomosis; alignment of this anastomosis is critical for unobstructed coronary blood flow in aortic atresia. The first marking suture is placed in the adventitia of the pulmonary trunk 1 to 2 mm above the sinutubular junction and circumferentially exactly where the small ascending aorta lies against it. The second suture on the aortic adventitia is placed so that its position coincides exactly with the pulmonary trunk suture (see Fig. 49-9, A ).
When the nasopharyngeal or tympanic membrane temperature reaches 16°C to 18°C after cooling with CPB for an appropriate period (see Section IV of Chapter 2 ), the snares around the brachiocephalic vessels are tightened and circulatory arrest established. Snares around the left and right pulmonary arteries are removed, and cannulae are removed from the pulmonary trunk and right atrial appendage after draining as much blood volume from the patient into the pump-oxygenator as possible.
Management of myocardial protection is variable. Some experienced centers use no specific cardioplegia and rely on profound hypothermia as the only form of myocardial management. Other experienced institutions use cardioplegia, which can be supplied in several ways. Cannulation of the ascending aorta can be achieved with an appropriately small needle and cardioplegia delivered directly into the aortic root. Alternatively, the cardioplegia system can be connected to the arterial cannula in the pulmonary trunk, and cardioplegia delivered into this cannula after circulatory arrest has been established while the brachiocephalic vessel snares and pulmonary artery branch snares are still in place. The only additional maneuver before proceeding with cardioplegia delivery using this method is to clamp the descending aorta distal to the ductal insertion site. The cardioplegic solution is delivered through the arterial cannula into the pulmonary trunk through the ductus and retrograde around the arch to the coronary arteries. All other peripheral runoff through this circuit must be reliably eliminated (see “Methods of Myocardial Management during Cardiac Surgery” in Chapter 3 ).
After myocardial protection has been addressed and circulatory arrest established, the ductus arteriosus is ligated distal to the origin of the left pulmonary artery. A small atriotomy is made, and through it the entire septum primum is removed to create an unrestrictive intraatrial communication. To avoid conduction problems, care is taken not to extend the resection beyond the septum primum. The atriotomy is closed ( Fig. 49-9, B ).
The pulmonary trunk is divided transversely as proximal as possible without risking damage to the pulmonary valve, leaving the previously placed marking suture proximal to the transection. As the transection is made, particular care is taken to avoid the orifice of the right pulmonary artery. The distal end of the divided pulmonary trunk is then closed with a patch (typically autologous pericardium or pulmonary artery allograft) using continuous 7-0 polypropylene. Alternatively, the distal pulmonary trunk may be closed primarily in transverse fashion ( Fig. 49-9, C ). Direct closure has the advantages of time efficiency and less bulk, and in experienced hands has shown no greater tendency to result in pulmonary trunk stenosis than the patch technique.
The ductus is transected just beyond the previously placed ligature. Redundant ductal tissue is cut away from the distal aorta, leaving a small cuff of ductal tissue at the level of the aortic isthmus. A 5- to 10-mm incision is made from the ductal orifice into the descending aorta to the level of the first set of intercostal vessels (see Fig. 49-9, C ). A proximal incision is made beginning at the ductal orifice and moving retrograde toward the aortic valve. This incision proceeds along the undersurface of the aortic arch and extends down the hypoplastic ascending aorta to within several millimeters of the atretic or hypoplastic aortic valve, terminating at the same level as the transected pulmonary trunk at the point of the previously placed marking suture (see Fig. 49-9, C ).
The aorta is then augmented throughout its length from the level of the aortic valve around the arch, to the first set of intercostal vessels, using a patch of pulmonary or aortic allograft tissue ( Fig. 49-9, D ). The patch is tailored to provide adequate but not excessive widening of the aorta. Suturing is begun at the distal end of the incision beyond the isthmus on the upper descending aorta and progresses retrograde until the proximity of the brachiocephalic artery is reached. The posterior suture line is developed first, using a running technique and 6-0 or 7-0 nonabsorbable monofilament suture, followed by the anterior suture line.
At this point, the allograft augmentation of the arch is temporarily set aside, and the proximal end of the divided pulmonary trunk is anastomosed side to side to the incised hypoplastic aorta ( Fig. 49-9, E ). This portion of the anastomosis is typically performed with five to seven interrupted 6-0 or 7-0 monofilament nonabsorbable sutures. The first of these connects the end of the aortic incision to the cut edge of the pulmonary trunk exactly where the previously placed marking suture was positioned. On each side of this interrupted suture, two to three other interrupted sutures are placed, attaching first the posterior and then the anterior edge of the longitudinally incised aorta to the circumference of the proximal pulmonary trunk. Care should be taken with small aortas (<3 mm diameter) not to connect them to too broad a segment of pulmonary trunk circumference, because this can stretch the aortic tissue and flatten and obstruct the orifice leading to the coronaries.
