Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]()
The pituitary is located in the sella turcica, which is a part of the sphenoid bone of the skull. It is attached to the base of the brain by a stalk, known as the infundibulum, and is contained in a capsule that is continuous with the dura mater, thereby placing it technically outside of the blood-brain barrier. The gland itself is composed of two parts, the anterior pituitary (adenohypophysis) and the posterior pituitary (neurohypophysis). The adenohypophysis has three anatomic components: the pars distalis (where the hormone-secreting cells are situated); the pars tuberalis (which hugs the infundibulum); and the pars intermedia, which is present in children but essentially absent in adulthood. The infundibulum has direct neural fibers that connect to the neurohypophysis, and, in a network of blood vessels, the infundibulum communicates with the adenohypophysis. The inferior border of the pituitary rests on the floor of the sella turcica, and the superior border is located just under the optic chiasm. Growth of the pituitary inferiorly can lead to erosion of the floor of the sella turcica and invasion into the sphenoid bone and sphenoid sinus, whereas the cavernous sinus and carotids are found laterally. Abnormal superior growth of the pituitary explains the visual problems encountered when the pituitary is enlarged by tumor, bleeding, or infiltration because of impingement on the optic chiasm.
The superior and inferior hypophyseal arteries provide the main arterial supply to the anterior and posterior pituitary, respectively ( Fig. 205-1 ). These vessels are branches from the internal carotid arteries. The superior hypophyseal artery forms the primary plexus of the hypophyseal portal system at the origin of the infundibulum. Blood from this plexus flows down the portal vessels. The pars distalis receives very little blood from the internal carotid and instead is mainly supplied by the venous system—long portal veins from the plexus and from the neurohypophysis through short portal vessels. This anatomy allows the pars distalis to be exposed to hormones from the hypothalamus, posterior pituitary, and the general blood circulation. Lack of direct arterial supply to the pars distalis makes it vulnerable to ischemia secondary to hypovolemia and hypotension. For the anterior pituitary, venous drainage is by the hypophyseal veins; for the posterior pituitary, it is via the short portal and hypophyseal veins to the cavernous sinus. The cavernous sinus drains into the inferior petrosal sinuses and then the internal jugular vein.
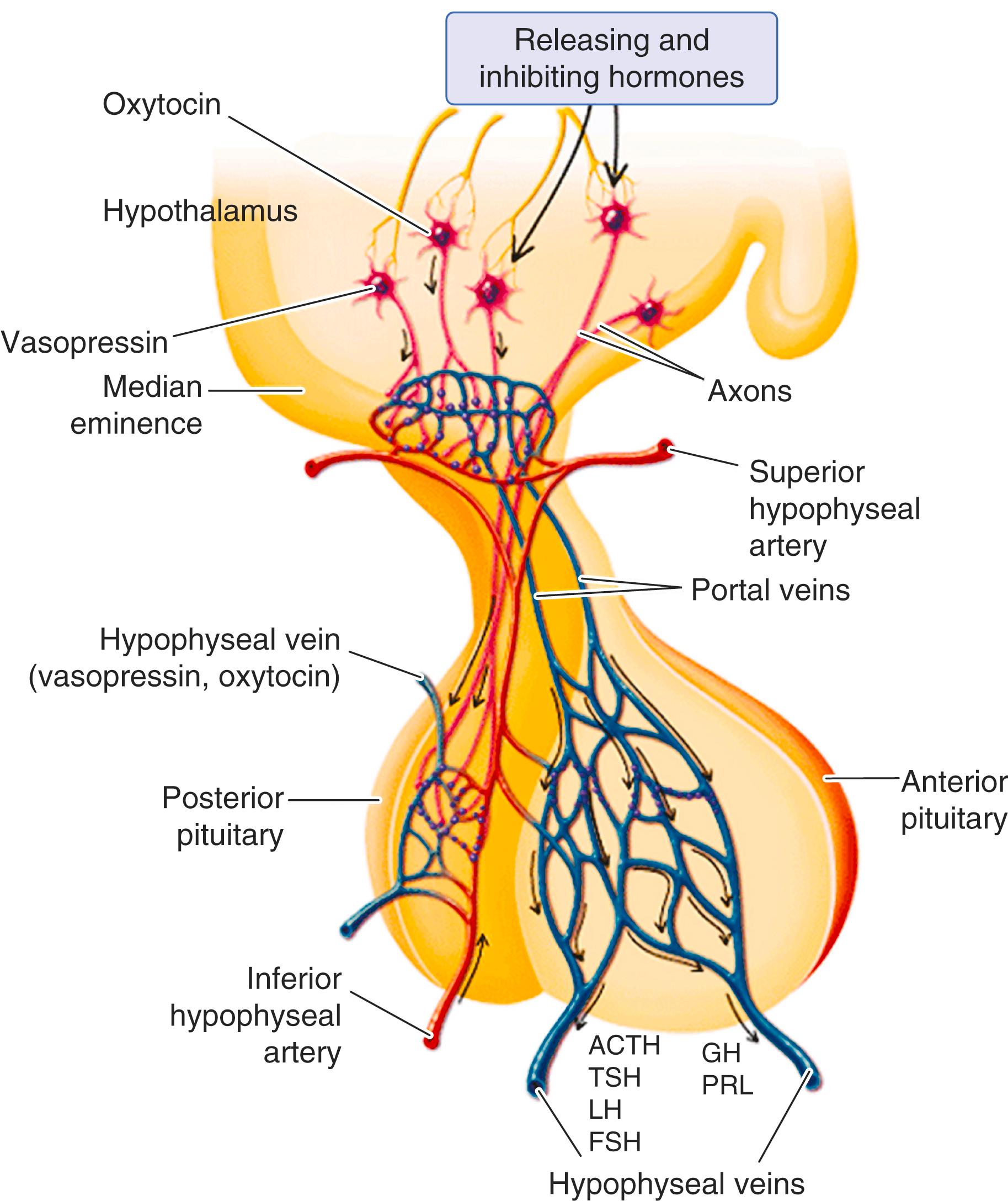
The pituitary gland secretes adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and growth hormone, largely regulated by signals from the hypothalamus ( Chapter 204 ), and it also secretes prolactin, largely regulated by dopamine as well as by thyrotropin-releasing hormone (see Fig. 205-1 ). The multitude of effects that the hormones of the pituitary have on the physiology and metabolism of the entire body provides exquisite control of hormone secretion regulated via multiple feedback loops and stimuli, not only from the hypothalamus, but also from higher in the brain and in the environment. All pituitary hormones are regulated and secreted with a highly reproducible diurnal variation and with superimposed episodic secretion throughout the day and night and influenced by sleep and wakefulness ( Chapter 204 ). Therefore, measurement of hormone levels must account for the episodic secretion of the hormones, and a single point in time may not reflect the overall activity of the axis. For example, the stress and pain (although minimal) of phlebotomy itself can be sufficient to cause an increase in prolactin and cortisol.
