Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The molecular pathogenesis of human cancer is a complex process that often requires the cooperation of genetic mutations within many cellular pathways that ultimately lead to tumorigenesis. Simple model organisms with conserved genes and developmental pathways offer systems with which to dissect the role of individual genes and their contribution to the development of cancer in vivo. From single-celled yeasts to vertebrate fish such as the zebrafish, each model system provides its own unique strengths with which to identify new genes and to elucidate genetic interactions required for the development of cancer.
Cancer develops as a result of disruption of the normal physiological processes of cell growth, differentiation, and proliferation. Genes involved in these processes encode transcription factors and other regulatory proteins controlling the cell cycle, apoptosis, and survival. Many of these genes, found in the simplest eukaryotes, are conserved with higher species. Over several decades the development of tools for forward genetic analysis based on phenotype in simple organisms has led to yeasts (Saccharomyces cerevisiae) , the fruit fly (Drosophila melanogaster) , and more recently the zebrafish (Danio rerio) emerging as the key simple organisms for investigating cancer genetics ( Figure 8-1 ).
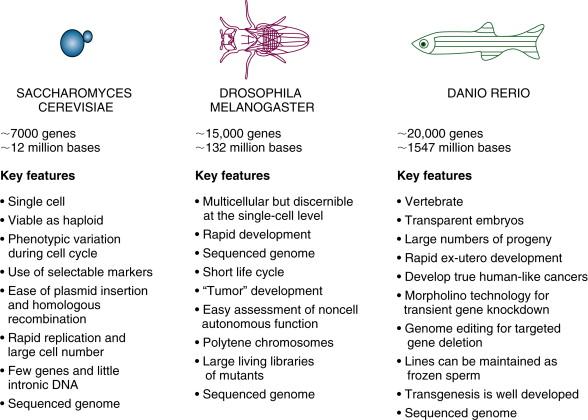
In addition to their individual strengths for investigating conserved pathways there are three main practical reasons to use a simple model organism: time, space, and tractability. Yeasts such as Saccharomyces cerevisiae (S. cerevisiae) and Schizosaccharomyces pombe (S. pombe) are single-cell organisms. They have comparatively few genes and little intronic DNA. They replicate rapidly by budding (S. cerevisiae) or fission (S. pombe) and can be maintained in large numbers in both haploid and diploid states, facilitating the isolation and investigation of recessive mutations. Yeast cell numbers double every 100 minutes (given adequate nutrition), and these organisms are safe and cheap to maintain. The whole organism can be readily visualized by light microscopy, and the incorporation of fluorescent proteins allows subcellular localization of specific proteins in real time. Similarly, Drosophila melanogaster has a life cycle of 10 days, and large numbers of animals can be maintained in a small space. This multicellular organism can be used to examine cell-cell interactions and the roles of non-cell autonomous gene function in the development of cancer. Although Drosophila melanogaster does not develop cancer in its classical form and lacks the closed blood system of vertebrates, ingenious genetic techniques have been applied to model pathways involved in the development of cancer and invasive metastases in this organism, which has the added advantage of being both multicellular and tractable to single-cell resolution. The zebrafish is relatively new to the field of well-characterized model organisms but has rapidly gained popularity. As a vertebrate with a closed vascular system (and beating heart), it provides an ideal intermediate model system between studies in invertebrates and small mammals such as the mouse. In contrast to the mouse, zebrafish development occurs rapidly outside the mother in transparent embryos, allowing direct visualization of the evolving systems and easy analysis of incorporated fluorescent markers. Its small size and high fecundity allow it to be easily maintained. The ability to directly visualize development in the embryo is particularly beneficial given that proto-oncogenes often have a crucial role during embryonic development.
