Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There are probably more than 1 billion people worldwide with raised blood pressure. It is one of the most common chronic medical conditions internationally (US National Center for Health Statistics, 2005), and occurs almost twice as often in African-Americans than in whites. The incidence of hypertension increases with age, with a slightly greater incidence in men than in women. In the United States, hypertension affects about 25% of all adults older than 40 years of age. More importantly, the prevalence of undiagnosed hypertension is about 1 in 15. In the United Kingdom, there are about 7.5 million patients suffering from raised blood pressure; 80% to 85% of these patients are either not treated or are being inadequately treated.
Blood pressure has been classified into four categories in the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure, 7th Report (JNC 7 Report) based on systolic (left) and diastolic (right) pressures :
Normotension less than 120 and less than 80 mm Hg
Prehypertension 120 to 139 or 80 to 89 mm Hg
Stage I hypertension 140 to 159 or 90 to 99 mm Hg
Stage II hypertension greater than 160 or greater than or equal to 100 mm Hg
In addition, patients with isolated systolic hypertension (ISH) are classified into ISH 1 (140–159/< 90 mm Hg) and ISH 2 (> 160/< 90 mm Hg). In 2014, JNC 8 recommended treatment of systolic blood pressure over 150 mm Hg or diastolic blood pressure over 90 mm Hg to a goal of less than these values for patents aged > 60 years, and treatment for systolic blood pressure over 140 mm Hg or diastolic blood pressure over 90 mm Hg for patients aged <60 years.
Many patients with hypertension have associated metabolic disorders (decreased high-density lipoproteins, increased triglycerides, increased urates, and reduced glucose tolerance). These of all contribute to the increased cardiovascular risk associated with high blood pressure and to the possible development of type 2 diabetes mellitus. These additional comorbidities influence the choice of drug therapy for treatment of raised blood pressure.
In the United States, strategies for treatment have included initiation of treatment if blood pressure is more than 20 mm Hg systolic or 10 mm Hg diastolic above the target pressure, but this has been modified by the JNC 8 criteria to include specific thresholds. Many studies have shown monotherapy to be generally inadequate and to be associated with an increased incidence of cardiovascular events occurring early after starting treatment. The use of combination therapy is not new; it originates from the mid-1960s when studies showed the efficacy of fixed doses of reserpine, hydrochlorothiazide, and hydralazine.
The causes of arterial hypertension are multiple but can be divided into 3 distinct subgroups ( Table 26.1 ). There are various ways of reducing blood pressure and hence of treating hypertension. The history of the treatment of raised blood pressure shows how different drugs have been used to act on different effector sites or receptors. The dates of introduction of some of these early antihypertensive therapies are listed in Table 26.2 .
| Essential |
| Secondary |
|
| Drugs |
|
|
One of the earliest treatments for hypertension was that described by Pauli using the sedative and hypotensive properties of sodium thiocyanate to obtain relief from the subjective symptoms of hypertension. Another drug used for the treatment of malignant hypertension was sodium nitroprusside. In the 1930s, the beneficial effects of extracts of Rauwolfia serpentina were first described with its first clinical use in 1955. In the 1940s, sympathectomy was used to treat high blood pressure, often preceded by use of tetraethylammonium as a prognosticator to judge the likely success of the surgery, but was associated with a fairly high surgical mortality.
Real advances in management came with the introduction of ganglion blocking drugs (e.g., mecamylamine, pempidine, hexamethonium, and pentolinium) in the 1950s. Other drugs introduced over the next decade included reserpine (a rauwolfia alkaloid) and hydralazine. The combination of a ganglion blocker and general anesthesia resulted in the development of significant bradycardias and hypotension. Diuretics were also introduced for treatment of hypertension (initially chlorothiazide). The reduction in circulating fluid volume produced by natriuresis enhanced the hypotensive effect of ganglion blocking agents and reduced some side effects of drugs such as reserpine. These synergistic interactions between drugs allowed a smaller dose of each drug and hence a less severe side-effect profile.
In the late 1950s and 1960s, two new important classes of drugs were introduced—adrenergic neuron blockers and the first generation of β-adrenoceptor blockers (see Chapter 14 ).
The antihypertensive effect of adrenergic neuron blockers (bretylium being the first) depends on inhibition of transmitter release from adrenergic nerve terminals in response to sympathetic nerve impulses. This results in a decrease in cardiac output, decrease in peripheral vascular resistance, and increase in venous capacitance. The main side effects of neuron blockade was postural hypotension, exertional hypotension, and hypotension in hot environments (all due to a failure of sympathetically mediated circulatory control).
α-Methyl DOPA (a false transmitter), introduced in 1961, reduces blood pressure by inhibition of norepinephrine synthesis. A slowly developing antihypertensive effect develops after repeated administration of small doses that do not exert a significant pressor effect. It is not established whether its hypotensive action is due to a peripheral effect (from impaired neuroeffector transmission) or to a central effect (involving the norepinephrine synapses in the medulla oblongata).
First-generation monoamine oxidase inhibitors (MAOIs), such as pargyline, were introduced in 1963. The antihypertensive effect of MAOIs has a slow onset that persists for a considerable period after cessation of treatment. In time, tolerance develops, probably due to fluid accumulation. The underlying mechanism for their antihypertensive effect is probably due to a change in composition of the monoamine stores of adrenergic neurons with a relative increase in amines other than norepinephrine (in particular, dopamine).
Other centrally acting drugs introduced in the 1960s include the α 2 -adrenoceptor agonist clonidine and the second generation of adrenergic neuron blockers typified by bethanidine and debrisoquine. One of the first β-adrenergic blocking drugs introduced into clinical practice was pronethalol in 1962, followed by propranolol in 1965, alprenolol in 1967, and oxprenolol and practolol in 1968.
At the same time, a major controversy and debate developed regarding whether antihypertensive drugs should be withdrawn from patients before anesthesia and surgery because of their effects in obtunding the normal mechanisms of cardiovascular control. The value of maintaining therapy was emphasized in a series of controlled studies of treated and untreated hypertensive patients. Patients with well-controlled hypertension maintained on their drugs up to and including the morning of surgery experienced less cardiovascular instability than their untreated counterparts, especially in the postoperative period. Recent evidence suggests that withholding angiotensin converting enzyme inhibitors or angiotensin II receptor blockers before major noncardiac surgery reduces intraoperative hypotension and risk of death.
