Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Antibiotics provide a backbone for modern interventional medicine, in which surgery, central venous catheters, urinary catheters, implantable devices, and mechanical ventilation have become commonplace. These interventions support seriously ill patients, but they also give bacteria access to normally sterile areas. Therapies for cancer and immune-mediated disease often leave patients severely immunosuppressed. Bacterial infections in such patients are serious, and antibiotics provide critical life-saving support for such patients.
Unfortunately, overuse and poor use of antibiotics have allowed many organisms to develop antibacterial resistance ( Chapter 261 ). Multiresistant Staphylococcus aureus has become a plague both in the hospital and in the community. Extended-spectrum β-lactamases and Klebsiella pneumoniae carbapenemase enzymes have mediated resistance to many of the most potent and broad-spectrum β-lactam agents, including the carbapenems. Consequently, it is important to understand the principles of antibacterial chemotherapy to obtain the best clinical outcomes for the patient at hand, but also, in a broader sense, to lower the probability of the emergence of resistance so as to maintain the potency of the drugs currently in the therapeutic armamentarium. In this context, the significant decline in the use of antibiotics in the United States in recent years is a good sign.
As in all of clinical medicine, a thorough history and physical examination are central to proper decision making and to achieving optimal therapeutic outcomes in patients with infections. Key to choosing the correct drug, dose, and schedule of antibiotic administration is recognition that an infection exists. The next steps are to document where the infection exists, and then to identify the dominant organisms present at each site of infection. The final step is to determine whether any risk factors might predict the presence of drug-resistant pathogens.
As an example, it is known that community-associated pneumonia ( Chapter 85 ) is caused by certain traditional bacterial pathogens. Streptococcus pneumoniae ( Chapter 268 ), Haemophilus influenzae ( Chapter 277 ), and Moraxella catarrhalis are among the classic bacterial pathogens associated with this entity ( Table 266-1 ). “Atypical” pathogens such as Legionella species ( Chapter 290 ), Mycoplasma pneumoniae ( Chapter 293 ), and Chlamydophila pneumoniae ( Chapter 294 ) also may be seen. In contrast, intra-abdominal infections are dominated by Escherichia coli ( Chapter 280 ) and other Enterobacteriaceae ( Chapter 281 ), and anaerobic organisms such as Bacteroides species. Consequently, it is important to understand the dominant pathogens present at different infection sites so that the best drug or combination of drugs can be chosen to treat the infection.
| SITE | BACTERIA |
|---|---|
| Complicated skin or skin structure infection | Staphylococcus aureus and Streptococcus spp |
| Diabetic foot ulcer | Organisms above plus Enterobacteriaceae |
| Intra-abdominal infections | Escherichia coli and other Enterobacteriaceae plus anaerobes |
| Community-acquired bacterial pneumonia | Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis , “atypical” pathogens |
| Hospital-acquired pneumonia | S. aureus, Klebsiella pneumoniae, Enterobacter spp, Pseudomonas aeruginosa, Acinetobacter spp |
| Meningitis | S. pneumoniae, H. influenzae (nontypable), meningococci; in hospital settings, S. aureus and gram-negatives |
| Urinary tract infections | Enterobacteriaceae, particularly in sexually active women; multiresistant gram-negatives in patients with complicated urinary tract infections or those instrumented; enterococci, particularly in elderly men |
| Prostatitis | Enterobacteriaceae, enterococci, atypical pathogens |
Knowing the site of infection is also critical because drugs penetrate differently into different body sites. Classically, penetration is poorer into spaces where there are tight junctions, such as the central nervous system, the eye, and the prostate. In general, the penetration of many classes of antibacterial agents is good into complicated skin and skin structure infection sites. What is often not appreciated is the divergent penetrations of different agents and even agents within the same class into the lung to treat bacterial pneumonia. For instance, the penetration by macrolide antibiotics into skin infection sites is modest, but their penetration into the lung is good, with penetration ratios (area under the concentration-time curve [AUC] in lung epithelial lining fluid/AUC in plasma) ranging from 4 to 20. The penetration of β-lactam drugs can range from 15 to 100%, but no set of variables explains such a range of penetrations.
It is also important to understand other factors that increase the probability that a resistant organism is present at the primary infection site. An example is a patient who has recently taken antibiotics before acquiring the present infection. Other examples are the acquisition of an infection in the hospital or in an extended care facility or a patient who is immunosuppressed. In such patients, the choice of antimicrobial agents must be careful to cover more resistant pathogens.
