Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The bony thoracic cage and crowded vital organs in the thorax make open surgical access difficult and challenging. For these same reasons, the thorax is ideal for image-guided interventions. In addition to procedural skills, a thorough knowledge of the anatomy, physiology, pathology, imaging findings, and preprocedure and postprocedure care is required for successful practice of various interventional procedures. Thoracic interventions can be broadly divided into vascular and nonvascular interventions. Nonvascular interventions include image-guided biopsy and drainage procedures, tumor ablation, lymphatic interventions, and esophageal and tracheobronchial interventions. Some of these procedures are covered elsewhere in this text. Vascular interventions involve the aorta and its branches, pulmonary arteries, and venous structures and include revascularization procedures (angioplasty, stent placement), vascular occlusion procedures (embolization and exclusion), and venous access procedures. This chapter provides a brief overview of some vascular and lymphatic interventions for a diagnostic radiologist.
For several years, catheter-based pulmonary angiography was the standard imaging technique for the evaluation of the pulmonary arteries. Technical advances in cross-sectional imaging, especially with the introduction of multidetector computed tomography (MDCT), have had a significant impact on imaging of the pulmonary arteries. CT pulmonary angiography is now the first-line imaging technique to evaluate pulmonary arterial diseases, including acute pulmonary embolism (PE). Catheter-based pulmonary arteriography is now essentially used as a problem-solving tool or as an a priori imaging technique for endovascular interventions. The current indications for performing catheter-based pulmonary arteriography are as follows:
Clinically suspected acute PE when CT pulmonary angiography is nondiagnostic
Chronic PE, especially prior to surgical endarterectomy
Suspected pulmonary vascular abnormalities, such as vasculitis, congenital and acquired anomalies, and tumor encasement
As part of a pulmonary arterial intervention
Clinical history, laboratory values, and medications should be reviewed. A clinical history of left bundle branch block places the patient at risk of a complete heart block while catheterizing the pulmonary arteries. Temporary cardiac pacing prior to the procedure may be considered in elective cases to mitigate this risk. Moderate pulmonary hypertension is a relative contraindication for pulmonary angiography. Iodinated contrast materials are known to cause pulmonary vasoconstriction and pulmonary edema, leading to increased right heart strain. The use of low-osmolar contrast material and limiting the injection volume can mitigate this risk. Similar to any other vascular procedure involving the use of contrast material, renal function tests, coagulation parameters, and platelet counts should be checked.
The procedure can be safely performed under local anesthesia. Moderate sedation is often administered to reduce anxiety. Patients who cannot lay flat or hold their breath for 10 seconds or have a poor airway are best intubated for pulmonary angiography. If a left bundle branch is known, a temporary pacer is placed. Jugular or femoral vein access is established after administering local anesthesia. The choice of venous access is dependent on the operator's preference and length of the catheter or devices anticipated for the procedure. Shorter catheters and devices will require a jugular access. Most of the catheters used for pulmonary angiography are between 6 and 8 Fr. Standard pulmonary flush catheters (Grollman, Van Aman, Berman) have a 90-degree curve to facilitate maneuverability through the right heart. A pigtail catheter and a tip-deflecting guidewire can also be used to catheterize the pulmonary artery.
Pulmonary arterial pressures are measured with the catheter positioned in the right or left main pulmonary artery. A nonselective pulmonary trunk arteriography is rarely performed. Selective arteriography with the catheter tip in the right and left pulmonary arteries in the frontal and lateral projections is typically performed. The contrast material is injected at a rate of 15 to 20 mL/s, for a total volume of 30 to 40 mL ( Figs. 10.1 and 10.2 ). The images are acquired at a rate of six frames/s using digital subtraction angiography during a single breath-hold.
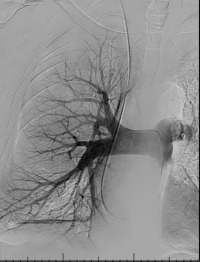
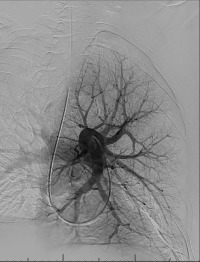
Minor complications, including access site complications, transient arrhythmias, and allergy to contrast materials occur in 5% of patients. Major complications include contrast-induced nephropathy, which occurs in 1%, and fatalities, which occur in 0.5%. With the advent of flexible catheters and nonionic contrast materials, the complication rates are even lower.
Image-guided interventions of the pulmonary arterial circulation involve treatment of pulmonary arteriovenous malformations (PAVMs), pulmonary artery aneurysms and pseudoaneurysms, pulmonary arterial embolism, and pulmonary arterial stenosis.