One example of axis regulation is the release of thyroid hormone from the thyroid under the control of thyroid-stimulating hormone from the pituitary thyrotrophs. The more TSH stimulates the TSH receptors on the thyroid cells (thyrocytes), the more thyroid hormone is released. The thyroid hormone then feeds back to the pituitary to downregulate further secretion of TSH, which then turns off thyroid hormone release from the thyrocytes. Superimposed on this process is the hypothalamus, which secretes TRH (thyrotropin-releasing hormone) and stimulates the release of TSH. However, thyroid hormone also provides feedback to the hypothalamus and, independent of TSH, turns off TRH secretion. Therefore the pituitary’s thyrotrophs need to integrate the positive stimulation of TRH and at the same time the inhibition by the thyroid hormone levels in order to modulate the level of TSH release. The story is still not complete, because dopamine and other neurotransmitters from centers in the brain above the hypothalamus can stimulate and inhibit TRH, thereby providing an additional level of control over thyroid hormone secretion. Similar multilevel feedback control mechanisms can be described for cortisol from the adrenal gland as well as other pituitary hormones such as the gonadotropins, prolactin, and growth hormone. Understanding the basis for hypothalamic-pituitary-target endocrine gland axis regulation is essential for the diagnosis of abnormalities at specific levels of the axis as interpreted from provocative and suppressive tests used clinically to assess the pituitary axes.
Hypopituitarism is a deficiency of one or more pituitary hormones. The prevalence is between 290 and 455 cases per million, with an incidence of 42 cases per million per year. Hypopituitarism can be congenital (with known and unknown genetic causes) and acquired ( Table 205-1 ). Panhypopituitarism refers to a defect in all pituitary hormone lines.
| CONGENITAL EMBRYOPATHIC DEFECTS |
| Anencephaly Midline cleft defects: septo-optic dysplasia, basal encephalocele, cleft lip and palate Pituitary aplasia Kallmann syndrome (GnRH defect with anosmia) |
| ACQUIRED DEFECTS |
| Tumors: pituitary adenomas, craniopharyngiomas, dysgerminomas, meningiomas, gliomas, metastatic tumors, hamartomas, Rathke cleft cysts Irradiation Trauma: surgery, external blunt trauma ( Chapter 368 ) Empty sella syndrome Vascular
Inflammatory and infiltrative diseases
Metabolic defects
Idiopathic |
The most common cause of hypopituitarism is a tumor, specifically a macroadenoma in the sella. Tumor enlargement within the confines of the bony sella turcica can compress the normal pituitary cells, thereby resulting in their failure to function. Irradiation of the pituitary, pituitary infarction (such as Sheehan syndrome, which is caused by severe postpartum bleeding ), surgical intrusion, and infiltrative disease also can compromise normal pituitary functioning.
Genetic defects have been reported at every level of the hypothalamic pituitary axis, including defects in the hypothalamic factors, receptors for the hypothalamic factors, and the genes for the pituitary hormones. Defects in the genes coding for the specific hormone almost always result in a low serum concentration of the hormone.
Although congenital hypopituitarism may involve deficiency of a single or multiple pituitary hormones, acquired types of hypopituitarism usually simultaneously involve more than one of the pituitary hormones. Defects in genes encoding for factors such as the PIT1 and PROP1 genes may impair pituitary cell differentiation. Furthermore, the pituitary hormone receptor in the respective glands may be defective, thereby resulting in resistance to the action of the pituitary hormones. Finally, genetic defects in the gene coding for the hormone or the hormone’s receptor can result in profound hormone deficiency.
Functional causes of defective pituitary function can be seen in severe malnutrition ( Chapter 197 ), in anorexia nervosa ( Chapter 200 ), or in critically ill patients who are in a state of severe calorie deprivation. In these situations, central (hypothalamic and higher) mechanisms and molecular mediators (e.g., cachexins and tumor necrosis factor) inhibit the release of pituitary hormone. This form of hypopituitarism is generally reversible when the caloric situation improves.
Traumatic brain injury ( Chapter 368 ) is an increasingly recognized cause of hypopituitarianism. Deposition and infiltration of amyloid protein in amyloidosis ( Chapter 174 ), iron in hemochromatosis ( Chapter 196 ) or granulomas (in sarcoidosis [ Chapter 83 ], tuberculosis [ Chapter 299 ], or granulomatosis with polyangiitis [ Chapter 249 ]) also can cause hypopituitarism. Invasion of the hypothalamus and pituitary by histiocytosis ( Chapter 155 ) or metasteses from breast cancer, lung cancer, melanoma, and rarely, colon cancer can impair pituitary function. Hypopituitarism is also a rare complication of infection with human immunodeficiency virus (HIV) or severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
In addition to congenital and acquired pituitary defects, hypopituitarism may result from a hypothalamic disorder ( Chapter 204 ). Concurrent diabetes insipidus, which is indicative of dysfunction of the posterior pituitary ( Chapter 206 ), usually suggests combined hypothalamic and pituitary defects. Hyperprolactinemia, which suggests interruption of the hypothalamic dopaminergic inhibition of prolactin, also can be a result of hypothalamic defects.
Congenital defects are usually noted soon after birth when an absent pituitary hormone results in growth retardation or failure to develop. Patients may also present with congenital failure of a specific pituitary function and manifest with early-onset growth defects, delayed puberty, or hypothyroidism.
Acquired hypopituitarism presents earlier in its course in women of reproductive age because any perturbation in the hypothalamic pituitary milieu will likely result in an abnormality of the menstrual cycle ( Chapter 218 ), which requires orchestration of multiple hormones that are exquisitely sensitive to changes in the pituitary hormones. By comparison, males of all ages and women of postreproductive age usually have hypopituitarism for several years before the diagnosis is made. In such patients, the symptoms can be nondescript (e.g., fatigue, decreased muscle strength and muscle mass, and increased body fat) and not appreciated for many years after the onset of hypopituitarism.
Although growth hormone deficiency can exist in up to 60% of patients with hypopituitarism, such deficiency in an adult is not likely to cause the same remarkable symptoms as the short stature that is seen in children. When multiple hormones are deficient, the manifestations in adulthood may be as subtle as skin changes, decreased sexual functioning, decreased muscle mass, and increased fat mass.
