Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver transplantation is a very successful and effective treatment for many different disorders of the liver. Improved techniques in surgery, anesthetic management, donor organ procurement, preservation, immunosuppression, and perioperative care have resulted in many centers reporting a 1-year survival rate for some recipient groups of nearly 90%. Increasing survival rates have led to the performance of a greater number of liver transplants and to broadened indications for transplantation. The numbers of transplant centers are expanding as the demand increases and the numbers of trainees graduating increases. The result of this is that many anesthesiologists involved in the management of liver transplants may have limited experience. The goal of this chapter is to provide a comprehensive up-to-date manual to serve as a reference resource for anesthesia providers. A further resource for the reader is the International Liver Transplantation Society Web site ( http://www.ilts.org ), which maintains a current database of the literature, interactive discussions, and educational monographs.
The American Society of Anesthesiologists ( http://www.asahq.org ) and the United Network for Organ Sharing ( http://www.unos.org ) have produced the specific requirements for providing anesthesia services for liver transplant procedures.
A director of liver transplant anesthesia must be able to establish a clear communication channel between the transplant anesthesiology service and services from other disciplines that participate in the care of liver transplant patients. The types of activities to be involved in include perioperative consultations, participation in the candidate selection process, attendance at morbidity and mortality conferences, and development of intraoperative guidelines based on existing knowledge. The director of anesthesiology for liver transplantation must be a designated member of the transplant team and is responsible for establishing internal policies for participation of the department of anesthesiology in the perioperative care of liver transplant patients. These policies are developed in the context of the institutional needs, transplant volume, and quality measures.The director must be a diplomat of the American Board of Anesthesiology (or hold an equivalent foreign certificate). Applicants for a directorship who are not board certified must attain this status within 2 years of approval as director. Clinical responsibilities of the director should include but are not limited to the following: preoperative assessment of transplant candidates, participation in candidate selection, intraoperative management, postoperative management, participation in the selection committee, consultation preoperatively with subspecialists as needed, and participation in morbidity and mortality conferences.
The director of transplant anesthesia must have experience demonstrated by one of the following:
Fellowship training in critical care medicine, cardiac anesthesiology, or pediatric anesthesiology that includes the perioperative care of at least 10 liver transplant patients
Experience within the last 5 years in the perioperative care of at least 20 liver transplant recipients in the operating room or intensive care unit (ICU). Experience acquired during postgraduate (residency) training does not count for this purpose
A minimum of 8 hours of Accreditation Council for Graduate Medical Education category 1 credits in transplant-related educational activities earned within the most recent 3-year period
The director is responsible for maintaining a team of well-credentialed anesthesiologists, and a basic template could be as follows: Liver transplant anesthesiologists will have undergone a fellowship in transplant anesthesia or have been involved in the anesthesia care of 12 liver transplant recipients or be proctored in at least 5 liver transplant procedures with sign-off by the director.
Lack of suitable donors is the current rate-limiting factor for performing more liver transplants. This has resulted in increasing numbers of marginal donors that are now being used, and also many liver grafts are now surgically divided to supply two recipients. The impact on the anesthesiologist and the operating room team is that this surgery is again becoming an emergent procedure that keeps the cold ischemia time of the donor organ to a minimum. This timing causes the surgery to be performed frequently in the middle of the night, when support teams may not be readily available. To meet the demand for organs and to address cultural reasons for the lack of a deceased donor organ supply, living donor procedures are increasing. The number of living donors providing right or left lobes of their livers for adult or pediatric patients may complicate the logistics of the procedures because two anesthesia teams need to be available at the same time. This scenario requires significant preparation and resources by the perioperative team.
The use of extended criteria or marginal donors has lead to criteria being put together to assess the risk of using these less-than-optimal grafts; one of the first scores to be validated was the Donor Risk Index. The Donor Risk Index includes only donor and transplant parameters found to significantly influence outcomes adversely after liver transplantation. These parameters included the donor’s age, race, height, and cause of death; split liver donor; donation after cardiac death; and cold ischemia time. Now other criteria have been added: ICU stay with ventilator for more than 7 days, body mass index greater than 30, steatotic liver greater than 40%, serum sodium level above 165 mmol/L, serum glutamic-pyruvic transaminase (SGPT) greater than 105 units/L, serum glutamic-oxaloacetic transaminase (SGOT) greater than 90 units/L, and serum bilirubin level greater than 3 mg/dL. The placement of an extended criteria donor graft into a high-risk recipient may result in a very challenging procedure.