The main center for neurogenic control of vascular tone and hence blood pressure is the vasomotor center in the midbrain. This affects both the sympathetic ganglia and parasympathetic nerves ( Fig. 26.1 ). There are 2 different central neural control systems involved in blood pressure regulation: neurogenic control (as outlined previously) and arterial baroreceptor reflexes.
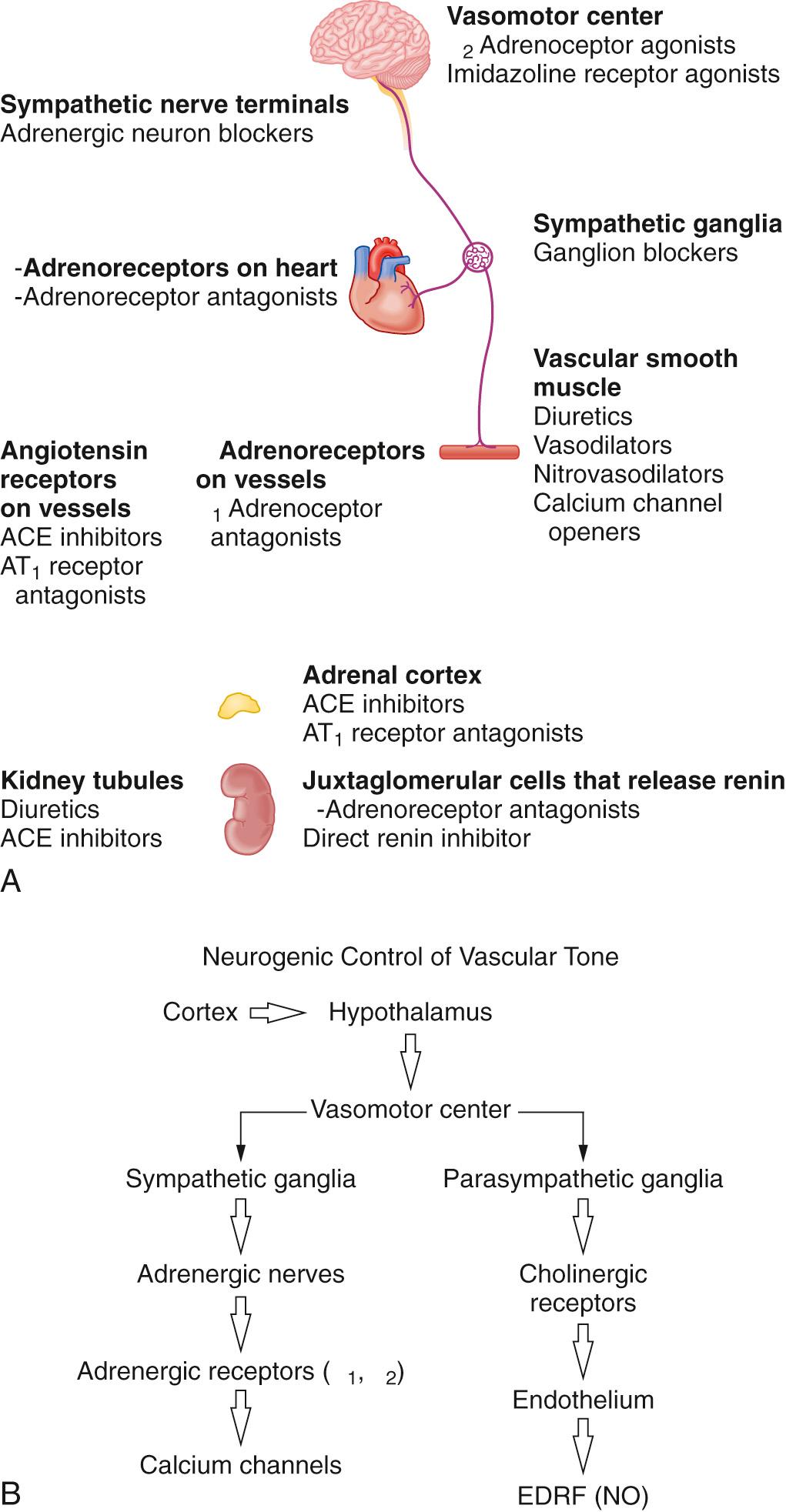
Other humoral and endocrine control systems include the renin-angiotensin-aldosterone system, atrial natriuretic peptides, renal control of Na + and water excretion, eicosanoids, the kallikrein-kinin system, adrenal steroids (both glucocorticoids and mineralocorticoids), the endothelin system, and medullipin in patients with hypertension secondary to uremia and renal failure.
There are 3 main mechanisms by which drugs act in treatment of high blood pressure: (1) effects on the autonomic nervous system, (2) inhibition of the renin-angiotensin-aldosterone system, and (3) peripheral vasodilation. Based on these mechanisms, there are a number of potential sites of action for antihypertensive agents ( Table 26.3 ).
|
Based on the 3 main antihypertensive mechanisms and the multiple sites at which drugs can act, there are different physiologic approaches to the reduction of blood pressure ( Table 26.4 ). Most drugs currently available do not act via a single mechanism. Within this physiologic approach to lowering blood pressure by vasodilation, the drugs available can be divided into 3 main subgroups ( Table 26.5 ). Most of these vasodilator drugs act either on the arterial or venous circulations, with very few acting on both ( Table 26.6 ).
| Decrease Systemic Vascular Resistance |
|
| Decrease Cardiac Output |
|
| Neurogenically Acting Hypotensive Agents |
|
| Neurogenically Acting Vasodilators Acting on Adrenergic Receptors |
|
| Other Vasodilators |
|
| Arteriolar | Both | Venodilators |
|---|---|---|
| Prazosin | Nitroprusside | Nitroglycerin |
| Phentolamine | ||
| Phenoxybenzamine | ||
| Tolazoline | ||
| Hydralazine | ||
| Diazoxide | ||
| Calcium channel blockers | ||
| Angiotensin-converting enzyme inhibitors | ||
These drugs have a direct dose-related effect on three separate functions of the heart (conduction, contraction, and inotropy), and on peripheral and coronary vascular tone. When used to treat high blood pressure, the major effect of these drugs is arterial vasodilation by blockade of Ca 2+ influx through L-type voltage-gated Ca 2+ channels in the smooth muscle cells of resistance vessels. They also reduce the increase in intracellular Ca 2+ in response to membrane depolarization and the secondary Ca 2+ -induced Ca 2+ release from intracellular stores. Some calcium channel blockers also reduce heart rate and myocardial contractility, which leads to decreased cardiac output and blood pressure.