Diagnosis of hypopituitarism in patients with secreting or nonsecreting pituitary masses usually involves a combination of static and stimulatory blood tests ( Table 205-2 ) beginning with morning fasting level of ACTH, TSH, FSH, LH, growth hormone, and prolactin. If multiple deficiencies are found, panhypopituitarism is likely. Isolated deficiencies in only one pituitary hormone can occur, but if two or more are deficient, it is likely that all are deficient. Testing ( Table 205-2 ) often depends on the age of the patient and the clinical setting. Oftentimes, no single gold-standard test is available for each hormone, so equivocal results on one test may require another test. For example, growth hormone reserve in children is usually best documented with an arginine stimulation test, whereas an insulin tolerance test would be more appropriate in an adult in whom the ACTH axis should be evaluated at the same time. Sometimes multiple tests may be performed if results of one test are equivocal.
| HORMONE | TEST | INTERPRETATION |
|---|---|---|
| Growth hormone (GH) | Insulin tolerance test: Regular insulin (0.05-0.15 U/kg) is given IV, and blood is drawn at −30, 0, 30, 45, 60, and 90 min for measurement of glucose and GH. | If hypoglycemia occurs (glucose <40 mg/dL), GH should increase to >5 µg/L ∗ |
| Arginine-GHRH test: GHRH 1 µg/kg IV bolus followed by 30-min infusion of l -arginine (0.5 g/kg up to 30 g) | Normal response is GH >4.1 µg/L | |
| Glucagon test: 1 mg IM with GH measurements at 0, 60, 90, 120, 150, and 180 min | Normal response is GH >3 µg/L | |
| Arginine-L-DOPA test: L-DOPA 500 mg PO at start of arginine 30 g in 10% solution IV infusion over 30 min, blood sampled at 30, 60, 90, 120 min | Normal response is GH >1.5 µg/L | |
| Adrenocorticotropic hormone (ACTH) | Insulin tolerance test: Regular insulin (0.05-0.15 U/kg) is given IV, and blood is drawn at −30, 0, 30, 45, 60, and 90 min for measurement of glucose and cortisol. | If hypoglycemia occurs (glucose <40 mg/dL), cortisol should increase by >7 µg/dL or to >20 µg/dL |
| CRH test: 1 µg/kg ovine CRH IV at 8 am , with blood samples drawn at 0, 15, 30, 60, 90, and 120 min for measurement of ACTH and cortisol | In most normal individuals, the basal ACTH increases two- to four-fold and reaches a peak (20-100 pg/mL). ACTH responses may be delayed in cases of hypothalamic dysfunction. Cortisol levels usually reach 20-25 µg/dL | |
| ACTH stimulation test: ACTH 1-24 (cosyntropin), 0.25 mg IM or IV. Cortisol is measured at 0, 30, and 60 min. | A normal response is cortisol >18 µg/dL. In suspected hypothalamic-pituitary deficiency, a low-dose (1-µg) test may be more sensitive | |
| Thyroid-stimulating hormone (TSH) | Basal thyroid function tests: free T 4 , free T 3 , TSH | Low free thyroid hormone levels in the setting of TSH levels that are not appropriately increased |
| Luteinizing hormone (LH), follicle-stimulating hormone (FSH) | Basal levels of LH, FSH, testosterone, estrogen | Basal LH and FSH should be increased in postmenopausal women. Low testosterone levels in conjunction with low or low-normal LH and FSH are consistent with gonadotropin deficiency |
| Prolactin | Basal level of Prolactin | The low range of prolactin levels in men and women is not established and depends on the assay. If prolactin is undetectable, deficiency is likely. Levels lower than 10 ng/mL indicate a possible deficiency |
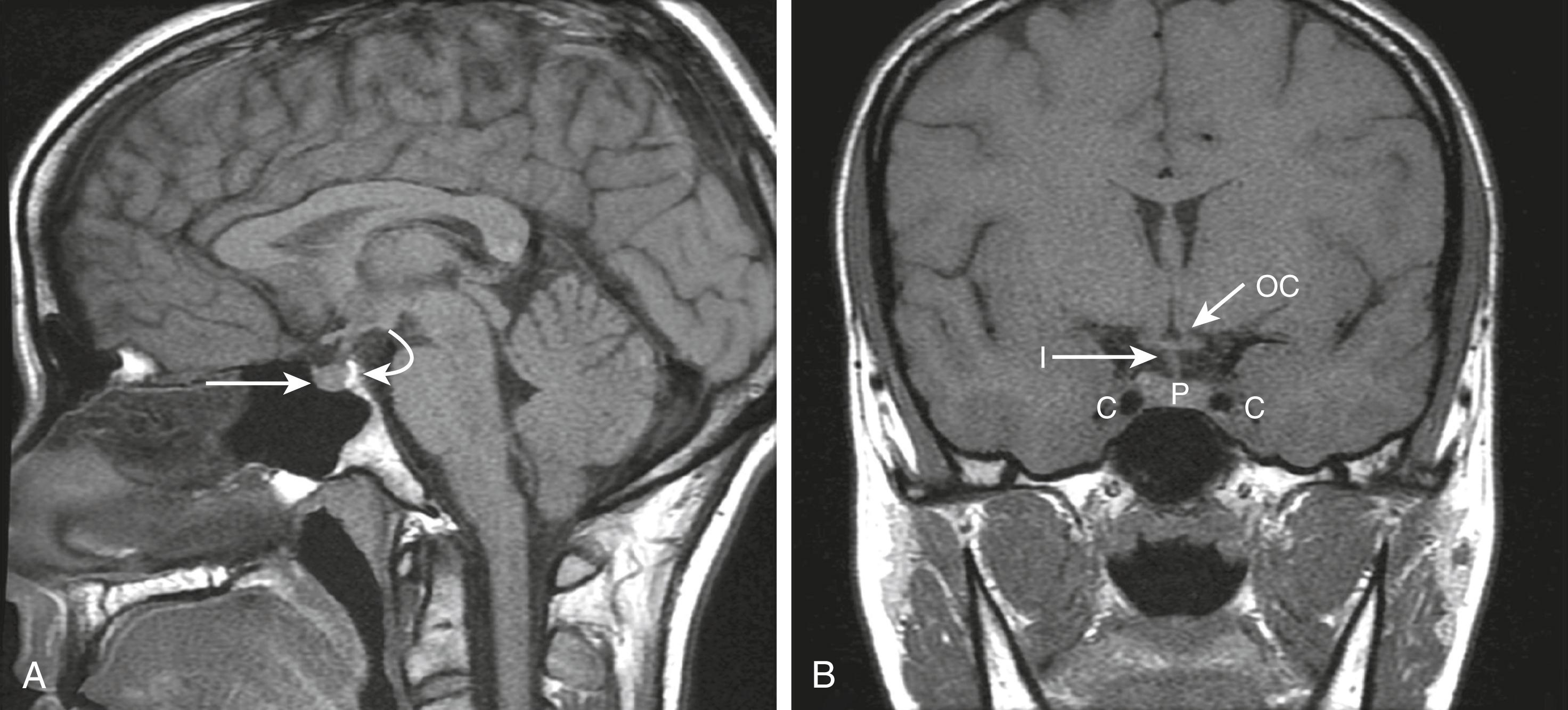
The next diagnostic imperative is to image the pituitary to determine the cause. Magnetic resonance imaging (MRI) is the preferred radiologic method for anatomic definition of the pituitary and its surrounding structures. The normal pituitary is less than 1 cm in height, and a small tumor (microadenoma) can be as small as 1 mm, so high-quality imaging is necessary. Sagittal and coronal images are the most useful to obtain ( Fig. 205-2 ). Intravenous contrast can enhance MRI images because normal pituitary generally enhances more than tumors. When the use of a contrast agent is not contraindicated, T2 images may be helpful to distinguish normal pituitary from a tumor ( Fig. 205-3 ). In such cases, helical computed tomography (CT) also can be useful with noncontrast and immediate postcontrast imaging.