Finally, the allograft patch suture line progresses from the level of the brachiocephalic artery down to the aortic-to-pulmonary anastomosis and around the remaining free edge of the proximal pulmonary trunk ( Fig. 49-9, F ). This completes the right ventricular–to–systemic arterial outflow reconstruction.
The last step in the operation is creating a source of pulmonary blood flow. At the choice of the operating surgeon, this can be achieved using either a restrictive systemic arterial–to–pulmonary arterial shunt or a restrictive right ventricle–to–pulmonary arterial conduit. If a shunt is chosen, typically a PTFE interposition graft is sewn into place from the junction of the brachiocephalic and right subclavian arteries on the systemic side to the proximal portion of the right pulmonary artery (see Fig. 49-9, F ). Both anastomoses are performed using end graft–to–side artery connections with running 7-0 monofilament or PTFE suture. Variation in positioning of the PTFE shunt must be considered, based on individual patient characteristics, to achieve an appropriate balance of ![]() and
and ![]() .
.
In patients weighing less than 3 kg and in those demonstrating very low Rp preoperatively, it may be necessary to perform the systemic pulmonary arterial shunt anastomosis at a more distal site on the right subclavian artery. In most cases, a 3.5-mm-diameter PTFE tube graft is used for the shunt procedure; a larger diameter is rarely necessary. Often in patients weighing less than 3 kg and most frequently in patients weighing less than 2.5 kg, a 3.0-mm-diameter graft should be considered, although risk of shunt thrombosis may increase with a smaller-diameter shunt. If circulatory arrest is prolonged, or by surgeon preference, the shunt may be placed after reestablishing flow on CPB.
If a right ventricular–to–pulmonary arterial conduit is chosen, a PTFE graft of appropriate diameter is selected. Alternatively, a composite graft consisting of a proximally positioned PTFE tube and a distally positioned small (6- to 7-mm diameter) allograft pulmonary or aortic valve can be used ( Fig. 49-9, G and H ). Regardless of whether a simple conduit or composite conduit is chosen, the diameter of the PTFE tube determines the resistance to flow into the pulmonary arteries. Typically, a 4-mm-diameter graft is used for patients weighing less than 3 kg, a 5-mm-diameter graft for those between 3 and 4 kg, and a 6-mm-diameter graft for those greater than 4 kg.
An incision is made in the infundibular portion of the right ventricle, just below the pulmonary valve. A 5- to 6-mm-diameter circular full-thickness resection of infundibular muscle is then made. It is extremely important that the caliber of the resulting hole in the infundibulum is maintained transmurally to prevent premature stenosis at this level postoperatively. Using a running 7-0 monofilament suture, the graft is first sewn end to side to the pulmonary trunk, either to the pulmonary artery directly, adjacent to the suture line that previously closed the distal pulmonary trunk, or to the center of the patch that was used to close the distal pulmonary trunk. The graft is then positioned to the left of the reconstructed aorta and tailored in length to reach the infundibulotomy. The proximal end of the graft is carefully beveled to the appropriate angle to ensure a smooth course around the large reconstructed aorta.
The anastomosis is performed using a running 6-0 monofilament suture. The right atrial and systemic arterial cannulae are reinserted, CPB is reestablished, and rewarming begun. If a systemic-to-pulmonary shunt has been placed, it is occluded with a vascular clamp during the rewarming phase of CPB. When the patient's tympanic membrane or nasopharyngeal temperature reaches 25°C to 30°C, perfusate ionized calcium concentration is measured and calcium chloride added to bring the ionized calcium concentration to a normal level (see Section III of Chapter 2 ). Separation from CPB is accomplished and decannulation achieved. Details of post-CPB management follow.
Whether the operation is performed using continuous perfusion or circulatory arrest, post-CPB management is generally the same. Before the patient is separated from CPB, inotropic support is initiated, and particular attention is given to complete reexpansion of both lungs. Endotracheal suctioning by the anesthesiologist is routine. If a systemic-to-pulmonary shunt was used, after complete rewarming has been achieved, approximately 5 minutes before discontinuing CPB, the clamp on the shunt is removed.