There are 3 classes of calcium channel blockers. Difference in subunit structure of Ca 2+ channels in vascular and cardiac tissues might explain the different selectivity of the drugs. The dihydropyridines act mainly on vascular smooth muscle of resistance vessels, while the nondihydropyridines act on the myocardium. All 3 classes act on voltage-gated Ca 2+ channels that are made up of 5 subunits (α 1 , α 2 , β, γ, and δ). The α 2 and δ subunit dimer and γ subunit are transmembrane, while the β unit is located intracellularly. The α 1 subunit forms the transmembrane ion channel.
Dihydropyridines (e.g., nifedipine and nicardipine) bind to the extracellular domain of the L-type voltage-gated Ca 2+ channel (Ca V 1) in the cell membrane, thus inhibiting Ca 2+ influx. These drugs primarily affect resistance vessels with minimal effects on veins owing to the low density of L-type Ca 2+ channels in capacitance vessels.
Phenylalkylamines (e.g., verapamil) act mainly on the heart, where inhibition of voltage-gated Ca 2+ channels is frequency dependent. The phenylalkylamines probably enter the cell and bind internally to produce their effects.
Benzothiazepines (e.g., diltiazem) bind on the extracellular side of the L-type channel. Their effects are greater on vascular compared with cardiac smooth muscle.
There are at least 3 distinct subtypes of β receptors (β 1 , β 2 , and β 3 ), each of which has different tissue distributions. Both β 1 and β 2 agonists mediate their cellular effects through increases in cyclic adenosine monophosphate (cAMP). β receptors are G protein–coupled receptors that traverse the lipid cell membrane 7 times. The N-terminal end of the protein is extracellular and the C-terminal end is intracellular. The region conferring β-receptor coupling is thought to lie in the third cytoplasmic loop between the fifth and sixth transmembrane segments (for further details on this receptor, see Chapter 1 ).
β 1 receptors are present mainly in heart muscle. The ligand–receptor complex is coupled to a Gs protein, which, in turn, activates adenylyl cyclase to produce cAMP. The cAMP activates protein kinase A, leading to enhanced influx of Ca 2+ and, in turn, muscle contraction.
β 2 receptors exist in heart muscle (hence the rationale for use of salbutamol in some patients with low-output states). However, they are more abundant in bronchial and peripheral vascular smooth muscle, where they lead to bronchodilation and vasodilation, respectively (see Chapter 30 ).
β 3 receptors are present in adipose tissue and heart muscle. In contrast to β 1 and β 2 adrenoceptors, activation of β 3 leads to a decrease in inotropic state of the myocardium via a Gi protein–dependent pathway. Thus, as well as having an effect on thermogenesis, β 3 activation can lead to cardiodepressant effects.
Administration of some β blockers restores receptor responsiveness to catecholamines after β receptor desensitization, leading to normal GRK2 activity (G protein–coupled receptor kinase 2; also known as BARK1, or β adrenoceptor kinase 1) and receptor protein subunit G α1 levels. Other β blockers allow intracellular signaling to return toward normal despite concurrent catecholamine stimulation. A third class of β blockers (including metoprolol and carvedilol) have an additional property in reversing the effects of cardiac remodeling. These differences in action could explain why some β blockers protect myocytes more effectively than others and why all are not equally effective in reducing perioperative ischemia, myocardial infarction, and cardiac mortality.
β receptors can exist in 3 distinct activation states: stabilized and inactivated, nonstabilized and inactivated, or stabilized and activated. Normally, there is a dynamic equilibrium between the activated and the 2 inactivated states. Drugs that stabilize the receptor in the activated state are either partial or full agonists; drugs that push the equilibrium toward the inactivated state are either full or partial agonists; drugs that block access to the receptor binding site without affecting the state of equilibrium are termed neutral (competitive) antagonists (see Chapter 1 ).
At the molecular level, some β blockers incompletely stabilize the activated state of the receptor, leading to a coupling of the receptor to the G protein (these are therefore partial agonists—bucindolol, xamoterol). They block the effects of potent agonists while generating low-level β adrenoceptor stimulation on their own. Other β blockers stabilize the receptor in the inactivated state, thus leading to receptor upregulation rather than the desensitization and downregulation seen with xamoterol. Such drugs are inverse agonists (metoprolol, nebivolol, sotalol). However, most of the β blockers are neutral or competitive antagonists in that they bind to the β receptor without affecting its activation state.
These different molecular actions give rise to drugs with different clinical profiles. Thus, the β 1 selective blockers (acebutolol, atenolol, bisoprolol, celiprolol, esmolol, xamoterol) diminish detrimental cardiac remodeling while preserving the protective effects of β 2 activation. The β 1 , β 2 , and α 1 blockers (e.g., bucinodolol, carvedilol, labetalol) decrease arterial vascular tone and serve as effective afterload-reducing drugs; they also block the deleterious effects of sympathetic hyperactivity. The partial inverse agonists (metoprolol and nebivolol) might reduce β receptor desensitization while maintaining functional β receptor responsiveness.
β adrenoceptor blocking drugs act by competitive antagonism. Their action to reduce blood pressure is by both decreasing cardiac output (through their effects on contractility and heart rate) and by inhibiting renin release from the kidneys. There are several ways of classifying the pharmacologic effects of β adrenoceptor blockers, depending on their receptor specificity: the presence of either intrinsic sympathomimetic or vasodilator activity and their lipophilicity or hydrophilicity ( Table 26.7 ).
| Antagonists * | Partial Agonists (ISA) * |
|---|---|
| Selective | |
| Atenolol (1) | Acebutolol |
| Metoprolol (3) | Practolol |
| Esmolol (0.02), landiolol | |
| Bisoprolol | |
| Nonselective | |
| Propranolol (1) | Oxprenolol (1) |
| Timolol (10) | Alprenolol |
| Nadolol | Pindolol (20) |
| Sotalol (0.1) | |
* Number enclosed in parentheses indicates potency relative to propranolol at the β 1 receptor.
The β 1 selective agents —such as atenolol, bisoprolol, and metoprolol—are cardioselective, although this selectivity is reduced at higher doses. The pure β adrenoceptor blocking drugs do not cause vasodilation. These agents diminish the detrimental effects of myocardial remodeling while preserving the protective effects of β 2 activity.
Nonselective (β 1 and β 2 ) agents have similar effects to the selective agents on blood pressure.
Partial agonists (pindolol) result in less resting bradycardia and some peripheral vasodilation.