The major disorders leading to transplantation are end-stage chronic liver disease, acute fulminant liver failure, primary liver malignancy, and certain liver-based metabolic disorders such as Wilson’s disease and α 1 -antitrypsin deficiency. In the latter category the donor liver not only replaces a damaged organ but also provides a source for the missing enzyme or protein. The contraindications for liver transplantation include active extrahepatic infections and extrahepatic malignancy. Viral hepatitis, alcoholic liver disease, and neoplasm are the most frequent causes for transplantation but remain challenging because the original disease frequently recurs unless adjunct therapy is provided.
The introduction of the Model for End-Stage Liver Disease (MELD) scoring system and Pediatric End-Stage Liver Disease (PELD) scoring system for the pediatric population has allowed organs to be transplanted based on medical urgency with a goal of reducing waiting list mortality. The MELD score is based on three laboratory results: serum bilirubin and creatinine concentrations together with the international normalized ratio (INR) ( Table 46-1 ). These laboratory values are predictive of 3-month mortality. These critically ill patients, with multiple-organ dysfunction and severe metabolic and coagulation disorders, present a significant challenge to the anesthesiologist.
| MELD score | 9 or less | 10-19 | 20-29 | 30-39 | ≥40 |
| In hospital | 4% | 27% | 76% | 83% | 100% |
| Outpatient | 2% | 6% | 50% |
∗ The MELD score: 10 × (0.957 × log e [creatinine] + log e [bilirubin] + 1.12 × log e [INR]).
The concept of a team approach to the care of the liver transplantation patient is an important factor in the development of a successful program. This approach starts with the preoperative selection and preparation of the patients and includes all facets of the perioperative care. Good communication among surgeon, hepatologist, pulmonologist, cardiologist, nephrologist, intensivist, anesthesiologist, nursing staff, and patient coordinators provides the basis for an optimal care team and a successful program.
Anesthesia management of the liver transplant recipient may involve taking into consideration a severely debilitated patient with multiple organ system malfunctions. There may be alterations in physiological and pharmacological responses, a severe coagulopathy, encephalopathy, cardiomyopathy, respiratory failure, massive abdominal ascites and pleural effusions, renal dysfunction, and severely deranged serum electrolyte levels, together with an emergent situation. The patient may have been admitted from home, having been evaluated months before the time the donor organ becomes available. The clinical status may have changed significantly from the time of initial evaluation until admission to hospital for the liver transplantation. An acute deterioration in the status of the patient may advance the patient on the waiting list or be serious enough to warrant admission to the ICU and to obtain the next available organ. Consequently, a careful preoperative assessment is necessary in the often short time available before transplantation.
Significant portopulmonary hypertension (see Chapter 39 ) may have developed in the months spent waiting for the availability of an organ. The patient may have been admitted in fulminant liver failure and be in the ICU. The patient may be comatose and require dialysis, mechanical ventilation, and intracranial pressure (ICP) monitoring. The pathophysiological processes of end-stage liver disease may affect all major organ systems, and therefore the anesthetic management must include measures for the optimal protection of all these organs, as well as the liver. Occasionally a patient may present with a hepatocellular carcinoma and still have good synthetic liver function.
A good understanding of the pathophysiology of end-stage liver disease is required to be able to develop an optimal care plan for the patient undergoing transplantation ( Table 46-2 ). The pathological condition of the liver will depend on the diagnosis and indication for liver transplantation. Genetic causes may affect more than just one major organ; an example is amyloidosis, with which significant cardiac amyloidosis may also occur. Hepatocellular cancers may develop especially in the presence of chronic viral hepatitis. The synthetic function of some of these livers may be well preserved, whereas in others severe liver dysfunction may be present.