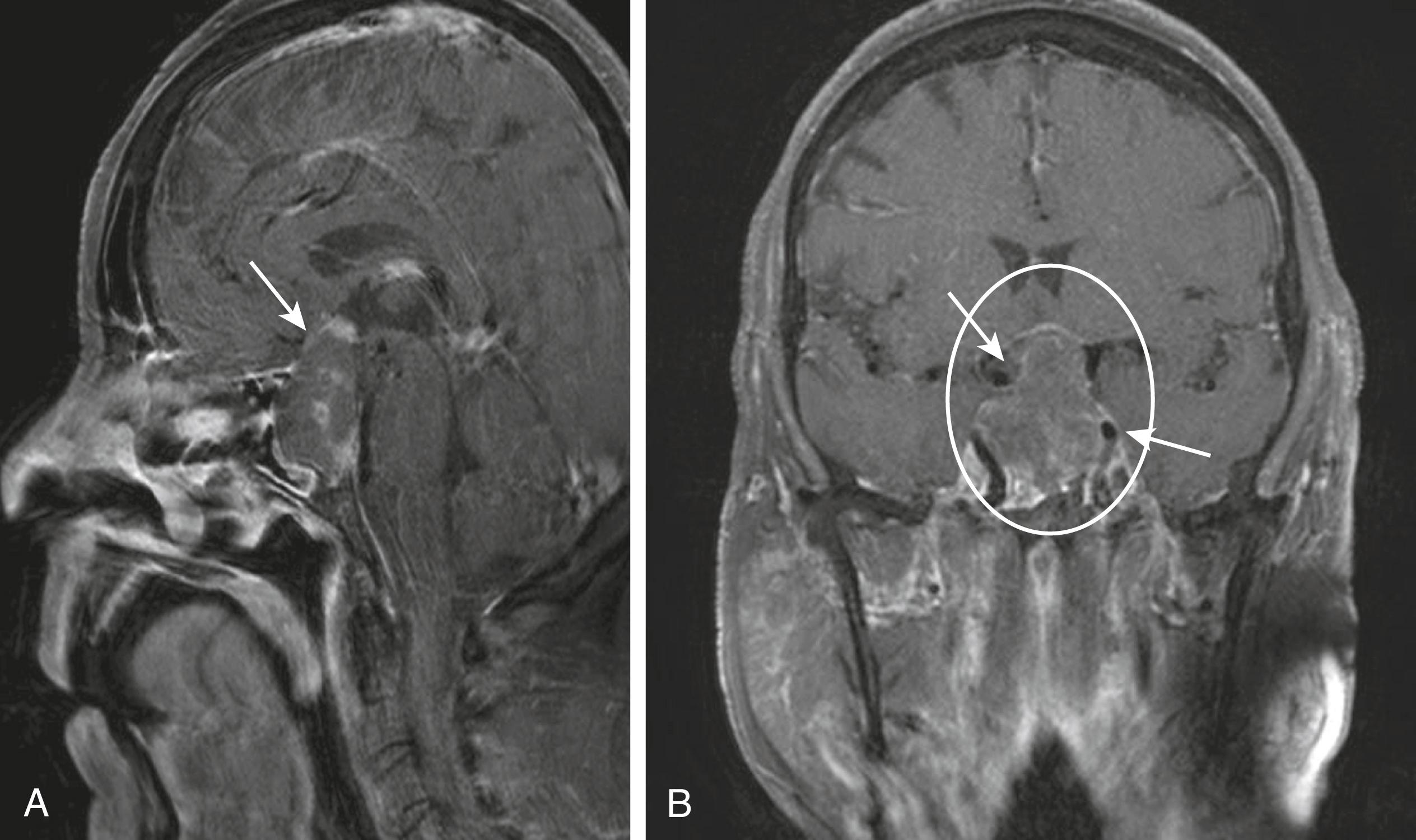
Treatment includes replacement of the deficient hormones ( Table 205-3 ) but also must address the underlying cause (see Table 205-1 ), such as hypophysitis, empty sella syndrome, pituitary apoplexy, infiltrating diseases, malnutrition, and pituitary tumors.
