Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although the liver is known to be a somewhat immunotolerant organ, pediatric liver transplantation (LT) would not have reached current success without efficient immunosuppressive drugs. Standard protocols include calcineurin inhibitors (CNIs), most often tacrolimus (TAC) and sometimes cyclosporine A (CsA). Additional induction with anti-interleukin 2 receptor antibodies (anti-IL2r) or a short corticosteroids (CS) course further decrease the risk of rejection. Specific situations may require immunosuppression adaptation, such as in ABO-incompatible graft, transplantation for immune disorders or retransplantation, abnormal renal function, or liver tumor. Ongoing research is attempting to minimize and produce tailor-made immunosuppressive schedules, aiming to decrease drug toxicity and, if possible, induce graft tolerance.
Induction of anti-rejection treatment includes the use of monoclonal antibodies given pre- or perioperatively. The goal is to decrease the severity of acute cellular rejection (ACR) by providing immediate blockade of the elements of the immune system while waiting for CNIs or other long-term immunosuppressant drugs to reach their therapeutic serum levels. The initial attempts with anti-thymocyte globulin (ATG) or orthoclone OKT3 (OKT3, anti-CD3 monoclonal antibody) did not demonstrate a clear efficacy and were associated with significant side effects. OKT3 is not available anymore, whereas ATG is usually not used as an induction therapy but can be indicated in the treatment of steroid-resistant (SRR) or chronic rejection (CR) after transplant (see later). In 2001, two reports from Hamburg and Atlanta were the first to demonstrate the value of anti-interleukin-2 receptor inhibition using anti-IL2r as induction anti-rejection treatment in liver transplantation (LT) in children, with basiliximab and daclizumab (both humanized anti-CD25 monoclonal antibodies), respectively. ACR was significantly decreased in the first 6 months after transplantation in the children receiving the treatment in comparison with the matched historical controls. Safety was not different between the groups. Moreover, these results were achieved while decreasing the target serum levels of CNIs or postponing their introduction. Medium-term results from the same groups confirmed the efficacy and safety at 2 years post-transplant. Additionally, use of the induction treatment allowed them to withdraw CS from the immunosuppressive scheme without impairing patient or graft survival at 1 year post-transplant. A meta-analysis showed that the induction with anti-IL2r was associated with a significantly lower risk of ACR and tendency for a decreased risk of SRR, graft loss, and patient death. Nowadays, daclizumab has been withdrawn from the market because of safety issues, but basiliximab is still used as induction therapy in LT in Europe and less frequently in the United States. The most commonly reported protocols include intravenous administration at days 0 and 4 ( Table 18.1 ).
| Drugs | Recommended Dose | Therapeutic Monitoring |
|---|---|---|
| Basiliximab | 10 mg IV < 35 kg weight 20 mg IV > 35 kg weight In 2 doses: D0 and D4 |
None |
| Cyclosporin A | 5–13 microg/kg/day in 2 doses Monitor and adapt to trough serum levels |
Month 1: 150–180 ng/mL Thereafter: 50–100 ng/mL |
| Tacrolimus | 0.1 microg/kg bid Monitor and adapt to trough serum levels |
Week 1–2: about 12 ng/mL; week 3–4: about 10 ng/mL; month 1–3: about 8 ng/mL Month 4–12: about 6 ng/mL Thereafter: about 4 ng/mL |
| Corticosteroids | Methyl-prednisolone D0 intraoperative: bolus 5–10 mg/kg D1–D6: 2 mg/kg/d Prednisolone Rapid tapering to 0.25 mg/kg/d and stop within 6 months of transplant |
Glycemia, blood pressure, proteinuria |
| Mycophenolate-mofetil | 10–15 mg/kg bid | No consensus |
| Azathioprine | 2 mg/kg/d | No consensus |
| Sirolimus | 1 mg/m 2 | 3–7 ng/mL |
| ATG | 1.5 mg/kg/d × 10–14 | None |
CNIs have revolutionized the field of organ transplantation, facilitating its clinical application and success. By inhibiting the activation of calcineurin inside T lymphocytes, CNIs decrease their production of several proinflammatory cytokines, including interleukin 2, and prevent their activation ( Fig. 18.1 ). The main problem of these drugs is their narrow therapeutic index. Subtherapeutic levels increase the risk of rejection, whereas overdosing could potentiate their side effects, which include infections, renal toxicity, hypertension, diabetes, dyslipidemia, and risk of malignancies. Therefore they need regular and very careful serum level monitoring. The most commonly reported protocols are described in Table 18.1 .