Careful attention is given to the mean arterial pressure on CPB at this point; typically a decrease of 10 to 15 mmHg should be expected, indicating adequate runoff into the pulmonary vascular bed. If this decrease is not observed, the cause must be identified. The systemic-to–pulmonary trunk shunt should be immediately assessed for obstruction due to a technical problem. If a right ventricle–to–pulmonary arterial conduit was used, diastolic blood pressure is not affected.
After rewarming has been completed, CPB is discontinued and the aortic and venous cannulae removed. Postoperative care begins immediately (see Special Features of Postoperative Care later in this chapter). Two separate polyvinyl catheters (or a single double-lumen catheter) are placed directly into the right atrial appendage and brought out through the chest wall to continuously monitor atrial pressure and provide reliable access for delivering blood products and pharmacologic support. Atrial and ventricular temporary epicardial pacing wires are placed. Chest drainage tubes are placed appropriately for neonates undergoing CPB (see “Completing Operation” in Section III of Chapter 2 ).
It may be beneficial to leave the sternum and soft tissue temporarily unapproximated during the early recovery period. This allows for maximal cardiopulmonary function during the first 24 to 48 hours postoperatively and easy accessibility to the mediastinum if aggressive resuscitative measures are necessary. When this “open chest” option is exercised, the skin is sealed with an oval silicone rubber sheet or some other appropriate material. After the patient's cardiopulmonary status has stabilized (48 to 96 hours postoperatively), the sheet is removed under sterile conditions, and the sternum and soft tissues closed in standard fashion. This can be accomplished routinely and effectively in the ICU without returning the patient to the operating room. At some institutions, the “open sternum” option is standard following first-stage reconstruction for hypoplastic left heart physiology.
A number of modifications of the standard ascending aorta and arch reconstruction described in the preceding text have been developed. However, the physiologic principles of providing unobstructed ventricular-to–systemic arterial output and appropriately balanced ![]() and
and ![]() remain the same. Several groups have introduced techniques of reconstructing the ventricular-to–systemic arterial outflow without use of patch material. Much experience has been obtained, and perioperative outcome and some midterm outcome data are available using these alternative techniques. Theoretical advantages include avoiding foreign patch material and the possibility of a modest reduction in circulatory arrest time. Disadvantages include potential problems with suture line tension, left pulmonary artery and left bronchus compression, and increased resistance to flow to the coronary system.
remain the same. Several groups have introduced techniques of reconstructing the ventricular-to–systemic arterial outflow without use of patch material. Much experience has been obtained, and perioperative outcome and some midterm outcome data are available using these alternative techniques. Theoretical advantages include avoiding foreign patch material and the possibility of a modest reduction in circulatory arrest time. Disadvantages include potential problems with suture line tension, left pulmonary artery and left bronchus compression, and increased resistance to flow to the coronary system.
Currently, there is no clear evidence that these alternative techniques are better or worse than the more standard arch reconstruction technique. Other modifications in the surgical treatment of neonates with hypoplastic left heart physiology have been reported. The right ventricle–to–pulmonary artery conduit, if used, can be placed to the right side of the reconstructed ascending aorta rather than to the left side as described. This is believed by some to have advantages.
Some surgeons prefer the hybrid procedure to the Norwood procedure or one of its variants as a primary procedure in high-risk patients (i.e., those with prematurity, low birth weight, associated genetic or other noncardiac comorbid conditions, extreme shock, or various real or perceived cardiac risk factors, such as severe tricuspid regurgitation, depressed right ventricular function, intact or highly restrictive atrial septum, aortic atresia with mitral stenosis, and very small-diameter ascending aorta). The procedure is ideally performed in a hybrid operating suite, essentially a cardiac catheterization laboratory that also has the dimensions and capability to support major surgery and use of CPB.
Preoperative management is the same as for a patient undergoing a Norwood procedure. The patient is anesthetized, prepped, and draped in the supine position, just as in a formal operating room. CPB support is available. Pulmonary artery branch bands are prepared from segments of PTFE tube grafts. For patients weighing 3 kg or more, 3-mm-diameter grafts are selected. For patients weighing less than 3 kg, 2.5-mm grafts are chosen. The bands are cut to a width of approximately 2 mm.