| PITUITARY AXIS | HORMONAL REPLACEMENTS |
|---|---|
| Growth hormone (GH) | In children, GH (0.25 mg/kg) SQ daily. In adults, GH (0.3-1.2 mg) SQ daily. Titrate dose to achieve IGF-I levels in middle to upper part of normal range. Women receiving oral estrogens require higher doses. |
| Prolactin | None; although not clinically available, human recombinant prolactin has been used |
| Adrenocorticotropic hormone–cortisol | Hydrocortisone (10-15 mg PO q am ; 5-10 mg PO q pm ) or prednisone (2.5 mg PO q am ; 2.5 mg PO q pm ). Dose adjusted on clinical basis. Stress dosing: 50-75 mg hydrocortisone IV q8h |
| Thyroid-stimulating hormone–thyroid | l -thyroxine (0.075-0.15 mg) PO daily |
| Gonadotropins–gonads | FSH and LH (or HCG) can be used to induce ovulation in women. HCG alone or with FSH can be used to induce spermatogenesis in men. |
| In men, testosterone enanthate (100-300 mg) IM q1-3 wk or testosterone cyclopentylpropionate (100-300 mg) IM q1-3 wk. Testosterone transdermal patches can also be used (5 mg daily). Testosterone gel 5-10 g daily † | |
| In women, conjugated estrogens (0.625-1.25 mg) PO days 1-25 each month, cycled with medroxyprogesterone acetate (5-10 mg) PO days 15-25 each month. Low-dose contraceptive pills may also be used. | |
| Estrogen-containing transdermal patches are also available. | |
| Posterior pituitary | Desmopressin, 0.05-0.2 mL (5-20 µg) intranasally once or twice daily, or tablets (0.1-0.4 mg q8-12h), or 0.5 mL (2 µg) SQ |
∗ Replacement therapy is dictated by the types of hormone deficiencies and by the clinical circumstances. In each case, the recommended preparations and doses are representative but need to be adjusted for individual patients. Other hormonal preparations are also available.
† Several oral formulations of testosterone undecanoate have recently been approved by the FDA based on single arm trials in hypogonadal men. ( https://secure.medicalletter.org/TML-article-1662b .)
Inflammation of the pituitary is known as hypophysitis and can be classified clinically as primary hypophysitis when isolated inflammation of the pituitary is not associated with other inflammatory conditions, infections, or medications, including checkpoint inhibitors. Lymphocytic hypophysitis, which is the most common form of this rare condition, occurs three times more frequently in women, typically in the fourth decade and often related to the end of pregnancy or the first few months after delivery. Lymphocytic hypophysitis is also seen in about 3 to 8% of patients treated with immune checkpoint inhibitors, specifically with the anti–CTLA-4 class of inhibitors, with peak onset at a median of 2.3 months after starting therapy. The other histologic types, granulomatous and xanthomatous hypophysitis, are not associated with pregnancy although they are more common in females. The least common form, plasmacytic or IgG4-related hypophysitis, occurs in males in a 2:1 ratio, usually presents in the seventh decade, and may include orbital inflammation, hypertrophic pachymeningitis, and central nervous system parenchymal disease.
Patients with hypophysitis usually present with headaches and multiple deficiencies of anterior pituitary hormones. Diabetes insipidus ( Chapter 206 ) is reported in about 50% of patients, and most have associated mild hyperprolactinemia.
On MRI, hypophysitis usually causes diffuse pituitary enlargement and thickening of the midline stalk, with a normal sellar size, but MRI cannot reliably differentiate hypophysitis from a pituitary adenoma.
Definitive diagnosis is usually made by biopsy. A clinical diagnosis can, however, be inferred based on radiologic and clinical characteristics during or just after pregnancy or if there is a proximal history of use of anti–CTLA-4 agents several months prior to the onset of symptoms (headaches and weakness).
Surgery is indicated when a biopsy is needed for diagnosis or if there is a mass effect. Careful pituitary function testing and repletion of physiologic hormone levels are mandatory, even in patients who do not undergo surgery because they might otherwise die from undiagnosed adrenocortical insufficiency. Stress doses of corticosteroids (e.g., 50 to 75 mg hydrocortisone IV every 8 hours) are indicated perioperatively.
The prognosis is unclear, but both the structural and functional abnormalities can occasionally resolve spontaneously. Glucocorticoids in physiologic doses may have a direct effect on limiting the lymphocytic invasion in lymphocytic hypophysitis, but there are no prospective treatment trials.
The pituitary can be involved with hemorrhage, infarction, or both. When abrupt, and sometimes catastrophic, hemorrhagic infarction occurs in the pituitary, it is defined as apoplexy.
Certain conditions predispose a patient to pituitary apoplexy ( Table 205-4 ). Although all large pituitary tumors are at risk for hemorrhagic infarction, certain functional pituitary tumors, such as those in Cushing disease or acromegaly, may be particularly prone. In women who have microprolatinomas treated with dopamine agonists, stopping the agonist while pregnant can precipitate pituitary apoplexy. Nearly 25% of all patients with apoplexy have inadequately treated hypertension. The main symptoms and consequences of apoplexy are due to the increased pressure present within the bony walls of the sella turcica in which the pituitary resides. A sudden increase in the sellar contents, due to blood and edema, results in increased pressure. This increased pressure and meningeal irritation are responsible for the neurologic symptoms, including the increased pressure in the cavernous sinus and the cranial nerve palsies, as well as bitemporal hemianopsia. Extravasation of blood into the subarachnoid space causes meningeal irritation. Asymptomatic hemorrhage and infarction into a pituitary tumor can occur in 10 to 25% of patients, but true apoplexy occurs in only 2 to 10% of pituitary tumor patients.
Pituitary Tumor
Hypertension and/or hypotension
Drugs
Head Trauma
Radiation therapy |
The most common presenting complaint, headache, can present variably from retro-orbital to unilateral to bilateral temporal headaches during the acute phase of apoplexy. The constellation of headache, vomiting, visual impairment, and altered consciousness with hemodynamic instability is not specific for pituitary apoplexy but raises suspicion for the diagnosis ( Table 205-5 ). Often, this dramatic presentation is the first time the patient is aware of a potential pituitary tumor. As the hemorrhagic infarction resolves, the patient often is left with hypopituitarism.
| SYMPTOM | APPROXIMATE INCIDENCE |
|---|---|
| Headache | 95% |
| Vomiting | 70% |
Vision Defects:
|
65% 50% 40-100% |
| Hemiplegia | Rare |
| Meningismus | Rare |
| Hypotension (cardiovascular collapse) | 95% |
Prompt recognition of patients presenting with the triad of headache, vomiting, and visual disturbances is required to prevent death or irreversible neurologic impairment. Clinical evaluation of the patient should begin with a thorough history from the patient, if sufficiently conscious to give one, or from family members. A history of a pituitary tumor should raise the suspicion of apoplexy. More subtle abnormalities associated with pituitary dysfunction (hypofunctioning of thyroid, adrenal, or gonadal systems) may be helpful.
The cornerstone for diagnosis is urgent MRI T2-weighted images. A CT scan can be useful when an MRI is not available or possible.
Urgent measurement of blood chemistries, including electrolytes, kidney function, liver function, complete blood count with platelets, and prothrombin time, can be useful. Because more than 80% of patients will have endocrine dysfunction, urgent measurement of free T 4 , TSH, prolactin, and random cortisol can be helpful. Less rapidly available and helpful (and less important in the initial diagnosis and management) are other pituitary hormones such as LH, FSH, estradiol or testosterone, growth hormone, and IGF-I.