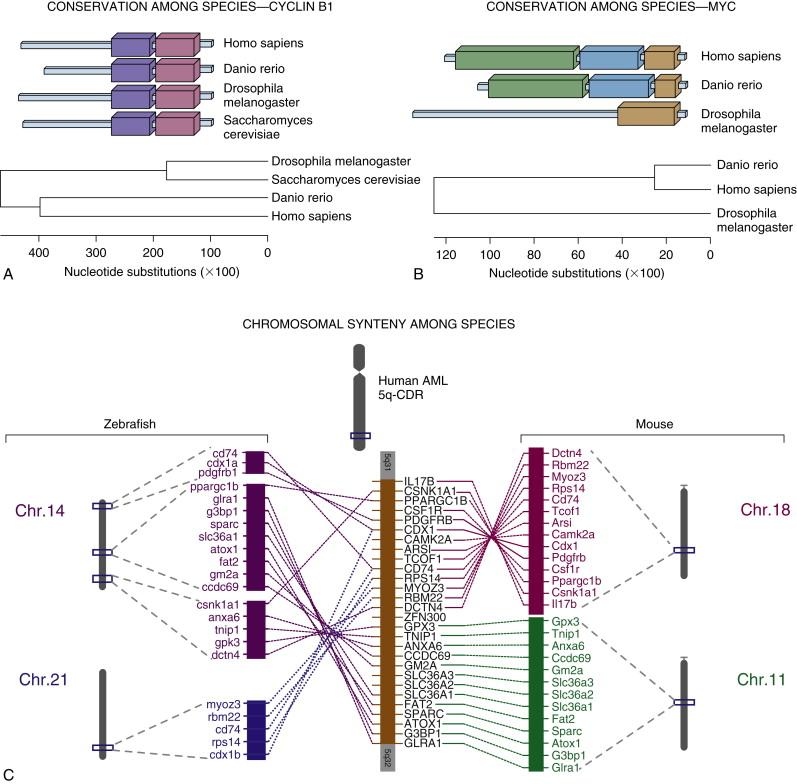
It has long been recognized that simple organisms carry genes that have functional equivalents in humans. Conclusive evidence for this phenomenon was determined when human DNA sequences expressed in yeast cells rescued the phenotypic defects arising from mutations in yeast or Drosophila genes, allowing the homologous human DNA sequence to be cloned. Conservation of a given gene through evolution suggests that its protein product performs essential functions that are similar across species. The more highly conserved the gene, the more important or critical the function of its product. For example, the amino acid sequence of the fission yeast ribosomal protein of the small subunit 14(rpS14) is 75% identical to the human RPS14. This suggests that from the millions of random mutations arising over millions of years of evolution, only 75% diversification of the amino acid sequence occurred, presumably because the majority of mutations resulted in deleterious effects on the fitness of the organism. Similarly, the most critical functional domains of a protein can be implied by determining the regions that are most highly conserved among species (illustrated in Figure 8-2 ). The genetic complexity of higher eukaryotes, however, indicates that not all genes are conserved. Indeed, even with the now-vast repositories of bioinformatics that house sequence data for hundreds of genes and species, it is not always straightforward to identify a single gene that is the functional ortholog of a human gene in a simple organism. As a result, in simple organisms there are often fewer genes performing a particular function than in humans. An example of this is the number of functionally conserved BCL2 family members in the intrinsic apoptosis pathway seen in worms and zebrafish (with none identified in yeasts). This is further complicated in zebrafish, where whole-genome duplication that occurred in teleost fish more than 150 million years ago has resulted in the presence of two or three copies (paralogs) of a large number of individual genes. Several methods permit determination of which copy of such genes is functionally homologous to the human gene. The analysis of genetic synteny using bioinformatic approaches has demonstrated that zebrafish orthologs of human genes can usually be found in chromosomal locations in the fish that reflect their location on conserved human chromosomal regions. In many cases divergence in promoter sequences leads to tissue-specific activities of different teleost orthologs of a single mammalian gene (see Figure 8-2 , C ).