Once the site of infection has been definitively identified or the most likely source of infection has been determined, it is critical to obtain microbial specimens from that site as well as to obtain blood culture samples. Increasingly, molecular testing is available to provide rapid diagnosis of certain bacterial infections from some sites. Nevertheless, the correct performance and interpretation of a Gram stain remains an important component for the diagnosis of many infections. Even though it is usually not possible to make a definitive diagnosis on the basis of the cellular morphology of the organism, it is possible to combine information on the morphology, the organism’s gram positivity or negativity, the site of the infection, and the most likely pathogens to make an initial antimicrobial choice with the highest probability of producing effective chemotherapy.
The initial antibiotic regimen chosen to cover organisms present at the primary infection site has a significant influence on the outcome of therapy, and such initial choices should always err on the side of caution. When the specific microorganism has been identified and even before antimicrobial susceptibility testing results are available, the chemotherapy usually can be streamlined.
Because infections occur at a specific place, it is important to have an understanding of the local antimicrobial susceptibility patterns. Oftentimes, these susceptibility patterns are different even within the same hospital. For example, patients on a general inpatient unit are generally (but not always) infected with pathogens derived from the community setting, whereas patients in an intensive care unit (ICU) are more likely to have hospital-acquired infections. In severely infected patients, particularly patients whose infections were acquired in the ICU, infections are likely to be resistant to multiple antimicrobial agents.
Antimicrobial susceptibility testing can determine the minimal inhibitory concentration (MIC), or can tell whether an organism is susceptible (S), intermediate (I), or resistant (R) to an antibiotic. The MIC is often misunderstood as the concentration of drug that prevents the pathogen from growing, but it is actually the concentration of drug that allows a tube (or well) containing the pathogen to remain clear by visual examination after 16 to 24 hours. This criterion can actually allow almost a 1 log 10 (colony forming units [CFU]/mL) increase in bacterial count over this time frame and still be read as the MIC. With all its limitations, the MIC provides critical information about drug choice. For several infections, such as meningitis, endocarditis, and perhaps bacteremia, knowing the minimal bactericidal concentration (defined as the concentration required to kill 99.9% of organisms during 16 to 24 hours) is