Examination of cerebrospinal fluid (CSF) is usually not diagnostic and is unnecessary if the diagnosis of apoplexy is certain. However, if there is bleeding into the CSF as a result of the apoplexy, red blood cells as well as an elevated protein level and xanthochromia can be seen.
The differential diagnosis of pituitary apoplexy should include other conditions that result in the symptoms of headache, vomiting, visual disturbances, and hemodynamic instability such as infection (meningitis [ Chapter 381 ]), cavernous sinus thrombosis ( Chapter 376 ), migraine ( Chapter 367 ), Rathke cyst hemorrhage ( Chapter 377 ), and hyperemesis gravidarum ( Chapter 221 ). Each of these conditions is itself a medical emergency that requires specific treatment.
The initial management is stabilization of the hemodynamic status with intravenous 0.9% NaCl boluses to maintain normal tissue perfusion, usually accompanied by high-dose parenteral glucocorticoids (100 mg hydrocortisone every 8 hours intravenously). Emergent surgical decompression may be required in patients who have progressive visual or neurologic impairment.
Although 80% of patients have residual hypopituitarism following apoplexy (with or without surgical decompression), some patients do not display immediate evidence of hypopituitarism. A month after discharge from the hospital and recovery from the acute event, patients should have repeat endocrine testing to determine if the endocrine defect persists. Repeat testing will confirm whether the patient needs to remain on lifelong hormone replacement therapy.
Recurrent apoplexy can occur. MRI of the pituitary should be obtained at 3- to 6-month intervals until the anatomy is stable and then yearly for 5 years.
Empty sella is a radiologic diagnosis that refers to the observation on imaging of the presence of CSF in the sella turcica accompanied by a flattened pituitary gland ( Fig. 205-4 ). An empty sella is usually noted as an incidental finding when an MRI or CT scan of the skull is performed because of problems unrelated to the pituitary. It can be primary or acquired when a defect in the diaphragma sella allows the arachnoid membrane to herniate into the sella. Patients with acquired empty sella may have a prior history of an infarcted pituitary, surgery, or radiation. If the herniation has been present for years, the sella enlarges, probably owing to persistent exposure to intracranial pressure. Primary empty sella is more frequent in women and may be accompanied by benign intracranial hypertension ( Chapters 175 and 367 ).
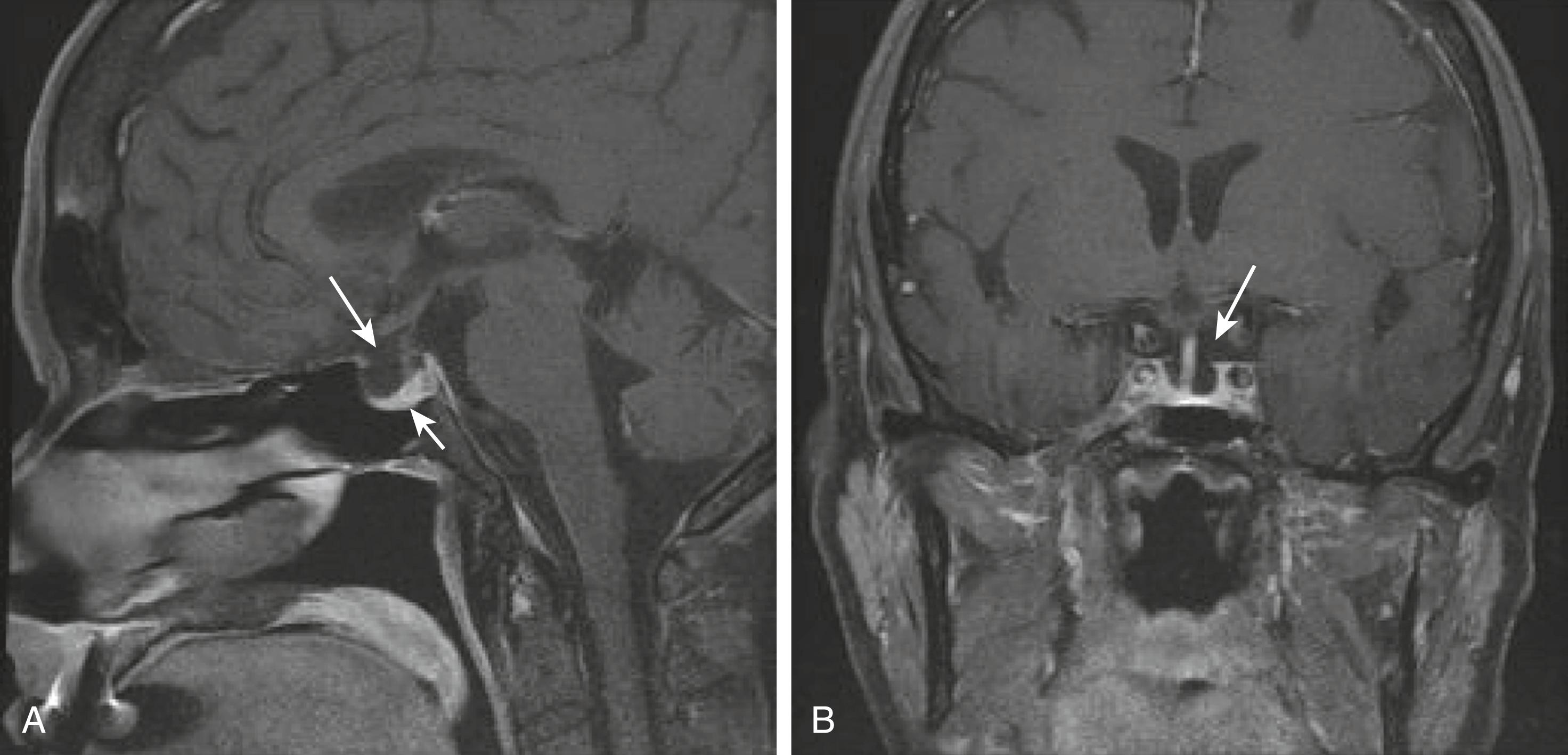
In asymptomatic patients, there is no need to measure hormone levels unless there is suspicion of a clinical abnormality related to the pituitary. Pituitary function is usually normal, but about 10% of patients also have mild hyperprolactinemia, probably owing to stretching of the pituitary stalk. Because pituitary function is usually normal, no specific treatment is required. However, if there is concern about possible hypopituitarism, investigation as outlined earlier for hypopituitarism (see Table 205-2 ) should be undertaken.