Vasodilator activity is also produced by drugs with antagonist action at α receptors (labetalol, celiprolol, carvedilol) or by agents that promote endothelial nitric oxide production via the L-arginine-NO (nitric oxide) pathway (nebivolol). The use of these drugs with vasodilatory properties can be advantageous when treating hypertension. One of the first drugs of this type (celiprolol) is formulated as a racemic mixture, is hydrophilic, and acts as a β 1 antagonist, mild β 2 agonist, and weak vasodilator. Celiprolol also causes an increase in myocardial inducible NO synthase activity.
Renin is a protease synthesized in the kidney that catalyzes the production of angiotensin I from angiotensinogen (a 225 amino acid prohormone). Angiotensin-converting enzyme (ACE) then biotransforms angiotensin I to angiotensin II—the latter being a vasoconstrictor through action at angiotensin receptors. The molecular mechanism of action for angiotensin II is through coupling to the phospholipase C Gq protein–IP 3 transduction pathway. Angiotensin II also affects norepinephrine kinetics through facilitation of its release and prevention of its reuptake at sympathetic nerve terminals. The renin–angiotensin pathway can therefore be interrupted at two separate sites (both leading to vasodilation): direct inhibition of ACE and angiotensin receptor antagonism ( Fig. 26.2 ).
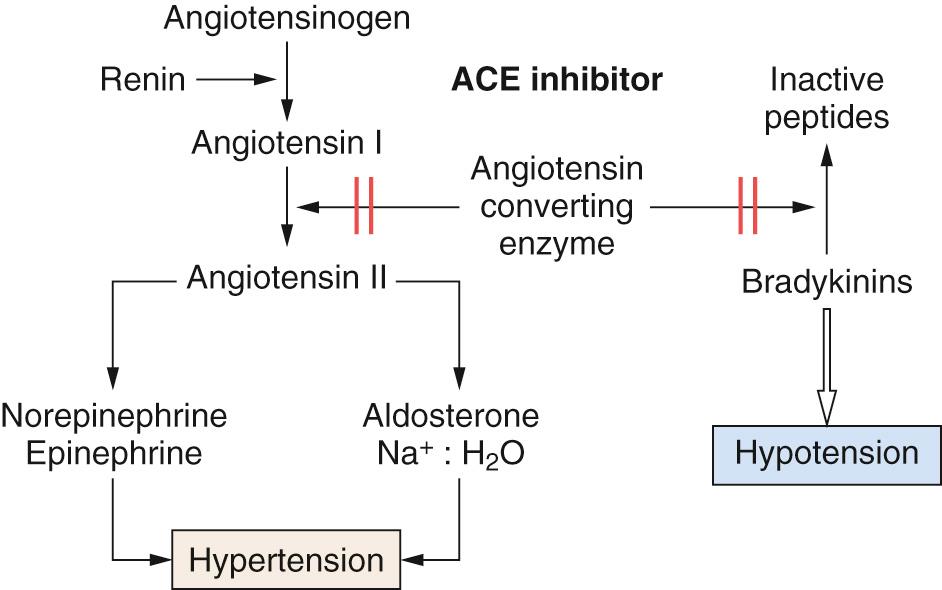
ACE inhibition occurs both in plasma and in the vascular endothelium. Inhibition also reverses arteriolar hypertrophy that occurs in hypertension and reduces left ventricular hypertrophy. ACE inhibitors decrease conversion of angiotensin I to angiotensin II, thereby reducing vasoconstrictor effects, aldosterone secretion, and sympathetic activation (see Chapter 40 ). Angiotensin II acts on AT 1 receptors via Gq to activate phospholipase C. This has the effect of increasing intracellular diacyl glycerol (DAG) and IP 3 , which increase Ca 2+ release from the sarcoplasmic reticulum (SR) to cause vasoconstriction of the efferent arteriole. Stimulation of the AT 2 receptor leads to smooth muscle proliferation and ventricular remodeling ( Fig. 26.3 ).
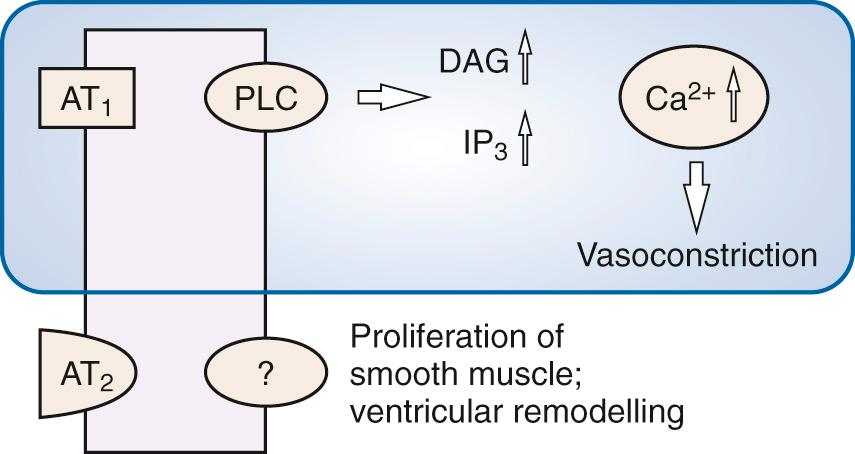
Both ACE inhibitors and AT receptor antagonists produce arterial vasodilation by limiting the direct effects of angiotensin II on vascular smooth muscle and minimizing its ability to increase sympathetic vascular tone. Their action is accompanied by an increase in parasympathetic tone. They also decrease the effect of aldosterone on the distal convoluted tubule of the nephron, thus promoting salt and water loss. They also block breakdown of bradykinin, which, in turn, contributes to the vasodilator effects of the drugs.
ACE inhibitors prevent the breakdown of kinins; the increased concentration of bradykinin is probably responsible for the unwanted side effects of cough and allergic reactions. Other side effects of ACE inhibitors include diarrhea. On the other hand, the angiotensin receptor antagonists prevent the vasoconstrictor effects of angiotensin II without affecting ACE activity. Thus, in contrast to ACE inhibitors, they do not affect kinin production (hence, cough is not a prominent side effect of these drugs). These drugs act as selective blockers at the AT 1 receptors, with no effect on AT 2 receptors.