A median sternotomy incision is made, the pericardium opened, and the branch pulmonary arteries exposed. If the patient is unstable because of pulmonary overcirculation, the right branch pulmonary artery can be temporarily occluded with a delicate neurovascular clip. Care must be taken not to distort the small ascending aorta, particularly if aortic atresia is present. The right and left branch pulmonary arteries are sequentially exposed and banded ( Fig. 49-10, A ). The bands are performed before the ductal stent is placed to prevent migration of the stent during manipulation of the pulmonary artery branches. Position of the bands is confirmed by angiography ( Fig. 49-10, B ). A purse-string suture is placed on the pulmonary trunk immediately proximal to the take-off of the right pulmonary artery, and a 5F or 6F sheath system is inserted through the purse string and advanced over a guide-wire through the ductus and into the descending aorta.

Accurate delineation of the ductal anatomy by angiography is important before deploying the ductal stent. After determining ductal dimensions with angiography, the stent is inserted and deployed ( Fig. 49-10, C ). Another angiogram is performed to assess stent position. If the stent does not cover the entire length of the ductus, ductal narrowing and systemic outflow obstruction may result. If the stent is too long, it could obstruct the origin of the pulmonary arteries or retrograde flow into the proximal arch and ascending aorta, which could be catastrophic in patients with aortic atresia ( Fig. 49-10, D ).
Once position of the stent is confirmed, PGE 1 infusion is discontinued. Based on hemodynamic and echocardiographic data, adequacy of the atrial septal communication is assessed. If deemed restrictive, and depending on the nature of the restriction, a balloon atrial septotomy or deployment of an atrial septal stent is performed. The completed procedure is shown in Fig. 49-10, E . Routine echocardiographic assessment is performed on arrival in the ICU and weekly until the patient is discharged. The chest is closed over a single drainage tube. Postoperative care is similar to that after the Norwood procedure.
Alternative techniques have been described for the hybrid procedure. These include a left thoracotomy approach, different methodology and materials for the pulmonary artery bands, and use of long-term PGE 1 (avoiding stents) to maintain ductal patency.
These procedures are performed as a second stage following the Norwood procedure. Bidirectional superior cavopulmonary anastomosis and the hemi-Fontan procedure are described under Technique of Operation in Chapter 41 . Because it is generally accepted that there remains substantial risk of mortality in the period between hospital discharge following a successful first-stage reconstruction for hypoplastic left heart physiology and creation of the bidirectional superior cavopulmonary anastomosis, the second-stage procedure should be performed relatively soon, typically between age 3 and 6 months. Following creation of the bidirectional superior cavopulmonary anastomosis, hemodynamic efficiency is markedly improved and mortality risk substantially reduced. Data suggest that somatic growth does not occur in patients with first-stage palliation after age 4 months.
Early construction of the bidirectional superior cavopulmonary shunt has two other advantages:
It allows use of a relatively small-diameter systemic-to-pulmonary shunt or right ventricular–to-pulmonary conduit at the time of first-stage reconstruction. This creates an ideal ![]() ratio during the first months following birth and does not require the initial shunt or conduit to have a life expectancy of more than 6 months. As a result, the initial shunt or conduit does not have to be “oversized” in anticipation of the growing infant requiring increased
ratio during the first months following birth and does not require the initial shunt or conduit to have a life expectancy of more than 6 months. As a result, the initial shunt or conduit does not have to be “oversized” in anticipation of the growing infant requiring increased ![]() in later infancy. This promotes early hemodynamic stability.
in later infancy. This promotes early hemodynamic stability.
It reduces duration of inefficient mixed circulation that is present with a shunt or conduit. This allows maximal preservation of right ventricular function by reducing right ventricular volume work, mortality risk, and chance of distortion of the pulmonary arteries caused by tethering from the PTFE graft.
This is a complex operation requiring CPB, aortic clamping, and (by preference) either antegrade cerebral perfusion or hypothermic circulatory arrest. Exposure is by median sternotomy with dissection of the aortic and pulmonary trunks, aortic arch, brachiocephalic arteries, ductus arteriosus, proximal descending thoracic aorta, and branch pulmonary arteries beyond the band sites. Cannulation technique and CPB management are similar to those used for the Norwood procedure described earlier.
Reconstruction involves removing the ductus arteriosus and stent, Norwood neoaorta and arch reconstruction, removing the pulmonary artery bands with possible branch pulmonary artery reconstruction, atrial septectomy or removal of atrial septal stent, and creating a bidirectional superior cavopulmonary anastomosis. In essence, it embodies most of the technical components of the Norwood operation, with the addition of a reoperative setting. Technical details of the procedure are shown in Fig. 49-11 .

The Fontan operation is described under Technique of Operation in Chapter 41 . Once the bidirectional superior cavopulmonary shunt has been constructed, considerations for completing the Fontan procedure, although somewhat complex, remain no different from those for any other single-ventricle anomaly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here