Pituitary adenomas have an incidence of about 4 to 7 cases per 100,000 and a prevalence of about 1/1000 population. Clonal proliferation of any of the different cell types in the pituitary result in the formation of tumors. Pituitary tumors are classified clinically as functioning if they produce one or more hormones in excess or nonfunctioning if they do not produce excess hormone in blood. Because most functional tumors do not have appropriate physiologic feedback owing to their oncologic nature, they usually cause the clinical syndrome of the excess hormone. However, some functional tumors do not result in excess blood levels of the hormone, owing to a defect in intracellular processing or release.
Prolactinomas, which may account for up to 50% of pituitary tumors, are likely caused when proliferation of the lactotroph results in excess prolactin, with subsequent galactorrhea and hypogonadism. Somatotrophs producing growth hormone, corticotrophs making ACTH, and gonadotrophs making LH and FSH together account for 10 to 20% of adenomas. Nonfunctioning tumors, which mainly cause a mass effect or hypopituitarism, account for 10 to 25%. In addition to functioning and nonfunctioning, tumors are classified by their anatomy based on imaging studies. Microadenomas are less than 10 mm in diameter, whereas macroadenomas are greater than 10 mm in diameter (and typically with extrasellar extension).
Pituitary adenomas are monoclonal and can be locally invasive, but they are rarely malignant. The somatic mutations causing most pituitary tumors remain unknown, but at least six types of inherited predispositions to pituitary tumors are recognized ( Table 205-6 ). When macroadenomas are found in children or when there is a family history of pituitary adenomas, genetic testing is warranted so that appropriate monitoring can occur before the patient is symptomatic. Local expansion of the tumor can cause headaches. The mass effect of the tumor is also responsible for the temporal visual field defects associated with macroadenomas that expand into the suprasellar region and abut against the optic chiasm.
| SYNDROME | PITUITARY TUMOR | MOLECULAR PATHOGENESIS | MODE OF INHERITANCE | OTHER MANIFESTATIONS |
|---|---|---|---|---|
| McCune Albright | F/NF | Gsα SU | Somatic mutation | Ovary, bone, thyroid dysfunction |
| Multiple endocrine neoplasias ∗ | F (30% PRL)/NF | Menin ( MEN1 ) and MEN4 ( CDKN1B ) | AD | Parathyroid and pancreas tumors |
| Familial isolated pituitary adenoma (FIPA) syndrome ∗ | F/NF | Arylhydrocarbon receptor interacting protein ( AIP ) | AD with variable penetrance | None |
| Carney complex (with or without PPNAD) | 1/3 with Cushing | Type 1A regulatory subunit of protein kinase A ( PRKARIA ) | AD | Atrial myxomas, spotty skin pigmentation, schwannomas |
| Primary pigmented nodular adrenocortical disease (PPNAD) | Cushing | Succinate dehydrogenase subunit ( SDH ) | AD | Pheochromocytomas, paragangliomas |
| Early childhood gigantism | GH | CD40LG, ARHGEF6, RBMX, GPR101 | X-linked | Gigantism |
∗ Tumors are generally more aggressive and occur at a younger age.
The clinical manifestations of pituitary tumors depend in large part on whether they are functional and whether their size compromises the normal function of the pituitary gland and thereby results in clinical symptoms of hypopituitarism. In general, the diagnosis of a tumor due to excess or diminishing hormone secretion presents the greater clinical challenge.
The size of the tumor usually is proportional to the amount of hormone it produces when functioning. Certain tumors are usually diagnosed sooner and therefore are usually smaller on diagnosis than others. For example, gonadotropinomas are usually diagnosed as macroadenomas because their symptoms are usually more subtle than TSH-secreting tumors, in which the symptoms present early so the tumors are usually smaller at the time of diagnosis.
As is true for hypopituitarism of all causes, pituitary tumors have historically been diagnosed more frequently in women of reproductive age because even a slight perturbation in hormonal milieu will disrupt the multiple hormone interactions necessary for a normal menstrual period ( Chapter 219 ). In men or postmenopausal women, slight perturbations in hormone levels (either excess or deficiency) are usually not noticed for several years. More recently, the diagnosis of pituitary adenomas is often an incidental finding on an imaging study obtained for unrelated reasons (e.g., a motor vehicle accident, headaches, or a nonpituitary intracerebral mass effect), and a pituitary microadenoma is an incidental finding. These findings are referred to as “incidentalomas” that otherwise would have avoided clinical detection because they are either silent or subclinical. Incidentalomas can be found in up to 10% of some autopsy series, so they are more common than pituitary tumors found during life. Even after careful endocrine evaluation of patients with incidental tumors, hyper- or hyposecretion of pituitary hormones is found in only a small percentage, and such incidental tumors usually do not increase in size over time.
Other than hormonal effects, reasons for suspicion of a pituitary mass are headaches and visual field abnormalities. A reported 33 to 72% of patients with a pituitary lesion have headaches, a percentage that is greater than the 22% of women and 11% of men in the general population who complain of headaches ( Chapter 367 ), but discerning which patients have a headache from the pituitary lesion and which ones have an unrelated cause for the headache is challenging. Headaches due to pituitary lesions are more common in females and in patients with Rathke cleft cysts and apoplexy.
Severely impaired visual fields can be revealed on examination by confrontation testing. The superior and temporal visual fields are most affected.
MRI is the cornerstone of diagnosis for nonfunctioning tumors and key for the evaluation of functioning tumors. If there is any evidence on MRI that the tumor is close to or abuts the chiasm, formal visual field testing should be performed. Specific diagnosis of pituitary adenomas that are actively secreting a hormone is based on basal hormone levels followed by provocative testing ( Table 205-7 ).