Simple model organisms, with their rapid development and large numbers, lend themselves well to the identification of new oncogenes, tumor suppressors, and novel therapeutic targets important in cancer. This is generally accomplished using classical (and variations on classical) genetic screens. Forward genetic screens are based on Mendelian inheritance of genes and the observation of a phenotype in cells or organisms where a gene has been disrupted ( Figure 8-3 ). The methods by which genes are disrupted and the assays used to screen for phenotypes are wide ranging, and some are species specific. Early screens looked for naturally occurring phenotypes of simple processes, such as inability of yeast to grow at a certain temperature, as a method of assaying genes involved in cell division. To increase the number of mutated organisms (mutants) with a phenotype of interest, methods of inducing mutations in genes, either chemically with retroviruses or with transposons (“jumping genes”), have been employed. Large numbers of organisms are screened to ensure that every gene within the genome has been mutated at least once—this is known as saturation of the genome. Forward genetic approaches allow unbiased isolation of phenotypically mutant organisms, after which the gene causing the mutant phenotype can be identified. The forward genetic screen remains the most powerful strength of model organisms as a cancer model, in particular for the discovery of novel tumor suppressors. Tumor suppressors are genes whose normal functions are to regulate uncontrolled or abnormal growth, or the growth of cells whose genetic integrity has been compromised. Because the cancer-causing effects of these genes can only be fully recognized when they are absent or nonfunctional, forward screening of large numbers of simple organisms is an attractive means to identify such genes in vivo. Historically, a potential drawback of such forward screens, particularly in the zebrafish, where there is somewhat greater genetic complexity, is that identification of the genetic mutation causing the phenotype can sometimes be time consuming and technically challenging: something of a “needle in a haystack” search. This is especially true if the mutation occurs in a regulatory or noncoding region of the genome or in telomeric regions of chromosomes where increased amounts of crossing over during meiosis further hamper conventional positional cloning techniques. However, the past decade has seen the emergence of massive parallel sequencing technologies that are now being used to facilitate rapid fine mapping of genetic mutants derived from phenotypic screens. These technologies allow hundreds of genes to be sequenced simultaneously, covering the entire genome in such a way that regions of the genome linked to the mutant phenotype can be identified when the fish have been crossed to a strain of a different genetic background. The rapidly decreasing costs and increasing efficiency and availability of these technologies will likely fuel a new wave of studies in coming years, accelerated by advances in deep sequencing for positional cloning.
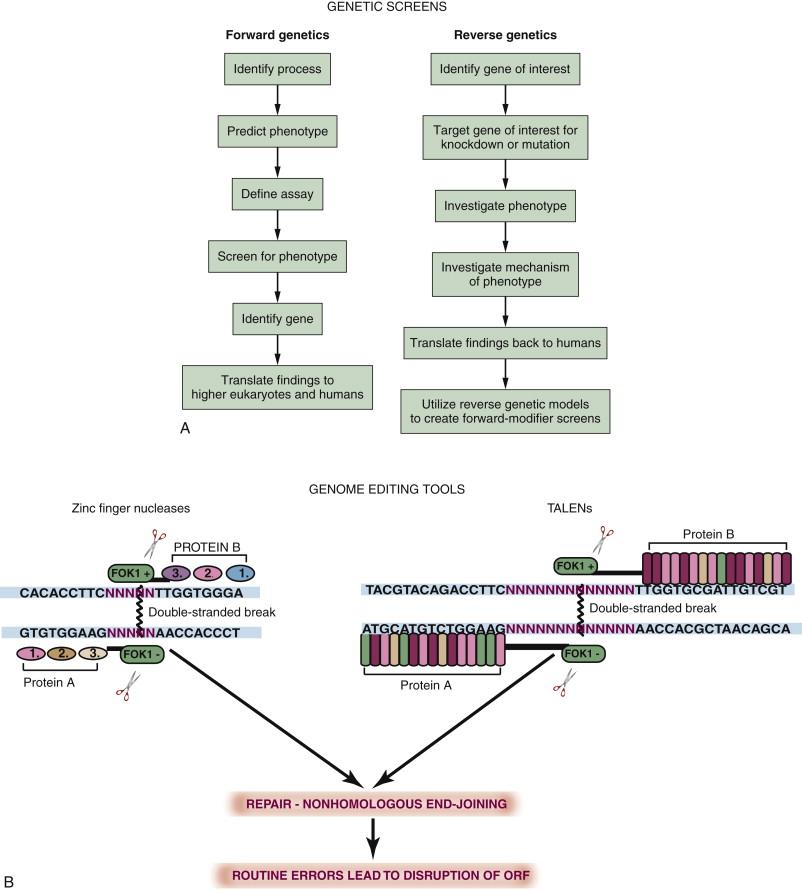
In addition to forward genetic modeling, it is also possible to disrupt specific known genes (such as known oncogenes or tumor suppressors) to investigate the phenotype produced. This process is termed reverse genetics . The most common reverse genetic studies are carried out using transient knockdown techniques. These include RNA interference (RNAi) in flies and antisense oligonucleotides (“morpholinos”) in frogs and fish. These techniques are particularly useful in determining epistatic relationships among genes giving rise to a particular phenotype, as several genes can be knocked down simultaneously. More recently, there