Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The anterior skull base is a region of interest because it is frequently breached by aggressive disease such as malignancy and infection or even chronic long-standing but progressive lesions such as mucoceles. This resultant transcompartmental spread has a significant impact in prognosis and management. Imaging plays a key role in the assessment of disease extent and management planning.
Most tumors that involve the anterior skull base originate in the sinonasal compartment. Malignant sinonasal tumors are relatively rare, comprising 3% of all head and neck malignancies. These tumors (ranging from the common squamous cell carcinoma [SCC] to the rare sarcomas) are usually aggressive. They generally have a poor prognosis because of extensive disease at presentation and high local recurrence rate. Benign sinonasal tumors are also common, and they include sinonasal polyps, juvenile angiofibroma, and inverted papilloma. These lesions are usually confined to their site of origin and rarely encroach or erode the skull base.
As most sinonasal tumors are amenable to biopsy, the primary role of imaging is in mapping the tumor extent. Information such as osseous erosion, meningeal and brain invasion, or orbital extension is crucial for management planning. Significant advancement in craniofacial surgical approach has enabled safe and reliable resection of sinonasal tumors. The craniofacial approach essentially allows for a wide exposure of the anterior craniofacial structures and can be modified to extend surgical accessibility (depending on the individual case and surgeon preference). The anterior craniofacial approach, as the name suggests, incorporates a combination of transfacial and transcranial procedures. It is important to note that the advances in craniofacial resection techniques are largely contributed by the wealth of information provided by preoperative CT and MRI.
The anterior skull base separates the cranial cavity above from the orbit and sinonasal compartments below. This boundary is formed by two bones: the cribriform plate centrally and the orbital plates of the frontal bone laterally ( Fig. 1.1 ). The cribriform plate is the part of the ethmoid bone that consists of two parallel grooves on which the olfactory bulbs sit, separated by a midline triangular process called the crista galli (because of its resemblance to the comb of a rooster). The crista galli serves as an attachment site for the falx cerebri. The floor of each groove has multiple tiny perforations that allow passage of olfactory nerves to the nasal mucosa. The frontal sinuses are located anterior to the cribriform plate.
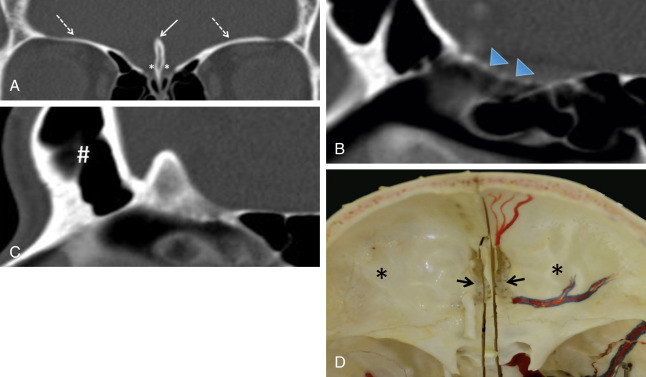
The thin cribriform plate is well demonstrated on coronal CT images. This structure is also visible on MRI as a linear hypointense signal on T1-weighted and T2-weighted sequences. It is frequently involved by tumors and fractured by trauma or surgery. The orbital plates of the frontal bone constitute a thick and robust barrier to disease spread. The lamina papyracea is the medial orbital wall that separates the orbit from the ethmoid sinus. It is thus named because of its paper-thin appearance and is easily fractured or eroded by tumors. This structure, like the cribriform plate, is easily evaluated on CT. The periorbita is the periosteum lining the walls of the bony orbit to which it is loosely connected. It is continuous with the periosteum of facial bones anteriorly and the dura mater posteriorly through the superior orbital fissure. This structure is indistinguishable from the adjacent orbital wall in normal patients but can be seen as a hypointense line on T1-weighted and T2-weighted MRI when lifted off the underlying bone by tumor, blood, or pus.
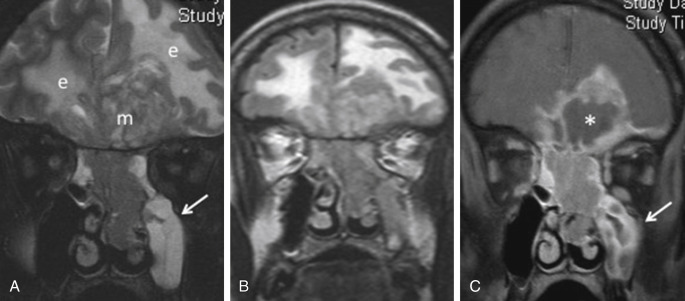
Intracranial invasion is recognized as the most adverse prognostic factor in sinonasal tumors and is a crucial feature requiring careful imaging evaluation. Dural invasion alone significantly decreases survival rate from 68% to 25% in a series of adenocarcinomas. On the other hand, orbital invasion by sinonasal malignancy is an independent prognostic factor with a significant impact on survival rates. The incidence of visual involvement is estimated at 50%. This is related to its close proximity to the sinuses and the presence of multiple routes of spread via anatomic foramina, traversing vessels, and nerves, as well as thin bones such as the lamina papyracea. Imaging plays a critical role in determining orbital disease extension ( Fig. 1.2 ).