PAVMs are rare vascular anomalies of the lungs, in which abnormal dilated vessels provide a direct high-flow, low-resistance, right-to-left shunt between the pulmonary artery and vein. PAVMs are usually part of hereditary hemorrhagic telangiectasia (HHT), although sporadic cases occur in 30% of patients. Conversely, one-third of patients with HHT harbor a PAVM. Uncommon causes of PAVMs include trauma, malignancy, and hepatopulmonary syndrome. Patients may be asymptomatic, especially with a small PAVM, but larger lesions may present with hypoxia or heart failure from a right-to-left shunt. Other presentations are usually secondary to systemic emboli; these include transient ischemic attacks (37%), stroke (18%), brain abscess (9%), and seizures (8%). Rupture leading to pulmonary hemorrhage or hemothorax is a rare but well-known and dreaded complication. Diagnosis is usually with CT pulmonary angiography and bubble echocardiography. Endovascular occlusion of the feeding artery with fibered coils or vascular plugs is the mainstay of treatment.
PAVMs are classified into simple (single pulmonary artery directly communicating with a single pulmonary vein, with or without an aneurysm), complex (two or more segmental arteries feeding the PAVM), and a telangiectatic type. The latter does not need treatment. Treatment of nontelangiectatic PAVM is indicated in all patients who are symptomatic and when the feeding artery of the PAVM is 3 mm or more in diameter, irrespective of symptoms. Patients require lifelong antibiotic prophylaxis for dental or surgical procedures, despite therapy.
Based on the location of the PAVM on CT pulmonary angiography, selective pulmonary angiography of the lung is performed. The feeding pulmonary arteries are identified ( Figs. 10.3 and 10.4 ). At our institution, the 7.0/5.0-Fr Lumax guiding catheter (Cook Medical, Bloomington, IN) is used to cannulate the feeding artery. Microcatheters are used if a more selective catheter position is desired. The feeding artery is occluded with fibered coils or vascular plugs ( Fig. 10.5 ). Coils or vascular plugs are selected based on the size of the feeding artery, with the coil usually oversized by 2 mm or 20% and vascular plugs by 30% to 50%. Embolization of the aneurysmal segment is not indicated. In the presence of a high-flow PAVM, a technique known as the anchor technique is used to prevent paradoxic embolization of the coil. In this technique, the initial segment of a long coil is first deployed in a proximal branch vessel (to anchor it to the branch) and the rest of the coil is then prolapsed into the feeding artery supplying the PAVM. The embolization procedure is much simpler with the use of detachable vascular plugs, especially in PAVMs with large but short feeding arteries.
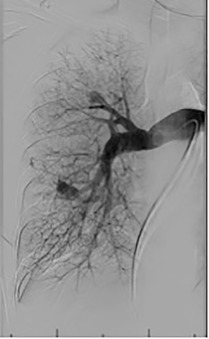
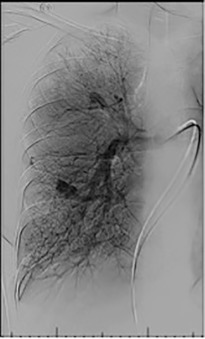
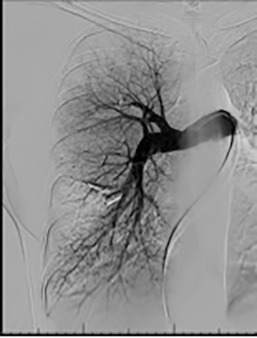
Embolization procedures are highly successful (close to 100%), but recanalization is known to occur in 5% to 19% of cases. With the use of the vascular plugs, the recanalization rates are much lower. In addition, smaller PAVMs may grow with time and may require therapy during follow-up. Posttreatment, contrast-enhanced CT is performed within 6 to 12 months and then at least every 3 years to assess reperfusion or growth of the PAVM. Minor complications include chest pain and pleural reactions, which occur in 10% to 14% of patients. These are self-limiting and resolve within 1 week, with supportive care and antiinflammatories on an outpatient basis. Other complications include hemothorax, pulmonary infarct, coil migration and nontarget embolization, stroke, severe pulmonary hypertension, access site complications, and persistent cardiac arrhythmia.
Systemic anticoagulation is the mainstay of therapy for acute PE. Systemic thrombolysis for massive and submassive PE has been well established. Intravenous infusion of tissue plasminogen activator (tPA) along with systemic anticoagulation decreases the risk of death (41% risk reduction compared to anticoagulation alone) and recurrence. However, systemic thrombolysis is contraindicated in the presence of a recent stroke or intracranial injury, intracranial malignancy, bleeding from any source in the body, and recent surgery. Intracranial bleeding is the most devastating complication of systemic thrombolysis, occurring with a frequency of 2% to 5%. Major bleeding complications, including retroperitoneal and gastrointestinal bleeding, occur in 9%. Catheter-directed thrombolysis (CDT) has been gaining acceptance in the treatment protocol for massive and submassive PEs by limiting the systemic side effects of thrombolysis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here