|
The metabolic function of the liver includes the processing of carbohydrates, fats, proteins, and other substances, including drugs. The end pathway for most carbohydrates is the conversion of glucose to produce energy in the form of adenosine triphosphate, carbon dioxide, and water. The liver also plays a critical role in protein metabolism, including amino acid deamination and formation of urea and plasma proteins. Combining two molecules of ammonia with carbon dioxide forms urea, and this can readily pass out of the liver and be excreted by the kidneys. Failure of this process will cause an accumulation of ammonia in the body and resultant ill effects. The plasma proteins produced by the liver include albumin and many of the coagulation factors. The hepatocytes also secrete bile salts, cholesterol, and conjugated bilirubin that are excreted into the intestinal lumen and are essential for the absorption of fats and fat-soluble vitamins A, D, E, and K.
Cirrhosis is a serious and progressive pathological disease that eventually results in hepatic failure. It usually is the result of chronic viral disease or chronic alcoholic disease, although now because of the epidemic of morbid obesity, nonalcoholic fatty liver disease is becoming more prevalent. Hepatocyte necrosis is followed by fibrosis and nodular regeneration. Distortion of the liver’s normal cellular and vascular architecture obstructs portal venous flow and leads to portal hypertension and impairment of the liver’s synthetic and metabolic functions that results in widespread endothelial dysfunction resulting in multiorgan system dysfunction. Most patients will eventually develop jaundice and ascites. Cirrhosis is generally associated with four major clinical complications: variceal hemorrhage from portal hypertension, which may be exacerbated by a profound coagulopathy, intractable fluid retention in the form of ascites, and pleural effusions, and all this compounded by the hepatorenal syndrome (HRS), cirrhotic cardiomyopathy, and hepatic encephalopathy.
The severity of liver dysfunction is the focus of two classification systems: the Child-Turcotte-Pugh (CTP) system, which most non–liver transplant anesthesiologists will be familiar with, and the MELD score, which is used for the allocation of liver organs. The CTP score relies on ascites, encephalopathy, prothrombin time, serum albumin and total bilirubin levels, and the INR ( Table 46-3 ).
| Score | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Prothrombin time (sec) prolonged | 1-4 | 5-6 | >6 |
| Encephalopathy | 0 | 1-2 | 3-4 |
| Ascites | Slight | Moderate | Severe |
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| International normalized ratio | <1.7 | 1.8-2.3 | >2.3 |
The 3-month mortality for a patient with a CTP score of 5 to 6 points is 4%; 7 to 9 points, 14%; and 10 to 15 points, 51%.
The MELD score is a more precise calculation that is based on serum creatinine and bilirubin levels and the INR (see Table 46-1 ). Both scores assess the patient’s risk for death.
Cirrhosis of the liver is associated with varying degrees of encephalopathy. It may be characterized by changes in mental status and fluctuating neurological signs—asterixis and hyperreflexia. Mild encephalopathy has been found in up to 84% of patients with chronic liver failure. The encephalopathy appears to be related to both the amount of hepatocellular damage and the degree of shunting of portal blood away from the liver. This allows the accumulation of toxins such as ammonia, mercaptans, short-chain fatty acids, and phenol, causing deleterious effects on the brain. Spectral electroencephalogram (EEG) analysis provides reliable quantitative information in the evaluation of subclinical encephalopathy. The intraoperative use of a brain function monitor that displays a multichannel EEG can guide the management of anesthesia in this set of patients, who may be very sensitive to anesthetic agents and changes in cerebral hemodynamics. The basic patterns of the EEG—awake, sedated, surgical anesthesia, burst suppression, and isoelectricity—are easily recognized by the practitioner and aid in managing the patient’s hemodynamics and anesthesia delivery ( Fig. 46-1 ).