have been a number of advances in the field of genome editing technologies that have made the generation of stable knockout lines at virtually any site within the genome accessible to researchers using simple organisms. This is beneficial because it allows specific domains within a protein to be targeted for mutation and allows propagation of mutations through the germline for studies in later stage juveniles or adult organisms. This is achieved by the creation of synthetic restriction endonucleases that fuse the cleavage portion of FokI, a naturally occurring bacterial restriction endonuclease, to a site-specific DNA binding domain generated using synthetic zinc fingers (ZFNs) or transcription activator–like effector nuclease (TALEN) repeats. Two DNA sequences are targeted, one on either side of the region of the genome where a mutation is desired (see Figure 8-3 , B ). Because FokI requires dimerization to induce cleavage at its target site, this produces site specificity and minimizes off-target mutations. Novel technologies allowing the generation of large numbers of TALENs are likely to make the use of this form of genome editing widespread not only in simple organisms but also in mammalian models and cell culture systems.
Forward and reverse genetic models may be used in combination. Reverse genetics in simple organisms may add tools for the investigation of the genetic interactions of a known gene by permitting the development of modifier screens where the mutant is subjected to forward genetic screening to identify genes that enhance or suppress its phenotype. In this way modifier screens may provide novel therapeutic targets in human cancers.
Another genetic tool used in simple model organisms is transgenesis. To obtain a transgenic organism, specific DNA sequences are typically introduced into the genome of an organism and expressed under the control of a specific promoter sequence to guide the cellular, temporal, and spatial localization of transgene expression. For example, in zebrafish, the introduction of the mouse Myc gene under the control of the rag2 lymphocyte-specific promoter leads to expression of Myc in the thymus and the subsequent development of T-cell leukemia/lymphoma. Adding the coding sequence of green fluorescent protein to the integrated construct has the additional advantage of permitting spatiotemporal visualization of the development of cancer in this system. A prime example is the recent use of dopamine β-hydroxylase promoter to drive MYCN and activated ALK expression in transgenic zebrafish to produce neuroblastoma, demonstrating the power of this model for detailed studies to reveal cellular mechanisms underlying synergy between oncogenes that are also activated together in human malignancies. Transgenic approaches are easily performed in yeasts, which undergo efficient homologous recombination into their chromosomes, allowing site-specific integration of the transgene. Using this tool in yeasts facilitates reverse genetics by replacing the normal functioning gene with a mutated or nonfunctional form (which may be genetically engineered or even derived from a different species).
Simple organisms can also be used for drug discovery in a variety of ways. Yeast can be manipulated to express a gene or protein of interest at a higher level than normal, or a heterologous human gene under control of a yeast-specific promoter. A differential effect of a drug on the normal-versus-mutated gene can also be assessed (e.g., attempting to find drug targets selective for certain oncogenes). More recently, drug screens using whole organisms, including both flies and zebrafish, have been successful in uncovering potential novel therapeutic anticancer drugs and for revealing previously unsuspected anticancer activities of existing drugs.