| HORMONE | TEST | INTERPRETATION |
|---|---|---|
| Growth hormone (GH) | Basal IGF-I | Elevated IGF-I levels are consistent with acromegaly when interpreted in the context of age and nutritional status. |
| Oral glucose suppression test: after 75-g glucose load, GH is measured at −30, 0, 30, 60, 90, 120 min. | GH should be suppressed to <1 µg/L in normal persons with polyclonal radioimmunoassays; <0.4 µg/L with two-site monoclonal assays. GH may paradoxically increase in acromegaly. | |
| Prolactin | Basal prolactin levels | Elevated prolactin (>200 µg/L) is consistent with a prolactinoma. When prolactin levels are between 20 and 200 µg/L, other causes of hyperprolactinemia should be considered. |
| Adrenocorticotropic hormone (ACTH) | Measurement of 24-hr urine free cortisol | Elevated level is suggestive of Cushing syndrome, but it has several other causes as well. |
| Midnight salivary cortisol: special tubes with cotton pledgets available to collect saliva at 11 pm to midnight | In normal persons, the midnight salivary cortisol is very low because of the normal diurnal variation. In patients with Cushing syndrome, the salivary cortisol is elevated. | |
| Overnight dexamethasone suppression test: dexamethasone (1 mg) PO at midnight, followed by 8 am plasma cortisol | In normal persons, am cortisol should be suppressed to <5 µg/dL. Normal test excludes Cushing syndrome. Other disorders can cause failure to suppress normally. | |
| CRH test: ovine CRH (1 µg/kg) is administered IV, and ACTH and cortisol are drawn at −15, 0, 15, 30, 60, 90, and 120 min. | In Cushing disease, there is usually a 50% increase in ACTH and a 20% increase in cortisol. Adrenal adenoma is associated with suppressed ACTH. Ectopic ACTH is associated with high basal ACTH and cortisol levels that are not affected by CRH. | |
| Petrosal sinus ACTH sampling: the inferior petrosal sinus is catheterized bilaterally, and plasma ACTH is compared with simultaneous peripheral samples. The sampling can be done in conjunction with CRH or vasopressin stimulation. | In Cushing disease, the ratio of ACTH in the petrosal sinus to the periphery is at least 2 basally and at least 3 after CRH. In ectopic ACTH, the ratio of petrosal sinus to peripheral level is <1.5. | |
| Thyroid-stimulating hormone (TSH) | Basal thyroid function tests | An inappropriate normal or elevated TSH in the setting of increased free thyroid hormone levels is consistent with a TSH-producing tumor or other causes of inappropriate TSH secretion. |
| Free α-subunit level | Elevated levels associated with inappropriately elevated TSH are suggestive of a TSH-producing tumor. | |
| Follicle-stimulating hormone (FSH), luteinizing hormone (LH) | Basal FSH, LH, testosterone | Increased LH and testosterone levels in males are consistent with LH-secreting tumors. Elevated FSH and low-normal testosterone are suggestive of an FSH-producing tumor if primary gonadal failure is not present. In females, assessment of excess hormone secretion is difficult because of changes during the menstrual cycle and at menopause. |
Except for prolactinomas, the first line of therapy for pituitary tumors that require treatment is surgery ( Video 205-1 ). The major exception is for prolactinomas (even those that are macroadenomas and result in visual field cuts, headaches, and evidence of selected hypopituitarism), for which medical therapy is the first mode of treatment. Indications for surgery include decompression of mass effects, prevention of further tumor expansion, and normalization of hormone levels. The endoscopic transsphenoidal approach is the standard for decompression or extirpation. Subfrontal craniotomy is reserved for tumors requiring extensive extrasellar exploration. Transsphenoidal surgery is effective, with a less than 5% complication rate and 1% mortality rate in experienced centers, but potential sequelae include sinusitis, hemorrhage, CSF leak, hypopituitarism, and optic nerve injury. About 5% of patients develop transient postoperative diabetes insipidus that rarely persists. About 80 to 90% of microadenomas and only 30 to 60% of macroadenomas are completely cured by transsphenoidal hypophysectomy, but most other patients substantially improve in terms of their hormone levels.
Irradiation (typically 45 Gy) can be adjunctive therapy after surgery or used in combination with medical therapy after curative surgery has failed, based on radiographic or endocrine testing, or when surgery is unable to remove the tumor completely. CyberKnife stereotactic radiotherapy can deliver the same total dose in one treatment as would be provided by traditional external beam radiotherapy over 5 weeks. CyberKnife therapy spares critical radiosensitive structures, but the response can take months up to several years. Proton beam therapy may be performed for intrasellar lesions but is not widely available. Irradiation provides complete remission, and it is most useful for nonfunctioning macroadenomas and for patients who have some residual postoperative tumor or visible tumor. Complications are generally dose related and include partial or complete hypopituitarism (50 to 70% of patients), second tumors in the radiation field (about 2% of patients over a 20-year period), cognitive dysfunction, damage to the optic nerve, and stroke. Stereotactic radiotherapy appears to cause similar complication rates of hypopituitarism but less stroke compared with conventional external beam therapy.
Medical therapy varies by the type of tumor (see later for details). Medical therapy is the primary treatment for prolactinomas but is a secondary option when surgery is not curative or is contraindicated by other pituitary tumors.
About two-thirds of incidental, asymptomatic pituitary microadenomas remain unchanged in size or even become smaller For other adenomas, however, the sooner treatment is initiated to decompress the chiasm (with surgery, or in the case of a medically responsive prolactinoma, with appropriate medication), the more likely vision will be restored. Nevertheless, visual improvement has been noted even after months of visual field impairment. Even after apparent surgical cure, about 10 to 20% of tumors recur within several years, as manifested by oversecretion of their hormones, and recurrence is more likely if the tumor extended into the suprasellar space or fragmented upon removal.
Growth hormone is the main regulator of the growth of bone and other tissues. The human growth hormone gene, along with four other related genes, is located at the growth hormone locus on chromosome 17 where, by gene duplication, these genes are interspersed in the same transcriptional orientation. The five genes not only have a high degree of sequence identity but also exist as multiple isoforms based on variable splicing. The variant expressed in the somatotrophs of the pituitary gives rise to two isoforms. Growth can occur in the fetus and early postnatal state independent of growth hormone; but soon after birth, growth hormone controls growth, and its deficiency results in dwarfism.
Growth hormone is a peptide hormone whose receptor is located on the cell surface. Growth hormone promotes the dimerization of two growth hormone receptors and activates JAK2 by phosphorylation of tyrosine residues. The activated JAK2 in turn activates STAT and an entire cascade of signal factors, thereby resulting in nuclear activation.
The metabolic effects of growth hormone are both direct, by interaction with its tissue receptors, as well as mediated by a serum factor secreted by the liver (somatomedin or insulin-like growth factor-I [IGF-I]). IGF-I in turn acts on a variety of tissues to stimulate body growth. IGF-I is also produced in a variety of tissues including smooth muscle, skin, lung, bone, and cartilage. However, in addition to IGF-I production in the various tissues, growth hormone causes hydrolysis of triglycerides in adipose tissue. In skeletal muscle, growth hormone enhances uptake of amino acids and retention of nitrogen, similar to what would be expected in exercising muscle. Growth hormone also stimulates glycogenolysis and gluconeogenesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here