The action of diuretics to reduce blood pressure is twofold. First, they cause an initial decrease in intravascular volume, although compensatory mechanisms are activated and this effect is reduced over time. They have a secondary mechanism to reduce blood pressure by direct arterial vasodilation. The exact mechanism involved is not clear but might be through a decrease in Ca 2+ entry into smooth muscle cells and through stimulating local vasodilator prostaglandin formation. Although the decrease in intravascular volume and total body Na + occurs within weeks of the start of diuretic therapy, an increase in renin release causes them to return to normal. The antihypertensive effects of diuretics are not just related to their effects on Na + and water balance, because more effective diuretic agents (e.g., furosemide) are not very effective hypotensive agents.
There are 3 main classes of diuretics (see Chapter 42 ):
Thiazides inhibit Na + /Cl − co-transporters in the proximal diluting segment of the distal collecting tubule and early collecting duct. The diuretic effect of the thiazides (except metolazone and indapamide) is less effective in patients with renal impairment. If used long term, thiazides inhibit maximal reabsorption of Na + to about 3% to 5% of the filtered load.
Loop diuretics have a short duration of action and hence are rarely used as first-line drugs for treatment of hypertension. They act on the medullary and cortical thick ascending limb segments of the nephron to prevent Na + , Cl − , and water reabsorption. There is some debate as to whether they also have other actions, such as systemic vasodilation and a reduction in left ventricular filling pressure secondary to preload reduction due to venodilation. However, they are useful if volume expansion contributes in part to the etiology of the hypertension (in patients with renal failure or from use of vasodilator drugs).
Of the potassium-sparing diuretics, both amiloride and triamterene have weak antihypertensive effects. However, spironolactone (an aldosterone antagonist) can be useful as an antihypertensive treatment in primary or secondary hyperaldosteronism or if the hypertension is treatment resistant. This class of diuretics has relatively weak natriuretic effects, with a maximal excretion of only about 1% to 2% of filtered Na + load.
Most centrally acting antihypertensive drugs are no longer widely used. However, identifying their sites of action illustrates the diversity of approaches used to reduce blood pressure.
Reserpine acts to deplete neuronal stores of catecholamines both centrally and peripherally at the postganglionic neuron. Its mechanism of action is related to inhibition of norepinephrine and dopamine uptake into terminal synaptic vesicles, leading to increased breakdown of norepinephrine and reduced conversion of dopamine to norepinephrine. Although reserpine crosses the blood-brain barrier, this is probably not the site of its antihypertensive effect.
Bethanidine, guanethidine, and debrisoquin are selective postganglionic neuron blockers that act to inhibit release of norepinephrine from peripheral sympathetic nerve terminals. Although the exact mechanism of action of these drugs is not clear, there is evidence that the drugs must be actively taken up into neurons, where they accumulate in synaptic vesicles, causing norepinephrine depletion. Their antihypertensive effects are through reduced adrenergic synaptic transmission.
α 2 Receptor agonists have several distinct pharmacologic effects—sedation, analgesia, and hypotension. The α 2 adrenoceptor is coupled to a Gi protein complex, which inhibits adenylyl cyclase and Ca 2+ and K + ion channels. The net result of α 2 agonist binding is reduced cAMP production, hyperpolarization, and decreased intracellular Ca 2+ . α 2 Receptors occur both presynaptically and postsynaptically. Stimulation of presynaptic α 2 receptor decreases central sympathetic outflow. Agonist stimulation of postsynaptic α 2 receptors mediates the sedative and analgesic effects.
Some of the earliest antihypertensive therapies were based on drugs acting on the control mechanisms in the central nervous system (CNS). There are α 2 adrenoceptors in the locus coeruleus in the floor of the fourth ventricle, where α 2 agonists act to cause sedation. The locus coeruleus also has afferent connections from the rostral ventrolateral medullary nuclei and efferent connections to noradrenergic fibers connecting to the thalamus and elsewhere. The α 2 agonists also block salivary gland secretion. Effects in the nucleus tractus solitarius lead to inhibition of sympathetic activity and reduce peripheral vasoconstriction. These drugs are no longer widely used in the treatment of hypertension, with the exception of α-methyl DOPA for treatment of preeclampsia of pregnancy.
Excessive hypotension secondary to α 2 agonist therapy can be reversed by vasopressors and inotropes. The use of ephedrine, phenylephrine, and dobutamine, but not norepinephrine, can be accompanied by an enhanced pressor response due to the β effects of these agents.
There are three main classes of α 2 agonists: (1) the phenylethylates (α-methyl DOPA, guanabenz), (2) the imidazolines (clonidine, dexmedetomidine, mivazerol), and (3) the oxaloazepines.
α-Methyl DOPA is converted in noradrenergic neurons to the false transmitter α-methyl norepinephrine, which is a potent α 2 agonist. It stimulates brainstem postsynaptic α 2 receptors to decrease sympathetic tone and systemic vascular resistance.
Clonidine acts as a presynaptic α 2 agonist on receptors in the brainstem, leading to reduction in sympathetic outflow and an increase in vagal activity. After intravenous dosing, there can be an initial transient hypertensive response due to peripheral vasoconstriction through α 1 and α 2B interactions. In contrast to α 2 agonists, such as α-methyl DOPA, imidazoline compounds produce hypotension following direct intramedullary injection. This led to the proposal of the presence of nonadrenergic receptors in the lateral reticular nuclei of the ventrolateral medulla (VLM). Two types of nonadrenergic imidazoline receptors have been identified: I 1 receptors in the brain and I 2 receptors in the brain, pancreas, and kidney. A number of the α 2 agonists also have activity at I 1 receptors. It is likely that they mediate their effects through a G protein–coupled mechanism that results in an increase in arachidonic acid and the subsequent release of prostaglandins. This led to the development of two specific I 2 receptor agonists with more ideal hemodynamic profiles: monoxidine and rilmenidine. Both drugs have a weaker affinity for central α adrenoreceptors than α-methyl DOPA and clonidine. Other α 2 agonists include dexmedetomidine, which has a greater selectivity and specificity for α 2 receptors than clonidine (α 2 : α 1 1620 : 1 compared with 200 : 1); and mivazerol (119 : 1; Fig. 26.4 ). This latter sympatholytic drug has been examined for a role in myocardial protection, but there are no published studies examining its use in the treatment of perioperative hypertension.