valuable. In these cases, it is important to obtain multi-log killing of the organism to ensure a high probability of a good clinical outcome. For meningitis and endocarditis, there is also the confounding problem of penetration of the drug into the primary infection site. In other circumstances (e.g., ventilator-associated pneumonia) in which bacterial burdens are high, optimizing the probability of cure requires highly bactericidal therapy.
The correct drug dose is the one that produces a high likelihood of achieving a good clinical response with a low probability of causing a concentration-driven adverse event. Another consideration is that the optimal dose should have a high probability of suppressing the emergence of resistant mutants. Confidence that a drug dose has a high likelihood of producing a good clinical outcome requires an understanding of the relationship between drug exposure and outcome, i.e., pharmacodynamics ( Chapter 25 ).
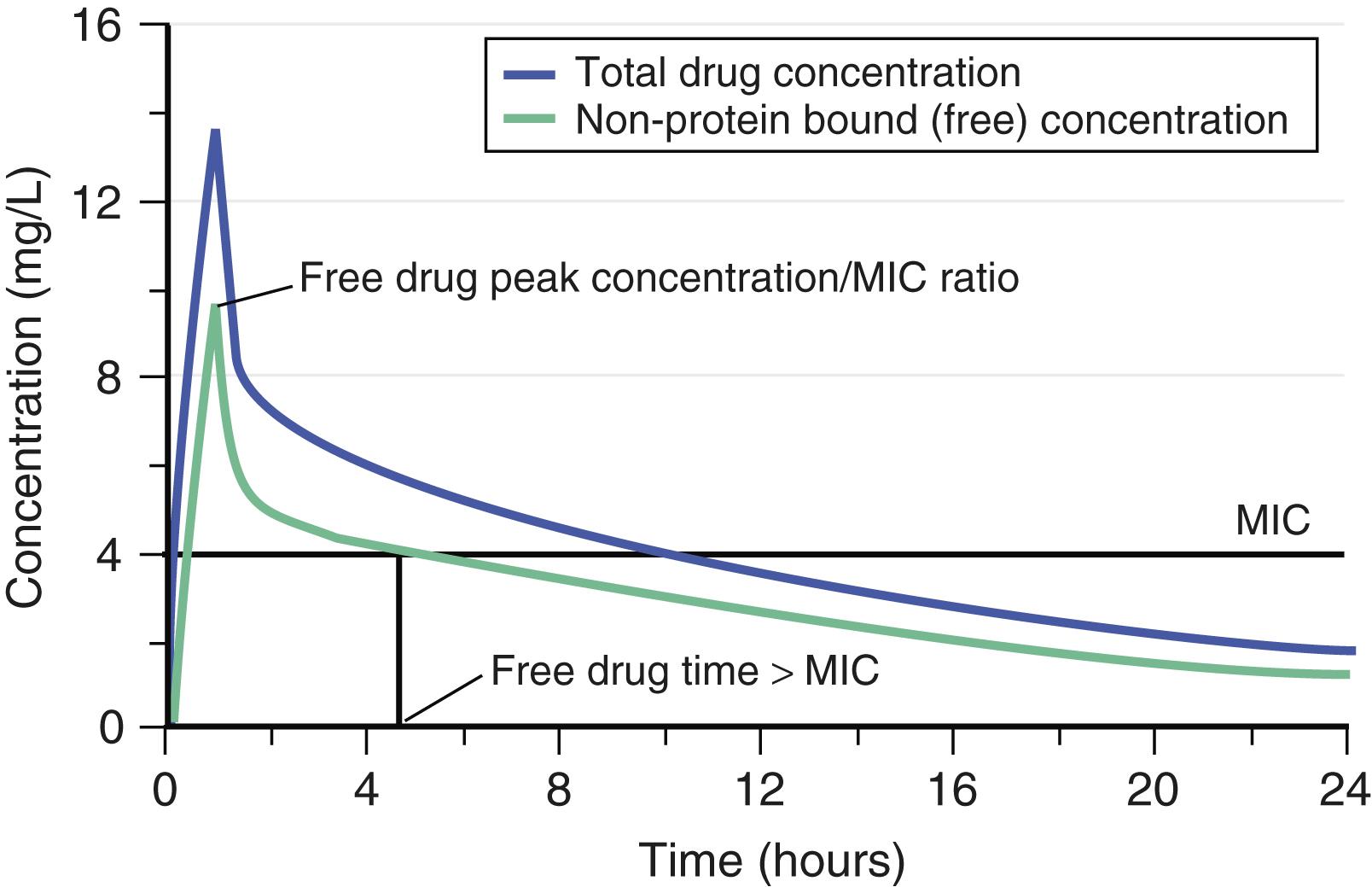
When a drug is administered intravenously or orally, the drug concentration starts low, increases to a maximal value, then declines over time until another dose of the drug is given (in a multidose regimen). This drug concentration–time profile should be seen relative to a measure of drug potency against the pathogen in question: the MIC (or minimal bactericidal concentration). Protein binding is also important because, in almost all cases, it is the free or non–protein-bound drug that kills the causative pathogen. So understanding the pharmacodynamics of a drug or class of agents first requires knowledge of the drug’s concentration–time profile, protein binding, and MIC ( Fig. 266-1 ). For drugs that kill organisms much faster as their concentrations rise (e.g., fluoroquinolones, aminoglycosides), the free drug AUC/MIC ratio is most closely linked to drug effect. For other drugs (e.g., penicillins, carbapenems, cephalosporins, monobactams), the rate of kill rises with the concentration and plateaus quickly, in which case the free drug time greater than MIC (T > MIC) is most closely linked to drug effect. On occasion, the free drug peak concentration/MIC ratio is linked to drug effect, as is seen when there is a rapid and frequent emergence of resistance, and the peak concentration helps suppress the amplification of resistant mutant subpopulations.
Antimicrobial resistance is a growing problem in both the hospital and outpatient settings. , If the drug is properly chosen, the organism should have a high probability of being susceptible to it. However, during the course of therapy, the organism has many mechanisms available to make it less susceptible (the MIC increases) to the administered drug, thereby making the drug less effective than was originally envisioned and resulting in a lower probability of cure.