Only a small number of human cancers can be attributed to the presence of an underlying inherited cancer predisposition mutation; thus the majority of human cancers arises from a series of somatic mutations acquired over time. Because many human oncogenes and tumor suppressors play a critical role in embryologic development, germline mutations affecting key genes of this type in all tissues frequently lead to death during development. Although investigation of the embryologic phenotype in model organisms continues to provide crucial information on gene function, the study of genetic interactions in specific tissues and in specific cancer models provides additional information on how the mutated gene contributes to tumorigenesis in vivo.
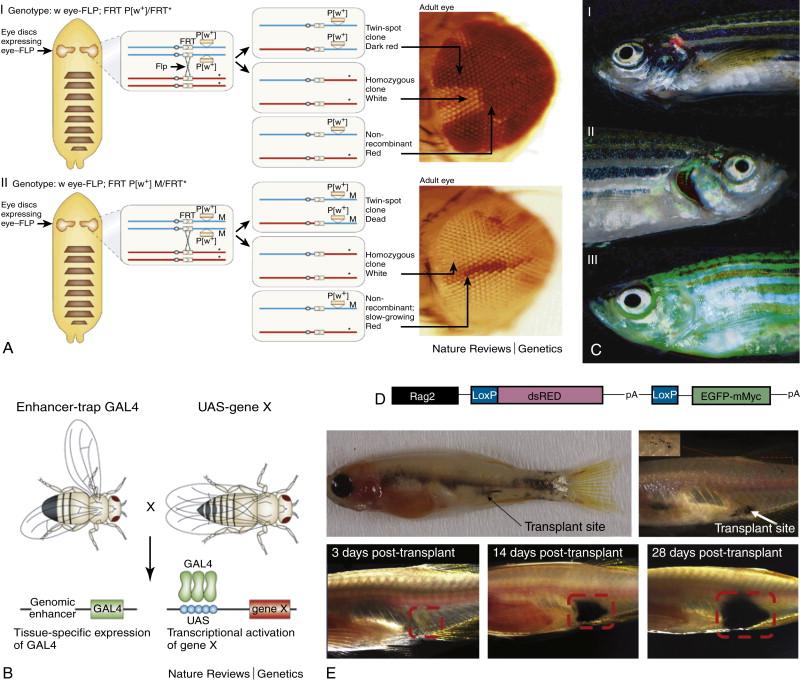
Over the past 20 years, genetic tools have been developed to engineer targeted conditional expression or (in some cases) knockout of a specific gene. There are three main conditional systems that facilitate directing gene expression to a specific cell type at a specific time. The first is based on an enzyme from P1, which is a bacteriophage that infects the bacterium Escherichia coli . This virus produces an enzyme called Cre recombinase that cuts DNA whenever it sees two identical 34-base-pair sequences known as Lox-P sites. The enzyme removes the DNA between the two Lox-P sites, and the sites are ligated together. In model organisms, this system can be used by driving the expression of Cre with a promoter that is only expressed at a certain site (tissue specific) or with a promoter that is activated by exposure to a specific stimulus such as heat shock or a particular drug (e.g., estrogen). Lox-P sites can be introduced into a transgene such that on Cre activation, the transgene is expressed (or removed) in a specific tissue. Fluorescent proteins can also be incorporated into transgenes to allow visualization of where the transgene is expressed and where the Lox-P sites have been removed ( Figure 8-4 ). In a similar fashion, Flp recombinase is an enzyme made by the 2-μm plasmid of Saccharomyces cerevisiae . This recombinase acts in a similar way to Cre recombinase, recognizing two 34-base-pair sequences termed Frt sites . This system has been extensively employed in flies, where instead of simply excising the intervening DNA sequence between two Frt sites, Flp recombinase results in the crossing over and exchange of genetic material between arms when Frt sites are located on opposite arms of the same chromosome. This allows, for example, the expression of mutant tissue in an otherwise wild-type background (see Figure 8-4 ). Tissue-specific overexpression of a gene can also be achieved by using the yeast transcription factor Gal4 driven by a tissue-specific promoter and its upstream activating sequence (UAS) driving the gene of interest. UAS can also drive a fluorescent protein–colored marker to allow spatial localization of cells expressing the gene of interest (see Figure 8-4 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here