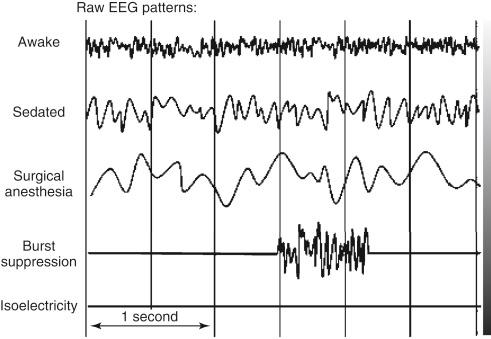
The pathogenesis of hepatic encephalopathy has been associated with increased γ-aminobutyric acid (GABA) neurotransmitter in the brain. This excess GABA neurotransmitter effect may be potentiated by benzodiazepine drugs such as diazepam; therefore this group of agents should be avoided in patients with encephalopathy because hepatic coma may be precipitated. The reversal agent flumazenil may improve the mental status of the hepatic encephalopathy patient. The liver performs a critical role in protein metabolism: the deamination of amino acids, and the formation of urea, by combining two molecules of ammonia with carbon dioxide. The kidneys may then excrete the urea produced. The failure of the liver to convert ammonia to urea results in the accumulation of ammonia, which exacerbates the encephalopathy. The liver is also responsible for the formation of plasma proteins with the exception of immunoglobulins. This includes not only the coagulation factors but also albumin, which is responsible for maintaining a normal plasma oncotic pressure and also the transport of a number of drugs. Therefore a reduction in serum albumin level will allow edema formation and also an increase in the unbound fraction of many drugs.
The patient in fulminant hepatic failure may develop deep coma, severe brain edema, and a marked increase in ICP. The anesthetic management of the comatose patient with raised ICP is greatly facilitated by the presence of an ICP monitor. However, because of the risk for bleeding in this patient population, many neurosurgeons defer placement of the monitor. An aggressive correction of the coagulopathy is required before the placement of a monitoring device. The administration of fresh frozen plasma may be all that is needed, but when this is inadequate, recombinant activated factor VII will temporarily correct the coagulopathy of liver failure and allow the placement of an ICP monitor. As the encephalopathy becomes worse and the patient becomes slowly obtunded, early intervention to maintain and secure the airway and oxygenation is indicated. Cerebral edema is best monitored and controlled by the direct measurement of ICP. Intraoperative periods of increased risk are when hemodynamic instability occurs and at the time of the new graft reperfusion. Very small changes in hemodynamics may cause major changes in cerebral perfusion pressure. The aim of anesthetic management is to keep the ICP less than 20 mm Hg, the cerebral perfusion pressure more than 50 mm Hg, and the mean arterial pressure more than 60 mm Hg. At reperfusion of the new liver graft, a significant infusion of acid and inflammatory, vasodilating cytokines may enter the circulation, together with an increase in cardiac output, resulting in an acute elevation in ICP. The infusion of a bolus of sodium pentothal or propofol at this time, guided by the ICP, may counter this effect.
Mild hyperventilation may be helpful by causing hypocapnia, resulting in cerebral vasoconstriction; however, this technique is controversial. Maintaining cerebral perfusion pressure is critical at this time, and an ICP transducer together with intra-arterial pressure and central venous pressure monitoring are vey useful management tools. The use of cerebral function monitors that display the cortical electrical activity of the brain may be helpful adjuncts in management. The prevention of volume overload and the increase in central venous pressure can be facilitated by the early introduction of continuous venovenous hemodialysis. Other measures that can help protect the brain include controlled hypothermia to 34° C and the use of osmotic diuretics and barbiturate or propofol coma.