Five factors account for the majority of cases of resistance emerging during therapy ( Table 266-2 ). The first is the mutational frequency to resistance of the organism being treated. Because mistakes are made by the organism during replication, mutations occur in the genome at a specific rate, which is organism-dependent. For antibiotic resistance, the mutational frequency rate is generally about 1 in 10 6 to 10 8 CFU. Some organisms, however—referred to as hypermutators—that have mutations in other parts of the genome that alter their DNA replication error–checking mechanisms. These organisms’ mutational frequencies are generally 10- to 100-fold higher than those of organisms without such mutations.
| ANTIBACTERIAL AGENT | MECHANISM | REPRESENTATIVE ORGANISM |
|---|---|---|
| β-Lactams (penicillins, cephalosporins, carbapenems, aztreonam) | Altered target (penicillin-binding proteins) | Methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, Enterococcus faecium |
| Reduced permeability | Enterobacter spp, Pseudomonas aeruginosa, Acinetobacter spp | |
| Enhanced efflux | P. aeruginosa, Acinetobacter spp | |
| β-Lactamases | S. aureus , Enterobacteriaceae (includes ESBLs), Haemophilus influenzae, Moraxella catarrhalis , Neisseria gonorrhoeae , Enterococcus faecalis , P. aeruginosa , Acinetobacter spp | |
| Aminoglycosides | Inactivating enzymes (acetylation, adenylation, phosphorylation) | S. aureus , enterococci, P. aeruginosa, Enterobacteriaceae |
| Reduced permeability | Enterobacteriaceae , P. aeruginosa , enterococci | |
| Enhanced efflux | P. aeruginosa | |
| Decreased ribosomal binding | S. aureus, E. faecalis, mycobacteria (streptomycin), gram-negative pathogens (aminoglycoside ribosomal methylase) | |
| Chloramphenicol | Enhanced efflux | H. influenzae |
| Reduced permeability | Enterobacteriaceae | |
| Inactivating enzyme (acetylation) | S. aureus, S. pneumoniae, enterococci | |
| Daptomycin | Altered target | S. aureus |
| Glycylcyclines | Enhanced efflux | Enterobacteriaceae, especially Proteus |
| Macrolides, clindamycin, ketolide, quinupristin | Altered target (methylation of ribosomal RNA) | S. aureus, S. pneumoniae (not ketolide), streptococci, Bacteroides fragilis |
| Enhanced efflux (not clindamycin or ketolide) | S. pneumoniae , streptococci | |
| Reduced permeability | Enterobacteriaceae | |
| Inactivating enzymes | Escherichia coli, Klebsiella pneumoniae, S. aureus | |
| Lefamulin | Altered target | S. aureus , Mycoplasma spp |
| Oxazolidinones | Altered target | Streptococci, enterococci, S. aureus |
| Quinolones | Altered target (DNA gyrase, topoisomerase IV) | Enterobacteriaceae, P. aeruginosa |
| Reduced permeability | Enterobacteriaceae, P. aeruginosa | |
| Enhanced efflux | E. coli, P. aeruginosa | |
| Tetracyclines | Altered target (ribosome) Enhanced efflux Reduced permeability Drug inactivation |
N. gonorrhoeae, streptococci E. coli, S. pneumoniae Enterobacteriaceae B. fragilis |
| Rifampin | Altered target (β-subunit of polymerase) | E. coli, S. aureus, Mycobacterium tuberculosis |
| Sulfonamides, trimethoprim | Altered target (dihydropteroate synthase or dihydrofolate reductase) | Enterobacteriaceae, M. catarrhalis |
| Enhanced p -aminobenzoic acid production | S. aureus, N. gonorrhoeae | |
| Reduced permeability | P. aeruginosa, Enterobacteriaceae | |
| Vancomycin and lipoglycopeptides | Altered target (peptidoglycan precursor binding site) | E. faecium, E. faecalis, S. aureus |
The second factor is related to the bacterial burden. As the bacterial burden increases and ultimately surpasses the inverse of the mutational frequency to resistance, it becomes increasingly more likely that a preexistent antibiotic-resistant mutant already exists in the population. For example, if the mutational frequency to resistance is 1 resistant organism per 10 7 CFU and the patient has a bacterial infection in which the total bacterial burden is 10 9 organisms, it is highly likely that a resistant organism is present in the population a priori. Antibiotic pressure provides these mutants with a selective advantage; they are amplified, whereas the more susceptible bacteria are killed by the antibiotic. A calculation of the actual probability can be performed by a Poisson distribution, the bacterial burden, and the mutational frequency to resistance. Consequently, infections in which the bacterial burden is high are more likely to generate resistance during therapy. For example, in clinical trials of ventilator-associated pneumonia, single-agent β-lactam drugs or fluoroquinolones allow the emergence of antibiotic resistance during therapy 33 to 50% of the time.