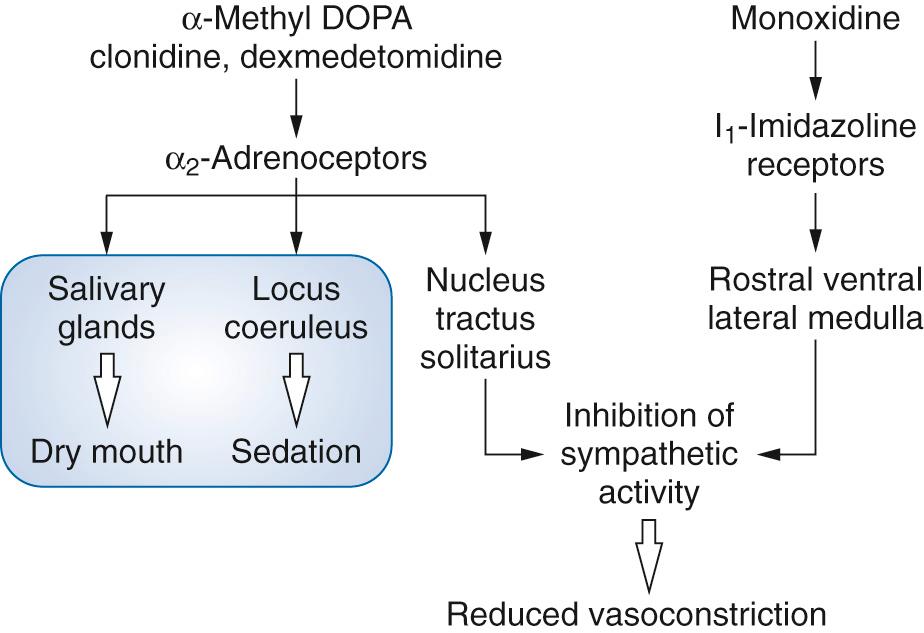
Monoxidine is a selective imidazoline receptor agonist that stimulates I 1 receptors in the VLM, thereby decreasing sympathetic outflow and lowering blood pressure. In contrast to some other drugs, its use is not associated with a reflex tachycardia.
Agonists of the α 1 adrenoreceptor leads to an increased intracellular Ca 2+ . The α 1 agonist–receptor interaction leads to activation of phospholipase C (linked to Gq), which increases hydrolysis of phosphatidylinositol bisphosphate (PIP 2 ) and increases synthesis of the second messengers DAG and 1,4,5-inositol trisphosphate (IP 3 ). DAG activates protein kinase C while IP 3 increases the intracellular Ca 2+ concentration by releasing Ca 2+ from the SR. There is also evidence that IP 3 causes an increase in the Ca 2+ sensitivity of the contractile proteins. The phosphorylated product of IP 3 (IP 4 —1,3,4,5-inositol tetraphosphate) has been suggested as the messenger that enhances Ca 2+ flux across the cell membrane, causing vasoconstriction.
The α 1 selective antagonists block α 1 receptors to prevent Ca 2+ increases, thus leading to smooth muscle relaxation in arterioles and venous capacitance vessels.
There are two types of α 1 antagonists:
Selective α 1 antagonists: doxazosin, terazosin, prazosin, and indoramin
Nonselective α 1 antagonists: phentolamine and phenoxybenzamine
While the selective antagonists act in a competitive manner, phenoxybenzamine combines irreversibly with the α 1 receptors and so has a prolonged duration of effect. Phentolamine is a short-acting, reversible antagonist.
NO was identified as the “endothelium-dependent relaxing factor” by Furchgott and Zawadski in 1980 and its mechanism was defined by Palmer and colleagues. NO is an endogenous vasodilator that causes renal vasodilatation, leading to a diuresis and natriuresis and resulting in reduced blood pressure. There is some suggestion that deficiency in NO could contribute to the etiology of high blood pressure.
NO stimulates guanylyl cyclase to produce cyclic guanosine monophosphate (cGMP), which activates cGMP-dependent protein kinase (PKG). PKG then activates myosin light chain phosphatase (MLCP) to dephosphorylate myosin light chains and hence produce vascular relaxation. NO also activates sarcoplasmic and endoplasmic reticular Ca 2+ -ATPase to enhance reuptake of Ca 2+ , an effect that is cGMP independent. NO might also activate K + channels, resulting in membrane hyperpolarization, thus closing voltage-gated Ca 2+ channels ( Fig. 26.5 ).
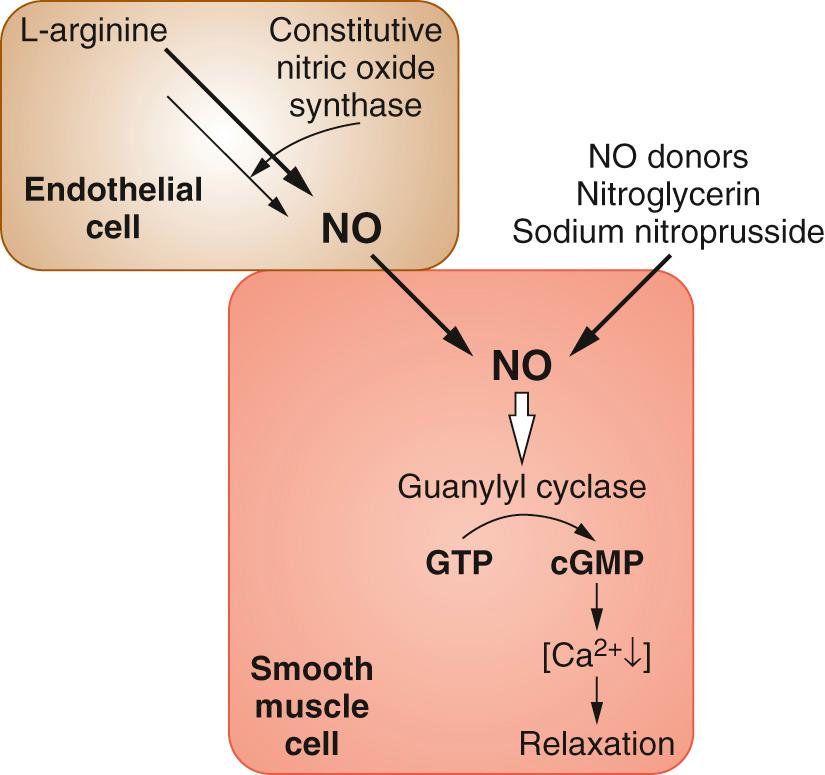
There are a number of NO donors. Some vasodilators release NO spontaneously (sodium nitroprusside) while others (organic nitrates such as nitroglycerin) need active reduction of the nitrate radical by intracellular sulfhydryl groups. All NO donors are potentiated by phosphodiesterase-5 inhibitors that reduce hydrolysis of cGMP (see later discussion).
Nitrites act as both arterial and venous dilators. They are usually associated with a reflex increase in heart rate; hence they are often coadministered with β blockers.