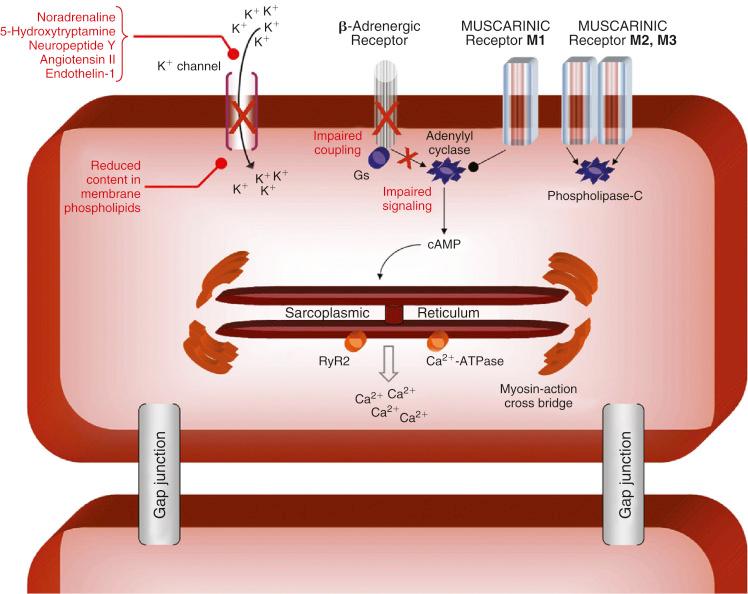
The cardiovascular system should be carefully evaluated. It has been recognized for more than 50 years that a high cardiac output state with extremely low systemic vascular resistance (SVR) typically is present with liver cirrhosis. This state can make the assessment of ventricular function difficult to interpret accurately. A reduced afterload can allow a ventricle that is functioning poorly to appear to be functioning well. This is important to know because severe cardiomyopathy is frequently associated with liver cirrhosis. Cardiomyopathy has been reported to exist to some degree in all patients with liver cirrhosis. Cardiomyopathy may result in impaired contractility, especially under stress conditions, and altered diastolic relaxation. It also presents as a blunted responsiveness to β-adrenergic receptor agonists because of diminished β-receptor function that may be exacerbated by the chronic use of β-receptor blocking agents ( Fig. 46-2 ). The cardiomyopathy may be further compromised in the alcoholic patient by the presence of an alcoholic cardiomyopathy. The performance of a transjugular intrahepatic portosystemic shunt stent placement will result in a sudden increase in cardiac output and may precipitate cardiac failure in the cardiomyopathic patient. Similarly, because this is a high–cardiac output cardiomyopathy, reperfusion of the liver graft at the time of transplantation, which usually results in a further acute increase in cardiac output, may precipitate ventricular dysfunction, both right and left, causing graft dysfunction.
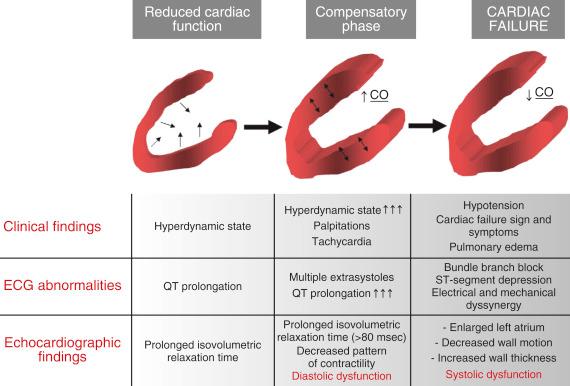
Cirrhotic cardiomyopathy is characterized by an increased cardiac output, attenuated response to inotropic stimuli, mild chamber dilatation, and repolarization changes. The examination by echocardiography may initially be interpreted as normal cardiac function because of the significant reduction in afterload caused by the low systemic vascular resistance. However, on closer examination both systolic and diastolic dysfunction may be demonstrated. The diagnostic features are an early to late diastolic filling ratio (E/A ratio) of less than 1, a prolonged deceleration time above 200 msec, a prolonged isovolumetric relaxation time of more than 80 msec, enlarged left atrium, overall decreased pattern of contractility, decreased wall motion, increased wall thickness, resting ejection fraction below 50%, left ventricular ejection time that is prolonged greater than 0.44 seconds (rate corrected) ( Fig. 46-3 ). If cardiomyopathy is suspected, a dobutamine stress echocardiogram may be performed to assess ventricular function. Any patient who presents for liver transplantation and is found to have a low cardiac index and elevated filling pressures must be closely examined for the presence of a cardiomyopathy. However, the most common cause of a low cardiac index in the immediate preoperative period is hypovolemia. The cirrhotic cardiomyopathy does improve after liver transplantation with disappearance of diastolic dysfunction and normalization of the cardiac response to stress.
Because the upper age limit for liver transplantation is being extended in most centers and is now based on physiological age as opposed to chronological age, careful screening for coronary artery disease (CAD) is necessary because the prevalence of this disease increases with age. In the past it was thought that there was reduced CAD in cirrhotic patients. However, recent data demonstrate a significant incidence of severe CAD in up to 16% of liver transplant candidates older than 50 years. The presence of CAD in patients undergoing liver transplantation is greater than in the general population and has been reported to increase the mortality rate to 50% and the morbidity rate to 81%. Therefore there should be close screening of at-risk liver transplant candidates for CAD and a low threshold for performing a dobutamine stress echocardiogram. If ischemia is producible, a coronary arteriogram should be obtained. If the coronary arterial obstructive lesions can be dilated and a bare-metal stent placed, liver transplantation may be an acceptable-risk procedure.