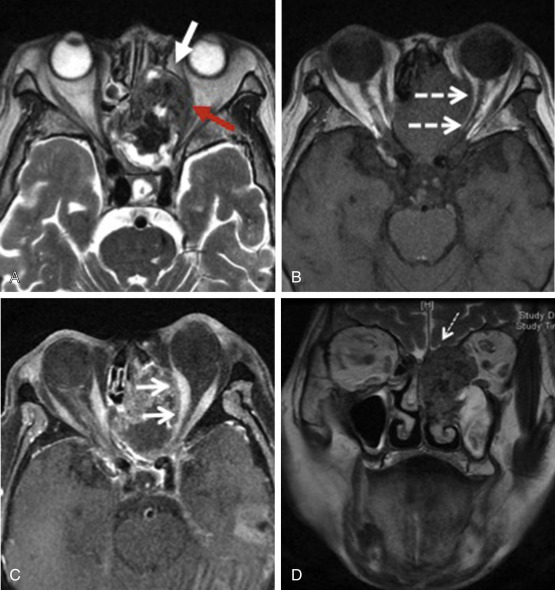
Intracranial invasion includes breach of the skull base, dural involvement, and brain parenchymal invasion. It is possible that the skull base integrity is compromised by disease without involvement of the adjacent dura mater ( Fig. 1.3 ).
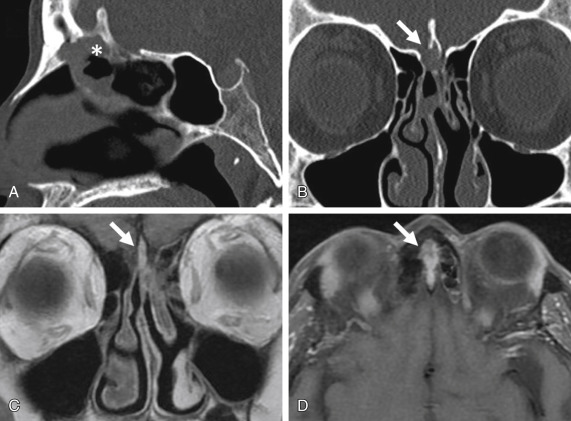
Breach of the anterior skull base is suspected when the cribriform plate is dehiscent on CT or the normal hypointense signal on MRI is lost. Thereafter, the dura should be inspected for evidence of disease such as dural thickening and enhancement. Dural involvement is considered a precursor to brain invasion and is associated with poor recurrence-free survival, disease-specific survival, and overall survival. Although dural spread can be seen on imaging, it may be difficult to differentiate neoplastic involvement from inflammatory change. In general, if the visualized dura appears grossly thickened and has a nodular contour, dural invasion can be assumed. As dural invasion is associated with a high recurrence rate, aggressive resection of potentially involved dura followed by radiotherapy is often undertaken, even though dural infiltration is not evident on MRI.
Tumors may indent the base of the brain without parenchymal invasion. In these cases, a thin cleft of cerebrospinal fluid (CSF) is seen at the interface between the tumor and the brain surface ( Fig. 1.4 ). Apart from the mass effect, the brain demonstrates normal signal characteristics. Aggressive lesions, such as SCC, often invade the frontal lobes, resulting in loss of CSF cleft and presence of brain edema and enhancement.
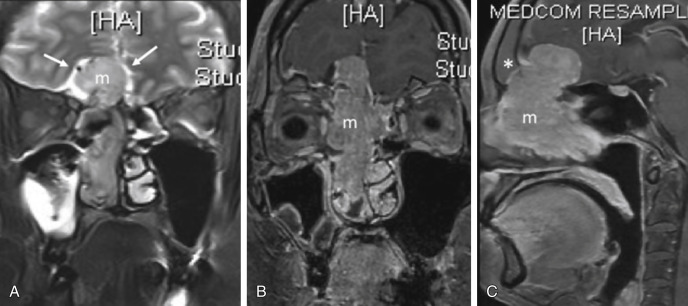
The periorbita is the periosteum lining the walls of the bony orbit and has traditionally been regarded as the decisive layer that determines the subsequent extent of surgery. Transgression of the periorbita means orbit evisceration is required. Eisen showed that tumor adjacent to the periorbita was the most sensitive predictor of orbital invasion. When the hypointense line is lost, orbital invasion is considered to be present. However, this structure can be difficult to visualize separately from the orbital wall even in normal cases, making interpretation extremely challenging. Furthermore, a meta-analysis showed no significant difference in 5-year survival rates between patients whose orbits were preserved and patients who underwent evisceration. Ianetti described three stages of orbital invasion: grade I, erosion of the medial orbital wall; grade II, extraconal invasion of periorbital fat; and grade III, invasion of the medial rectus muscle, optic nerve, ocular bulb, or skin overlying the eyelid. It is thus perhaps more feasible for the radiologist to use this grading system. Most surgeons now consider grade III invasion as an indication for orbital evisceration. A more conservative approach involves intraoperative microscopic dissection and frozen section of the periorbita before decision for or against evisceration is made.
The most common malignant tumor in the sinonasal cavity is SCC, accounting for 60%–75% of all sinonasal cancers. There is a male predominance, with a peak incidence in the fifth to seventh decades. The most frequent location is the maxillary sinus followed by the nasal cavity, ethmoid sinus, and, rarely, frontal or sphenoid sinus.
SCC often shows intermediate signal intensity on T2-weighted sequence, reflecting the hypercellular nature of this tumor. MRI may also show areas of T1-weighted hyperintensity (representing hemorrhage) and areas of T2-weighted hyperintensity (representing necrosis). Bone destruction is characteristic of SCC, but tumor extent is best appreciated on MRI. MRI with contrast agent can demonstrate the enhancing tumor, a feature that helps to distinguish it from benign inflammatory lesions, such as retained secretions, mucoceles, and sinonasal polyps ( Fig. 1.5 ). According to the TNM (tumor, node, and metastasis) classification system, SCC invading the medial orbital wall is classified as T3, whereas involvement of the anterior cranial fossa is T4.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here