![Fig. 18.1, The binding of an antigen to a lymphocyte will induce an increase of intracellular calcium, leading to activation of calcineurin. Activated calcineurin will dephosphorylate cytoplasmic NF-ATc, a transcription factor. Activated NF-ATc will translocate to the nucleus, where it upregulates the expression of multiple cytokines and costimulatory molecules necessary for full activation of T cells (interleukin 2 [IL-2], IL-4, and CD40-ligand). CsA (through binding with cyclophilins) and TAC (through binding with FK-binding proteins) will inhibit the activation of calcineurin and, therefore, the upregulation of IL-2 and the activation of T lymphocytes. Fig. 18.1, The binding of an antigen to a lymphocyte will induce an increase of intracellular calcium, leading to activation of calcineurin. Activated calcineurin will dephosphorylate cytoplasmic NF-ATc, a transcription factor. Activated NF-ATc will translocate to the nucleus, where it upregulates the expression of multiple cytokines and costimulatory molecules necessary for full activation of T cells (interleukin 2 [IL-2], IL-4, and CD40-ligand). CsA (through binding with cyclophilins) and TAC (through binding with FK-binding proteins) will inhibit the activation of calcineurin and, therefore, the upregulation of IL-2 and the activation of T lymphocytes.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/AntirejectionStrategies/0_3s20B9780323636711000185.jpg)
CsA was first used in the late 1970s and immediately brought LT to a genuine clinical use with early patient survival above 60%. CsA is a small cyclic polypeptide, poorly soluble in water. The first oil-based preparation (Sandimmune) had a variable bioavailability, dependent on, among other things, bile secretion and lipid content of the food. A microemulsion was then developed (Neoral) that was less affected by these factors. It is important to note that the pharmacodynamics of these two formulations is different during conversion from one to another. In addition to the discussed side effects, CsA has also considerable cosmetic drawbacks such as hypertrichosis and gingival hypertrophy. In modern transplant medicine, this drug is generally replaced by TAC. When used, CsA dosing is modified according to target serum levels for the maximal CsA peak, occurring 2 hours after the intake (C2) or, for the trough levels, 12 hours post dose (see Table 18.1 ).
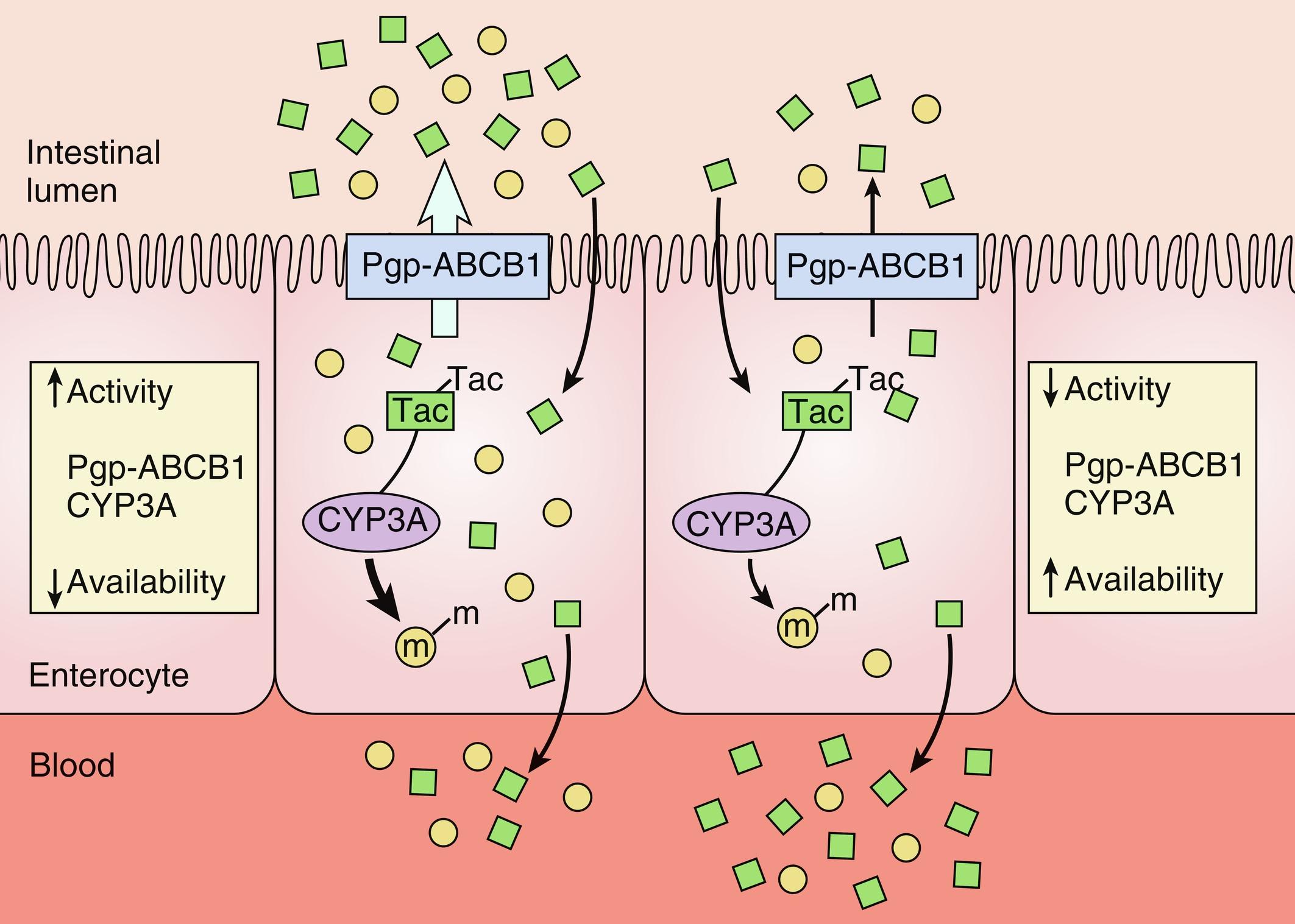
TAC is currently the cornerstone of immunosuppression after LT; it was shown to be more efficient than CsA in several reports, with no cosmetic side effects of CsA or CS. TAC is a macrolide usually taken orally as a capsule with solid dispersion of the medication and lactose monohydrate (Prograf). TAC granules also exist for younger children (Modigraf). For TAC, being a lipophilic molecule, the bioavailability is low, and pharmacodynamics shows considerable interindividual variability. In a cohort of 116 adult liver transplant patients, TAC interindividual variability between 35% and 40% during 2 years after transplant was noted and was positively correlated to the risk of rejection and to the detection of anti-donor-specific antibodies. Individual factors are responsible for about 50% of this high variability, including recipient age, race, body composition, organ function, intestinal motility, food intake, concomitant medications (proton pump inhibitors, antifungals, macrolides), donor age, and sex. The other 50% of the