Sodium nitroprusside (SNP) is broken down in red cells with liberation of cyanide and NO. SNP has a rapid onset of action and is of short duration (1–2 minutes). While breaking down to form NO, SNP interacts with oxyhemoglobin to form cyan-methemoglobin and cyanide. The latter is converted to thiocyanate through transsulfation by the liver enzyme rhodanase, with thiosulfate acting as the sulphur donor. There are still issues over the total safe dose of SNP. Prolonged administration leads to accumulation of thiocyanate and cyanide, with the development of venous hyperoxemia and metabolic acidosis.
Nitrates undergo denitration to produce NO, their active component. Denitration can involve reaction with sulfhydryl groups or depends on the action of glutathione-S-transferase, cytochrome P450, and xanthine oxidoreductase. A stepwise increase in the dose of infused nitrate leads to a reduction in venous tone and central venous pressure. This is accompanied by a gradual decrease in systemic pressure. Administration of bolus doses of nitrates causes significant and immediate decreases in arterial pressure.
Glyceryl trinitrate (GTN), or nitroglycerin, acts as an NO donor, with a greater action on venous rather than arteriolar vessels. Its use can lead to tachyphylaxis due to a decrease in NO formation through generation of superoxide and peroxynitrite free radicals that inhibit aldehyde dehydrogenase. Without this enzyme, the conversion of organic nitrates and nitroglycerin to NO is impaired. In addition to decreasing blood pressure, nitroglycerin also reduces preload and myocardial oxygen demand. Nitroglycerin is a coronary vasodilator (including relaxation of stenosed vessels) and brings about an increased collateral coronary flow, improved subendocardial perfusion, and improved diastolic function. Because nitroglycerin undergoes extensive first-pass metabolism after oral dosing, nitrates are administered orally as isosorbide dinitrate (ISDN) and its metabolite isosorbide mononitrate (ISMN). Although the main use of nitroglycerin, ISDN, and ISMN is for antiangina therapy, they can also be used by titration for the control of blood pressure in the cardiac patient.
Adenosine stimulates purinergic receptors (P 1 and P 2 ) on endothelial cells to stimulate NO production. Adenosine also has direct effects on smooth muscle via a G protein–coupled pathway and activates ATP-sensitive K + channels, leading to membrane hyperpolarization and relaxation. Adenosine receptor blockers prevent Ca 2+ entry during phase 1 of the action potential, thus causing vascular relaxation. Although predominantly a coronary vasodilator, it can decrease blood pressure. It is not absorbed from the gut; thus, it is given by the intravenous route. The half-life after intravenous dosing is less than 10 seconds. It is intracellularly phosphorylated to AMP and then deaminated to inosine, hypoxanthine, xanthine, and uric acid. Both theophylline and other xanthines are antagonists at both P 1 and P 2 receptors; hence, their concurrent use increases dose requirements for adenosine. The uptake of adenosine into coronary endothelial cells is inhibited by dipyridamole.
Minoxidil opens ATP-sensitive K + channels in vascular smooth muscle, hyperpolarizes the cell membrane, and closes voltage-gated Ca 2+ channels, leading to smooth muscle relaxation and vasodilation.
Hydralazine acts by promoting the efflux of K + from vascular smooth muscle cells, thereby causing hyperpolarization and muscle relaxation. It can also cause arterial vasodilation by promoting smooth muscle relaxation through activation of guanylyl cyclase, leading to an increase in cGMP. Other mechanisms of action include inhibition of Ca 2+ release from the SR, reduction in Ca 2+ stores in the SR, and stimulation of NO formation by the vascular endothelium.
Diazoxide is chemically similar to thiazide diuretics but causes Na + retention. It is a powerful antihypertensive agent due to peripheral vasodilation secondary to opening K + channels.
Nicorandil promotes vasodilation of both systemic and coronary arteries by opening ATP-sensitive K + channels, hyperpolarization of the membrane, and reduced opening of voltage-gated Ca 2+ channels and therefore vascular smooth muscle relaxation. This is enhanced by the local production of NO from a nitrate-like action that also causes venodilation.
Ganglionic blockers formerly played a significant role in the modulation of blood pressure. Now, only trimethaphan is used (rarely) in hypertensive emergencies or for the production of controlled hypotension during surgery. Ganglionic blockers act by inhibiting autonomic activity at both sympathetic and parasympathetic ganglia. The decrease in sympathetic outflow causes vasodilation.
Magnesium affects vascular tone by diverse mechanisms; it acts as a Ca 2+ channel antagonist, stimulates production of vasodilator prostaglandins and NO, and alters the response of vascular endothelium to vasoactive agonists.
Endothelin receptor antagonists reduce blood pressure by blocking the actions of endothelins. Endothelins act on both ET A and ET B receptors. Endothelin 1 (ET-1) causes vasoconstriction through activation of both types of receptor on vascular smooth muscle cells. The receptors are coupled to Gq, with activation leading to increased synthesis and release of IP 3 and Ca 2+ release from the SR. ET-1 synthesis and release is stimulated by a number of mediators: angiotensin II, arginine vasopressin, thrombin, cytokines, reactive oxygen species, and shear forces on the endothelium. Mixed ET receptor antagonists lower arterial blood pressure and prevent vascular and myocardial hypertrophy. Use of these drugs has also been studied in the treatment of pulmonary hypertension.
Peripheral dopamine (DA) agonists cause selective vasodilation of a number of vascular beds (splanchnic, cerebral, and coronary circulations) and can therefore be used in the treatment of hypertension. There are two subtypes of peripheral DA receptors. DA 1 receptors are postsynaptic Gs protein–coupled receptors and cause vasodilation following increased intracellular cAMP and reduced intracellular calcium. DA 2 receptors are presynaptic and coupled to Gi protein, leading to reduced cAMP, with membrane hyperpolarization and reduced norepinephrine release. The effects of dopamine on various catecholamine receptors are dose dependent: DA 1 interactions occur at low doses, β 1 at moderate doses, and α 1 with high doses (see Chapter 25 ).
Fenoldopam is a selective DA 1 receptor agonist without adrenergic receptor agonist properties. It is more potent than dopamine at DA 1 receptors and is useful for control of blood pressure. It also has no agonist effects at the DA 2 receptor. As such, it causes a decrease in blood pressure without increases in heart rate or contractility due to adrenergic effects. An advantage of the drug is that it does not cross the blood-brain barrier. It is available in the United States and some European countries, where it is recommended for acute or emergency control of blood pressure in the perioperative period.