The third issue is drug penetration. In some circumstances, such as an empyema, the bacterial burden is high, but the drug’s penetration to the infection site is reduced. In this case the emergence of resistance is more likely. In contrast, poor penetration with relatively low bacterial burdens (e.g., meningitis) generally does not result in a high probability of the emergence of resistance.
The fourth issue has to do with error-prone replication in bacterial pathogens. When resistance develops relatively late in therapy and the bacterial burden is modest, error-prone replication is often to blame. Antibiotics differ greatly with respect to their ability to induce the bacterial isolate to perform error-prone replication. Perhaps the best example is the fluoroquinolone antimicrobials, because these drugs strike at the heart of DNA replication. The organism senses the attack of the antibiotic, and a cascade of events ensues, the most important of which is the induction of error-prone polymerases (e.g., pol V). These polymerases markedly increase the error rate in DNA replication. Most of these errors are lethal to the organism and therefore unhelpful to it. However, because this process is totally random, by chance a mutation can occur in a gene that provides protection from the onslaught of the antibiotic (e.g., a mutation in the DNA gyrase when the patient is receiving a fluoroquinolone). This selective replication advantage (emergence of resistance) results in an increase in the MIC of the mutant organism relative to the baseline organism.
The fifth factor relates to mechanisms other than antibacterial target site mutations that allow the organisms to survive in the face of appropriate antibiotic chemotherapy. One extremely common mechanism seen in the majority of both gram-positive and gram-negative organisms is the upregulation of efflux pumps. These pumps are indiscriminate in their ability to pump molecules; they can eject multiple classes of antibacterials from the organism as well as other natural substances that can harm it, such as metal ions. These pumps keep drug concentrations at their target sites much lower than they would be in the absence of the pumps. The pumps can be induced and then downregulated once the threat has passed; or occasionally, the organism can have a mutation in the part of the genome where expression of the pump is regulated, so the pump is always expressed (constitutive expression).
A similar process is seen with the production of β-lactamases. Often, these enzymes are situated on plasmids and are produced continuously. Sometimes, as with the efflux pumps, the bacteria sense the β-lactam, and their β-lactamase production is markedly increased (the phenomenon of induction, seen with ampC-type enzymes, which generally reside on the chromosome). Sometimes, also like the pumps, the organisms have a mutation in the part of the genome that regulates production of the β-lactamase. This event is referred to as stable de-repression, and large quantities of the enzyme are made continuously. The enzyme hydrolyzes its substrate (the β-lactam drug), thereby preventing this antibiotic from binding to the target sites, the β-lactam–binding proteins.
Finally, in gram-negative organisms, the drug must cross the diffusional barrier of the outer membrane before binding to β-lactam–binding proteins (if the drug is a β-lactam) in the periplasm of the organism, or it must cross the inner membrane if its target site is actually inside the organism. For many agents, particularly those that are water soluble, a large percentage of their influx is due to passage through porin proteins. These proteins are water-filled channels that pass through the entirety of the outer membrane of gram-negative bacteria. Part of their function is to provide access to nutrients for the organism and allow the easy diffusion of waste products. These channels are also used to obtain passage by water-soluble antibacterial agents. The organism has the ability to downregulate these channels, either temporarily or permanently (by mutation). An example is Pseudomonas aeruginosa ( Chapter 282 ), in which downregulation of the porin channel oprD markedly diminishes the penetration of carbapenem antibiotics (β-lactam agents), with a resultant two- to eight-fold rise in MIC.
These mechanisms also interact. In most cases, resistance is caused by an alteration in the target site; by an enzyme that alters the drug, thereby resulting in a lack of activity; by the drug’s being pumped out of the organism; or, in the case of gram-negative pathogens, by the loss or downregulation of a transmembrane porin protein. These mechanisms, along with error-prone replication, can interact to cause large increases in the MIC. For example, a gram-negative pathogen that acquired a mutation in its gyrase enzyme would generally have only a four-fold increase in its MIC value for a fluoroquinolone antimicrobial; however, if this isolate also had an efflux pump upregulated, the MIC could change 8- to 16-fold. In the case of fluoroquinolone antimicrobials, where error-prone replication almost always occurs under pressure, the upregulation of efflux pumps relieves some of the antibiotic pressure, thereby allowing the organisms to undergo more rounds of replication per unit of time, giving the error-prone replication mechanism more time to find a mutation that is not lethal, and providing protection against the drug at the primary target site.
With all the mechanisms that can be brought to bear, it is not surprising that poor dosing and prolonged courses of therapy often lead to the emergence of resistance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here