variability includes differences in TAC metabolism, mainly through CYP3A5 polymorphisms, but also involving CYP3A4, CYP3A7, PXR, POR, and ABCB1 variants ( Fig. 18.2 ). Patients carrying CYP3A5*1 allele are described as CYP3A5 expressors or rapid metabolizers in comparison with those displaying CYP3A5*3, *6, or *7 alleles. The rapid metabolizers are supposed to need 1.5 times higher doses to reach the same drug levels in the serum. The lower prevalence of the CYP3A5*3 in the African-American population presumably explains their higher TAC dose requirements after kidney transplantation. It was also shown that they have a higher prevalence of the *6 and *7 alleles, leading probably to a TAC metabolism relatively similar to the Caucasian patients. The differences in the drug requirements could therefore be related to other factors such as diet, lifestyle, or concomitant medication. In a cohort of children who have had LT, TAC concentration at day 1 was significantly associated with the donor CYP3A5 polymorphism and gender and with the recipient age. The authors suggested that the initial dose should be doubled in the patients younger than 6 years who received an organ from a male rapid metabolizer donor. Despite the improved understanding, the genotyping of recipients and donors has not yet reached a practical clinical application or replaced therapeutic drug monitoring. Further studies could provide additional information and eventually lead to individual tailoring of CNI doses in the future. At present, TAC should be administered consistently (according to the described protocols [see Table 18.1 ]; at the same time of the day, consistently with or without food), and regular drug monitoring is mandatory. Interfering drugs ( Table 18.2 ) should be avoided where possible, and daily dose should be modified according to the serum trough levels.
| CYP3A inhibitors; may induce increased through levels | |
| Family | Examples |
| Antifungal agents | Fluconazole Itraconazole Ketoconazole Voriconazole |
| Antibiotics | Clarithromycin Erythromycin |
| Ca-channel blockers | Diltiazem Nifedipine Verapamil |
| Others | Protease inhibitors Grapefruit, pomegranate, star fruit, and Seville oranges |
| CYP3A inducers; may induce decreased through levels | |
| Anticonvulsants | Carbamazepine Phenytoin Phenobarbital |
| Antibiotics | Rifampicin |
A prolonged-release once-daily TAC (TAC-OD) formulation (Advagraf) is also available and is commonly used in adults after LT, having been demonstrated to be safe and efficient. A recent report in a pediatric population shows that a switch from TAC to TAC-OD is also feasible in children. Some 55 clinically stable children, with a mean age of 10.2 ± 3.6 years at a mean follow-up post-transplant of 6.4 ± 2.5 years, 32 of them on TAC monotherapy, were switched to the once-daily scheme without significant side effects after 1 and 3 years. Only one episode of rejection occurred 3 years after the switch because of poor compliance. Kidney function was impaired in 15 patients (calculated glomerular filtration rate, 60–80 mL/min/m 2 ) but significantly improved at 1 and 3 years’ follow-up. Deviation of the drug intake (time change or drug forgotten) was recorded in 32/55 patients before and in 21/55 after the switch. A once-daily TAC dose could therefore improve compliance during the challenging time of adolescence and decrease long-term nephrotoxicity of CNIs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here