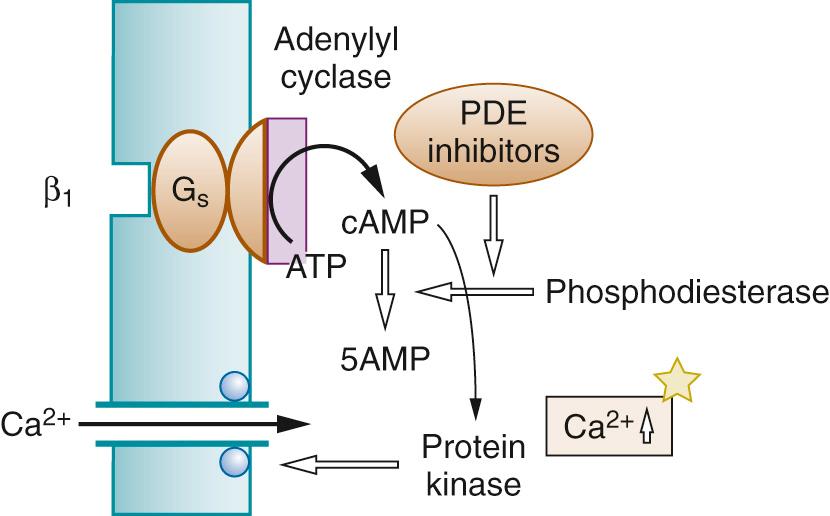
Phosphodiesterases catalyze the hydrolysis of cyclic nucleotides. Phosphodiesterase inhibitors (PDEIs) block the hydrolysis of cAMP or cGMP, prolonging the effects on smooth muscle of signaling pathways involving these second messengers. An increase in cAMP (or cGMP) leads to phosphorylation of myosin light chain kinase, leading to a reduction of its activity due to reduced affinity for the Ca 2+ -calmodulin complex. The resulting reduced phosphorylation of myosin light chains leads to smooth muscle relaxation and vasodilatation. There are five subtypes of phosphodiesterase; PDE3 is inhibited by milrinone and enoximone, and PDE5 is inhibited by sildenafil and related compounds (see later discussion).
PDE3 inhibition prevents the breakdown of cAMP. Specific phosphodiesterases also hydrolyze cGMP, which might contribute to the vasodilator effect of PDE5 inhibitors. As a result of the inhibition of cyclic nucleotide breakdown, PDEIs cause a decrease in systemic vascular resistance and a small but significant fall in arterial pressure ( Fig. 26.6 ).
Prostaglandins PGE 1 and PGI 2 (prostacyclin) have direct vasodilator actions via prostanoid receptors on vascular smooth muscle involving stimulation of cAMP. Although they decrease systemic vascular resistance (and, therefore, blood pressure), their main effect is on pulmonary vascular resistance (see later discussion).
A number of other drugs are also vasodilators. These include ganglion-blocking neuromuscular blocking drugs (tubocurarine derivatives), general anesthetics, and histamine.
All calcium channel blockers are effective in reducing blood pressure, although there is some controversy over the adverse effects of short-acting drugs. In general, they are all well tolerated and are becoming the first-line treatment for patients older than 55 years. The actions of the three classes of calcium channel blocking drugs differ. The relationship between vasodilation and negative inotropy is about 1 : 1 for both diltiazem and verapamil, 10 : 1 for nifedipine, and up to 1000 : 1 for the newer dihydropyridines. In the same way, the side effects of calcium channel blockers differ according to the class of drug.
Verapamil (a phenylalkylamine) is a racemic mixture that is well absorbed orally and has high presystemic metabolism (about 85%; L-enantiomer > D-enantiomer). Metabolism is extensive by O-demethylation (25%) and N-dealkylation (40%) followed by conjugation. Some of the metabolites are active, especially norverapamil, which has about one-fifth to one-tenth the activity of the parent drug. Verapamil has a half-life of about 5 hours (range, 2–7 hours), which can be prolonged in patients with liver disease. The clearance of L-verapamil is greater than that of the D-enantiomer, although the half-lives of the 2 enantiomers are similar. There is greater free fraction of L-verapamil; hence, it has a greater volume of distribution. Verapamil is excreted in the urine (70%; as metabolites and unchanged drug) and feces.
Diltiazem (a benzothiazepine) is well absorbed orally (> 90%), but has a smaller fraction undergoing presystemic metabolism (about 50%). Diltiazem is extensively metabolized, and some of these metabolites are active.
All dihydropyridines (nifedipine, amlodipine, felodipine, isradipine, nicardipine, lacidipine, lercanidipine, nisoldipine) are well absorbed by the oral route (> 90%), but because of their significant presystemic metabolism, sublingual dosing is preferable for some (e.g., nifedipine). Presystemic metabolism of nifedipine results in inactive metabolites with half-lives of about 9 hours, while that of the parent drug is about 2 to 6 hours, with plasma protein binding of more than 90% and a small volume of distribution (0.3–1.2 L/kg). Metabolites are excreted in both the urine (80%) and feces.
Chemical substitutions in nifedipine at the R1 position, with introduction of long side chains (as amlodipine), produce drugs with a longer duration of action and a half-life of up to 30 to 40 hours (felodipine 12–36 hours; isradipine 2–8 hours; nicardipine 3–12 hours; amlodipine 35–60 hours; Fig. 26.7 ). The dihydropyridines are metabolized by hepatic cytochrome P450 (mainly CYP 3A4). Hence, there are many examples of drug–drug interactions either with CYP 3A4 inhibitors (the -azoles, -avirs, erythromycin, clarithromycin) or with other drugs competing for metabolism by the same isoform (e.g., midazolam, alfentanil, cyclosporin). Nifedipine shows a bimodal polymorphism of metabolism by CYP 3A4/5.
The calcium channel blockers all demonstrate age-related kinetics due to reduction in cardiac output (and liver blood flow), leading to increased bioavailability and decreased systemic clearance. Other side effects of dihydropyridine include vasodilation with flushing, headaches, ankle edema, and reflex tachycardia. The use of calcium channel blockers is contraindicated in patients with heart failure owing to their negative inotropic effects (especially the nondihydropyridines) and slowing of sinoatrial and atrioventricular node conduction, leading to bradycardia and heart block. Pronounced antihypertensive effects are mainly observed with the dihydropyridines. The decrease in blood pressure is accompanied by reflex tachycardia and increased myocardial oxygen utilization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here