Occasionally angioplasty is not a viable option, and the decision must be made as to whether coronary artery bypass grafting or liver transplantation should be performed first or if they should be performed together. Cardiac surgery in the patient with severe liver dysfunction may result in an exacerbation of the preexisting coagulopathy and potentially a decrease in liver blood flow, precipitating fulminant liver failure. Conversely, the unavoidable major hemodynamic changes that may occur during liver transplantation make this option very hazardous if it is undertaken first. This therapeutic dilemma requires close consultation among the cardiologist, anesthesiologist, and surgeon. The role of coronary artery bypass surgery is a risk-benefit decision that will depend on the severity of the liver disease. The at-risk patient should receive a dobutamine stress echocardiogram so that CAD, ventricular function, and valvular function can be assessed and to determine if the pulmonary artery pressures are normal or elevated. Plotkin et al demonstrated that the dobutamine stress echocardiogram had a sensitivity of 100%, a specificity of 90%, a positive predictive value of 100%, and a negative predictive value of 100% when evaluating liver transplant candidates with cardiac risk factors. These data support the use of the dobutamine stress echocardiogram as a screening tool for CAD in those liver transplant patients when indicated by the guidelines of the American College of Cardiology and the American Heart Association. The data supporting the administration of perioperative β-blockers to patients undergoing surgery with a risk for coronary ischemia is compelling and should be applied to liver transplant recipients who meet the criteria. Many liver recipients are already taking β-blockers as treatment for portal hypertension. The combination of a cirrhotic cardiomyopathy and the administration of β-blockers can make catecholamine responses very blunted during liver transplantation.
The monitoring of the cardiovascular system should include intra-arterial and central venous pressure sensors. The role of the pulmonary artery catheter is controversial, but it can detect previously undiagnosed pulmonary hypertension and cardiomyopathy and may be used to guide the administration of vasopressors and volume in the face of hypotension. The differentiation among hypovolemia, low SVR, and poor ventricular function can be critical. The transesophageal echocardiogram (TEE) offers a comprehensive assessment of volume status, together with left and right ventricular function. In patients with pulmonary hypertension who are undergoing liver transplantation, the TEE can provide essential information on right ventricular function. On the rare occasion when pulmonary emboli occur, the TEE can be critical in early diagnosis and management.
Ventilation may be restricted by massive abdominal ascites compressing the diaphragm and by large bilateral pleural effusions. The mortality of patients with end-stage liver disease who develop adult respiratory distress syndrome is reported to be 100% unless transplantation is performed. Circulating endotoxins, the result of the liver disease, may initiate an inflammatory cascade, causing a panendothelial injury that causes multiorgan dysfunction. Cirrhotic patients present with diffuse bilateral pulmonary infiltrates and poor pulmonary compliance. This clinical picture of adult respiratory distress syndrome is thought to be the result of a sepsis syndrome and not active sepsis, which could be a contraindication to transplantation. Successful resolution of adult respiratory distress syndrome has followed orthotopic liver transplantation.
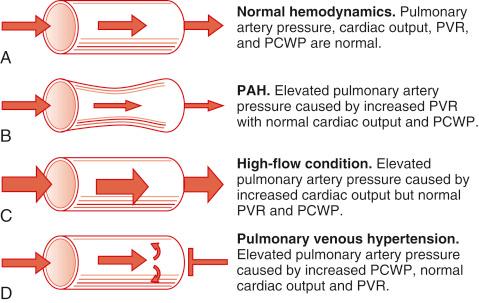
These pathological entities are discussed in detail in Chapter 39 . The key to understanding the clinical significance of high pulmonary artery pressure is to determine if it is the result of increased vascular resistance, cardiac failure, volume overload, or high cardiac output ( Fig. 46-4 ). The management then follows protocols.
An adequate volume of urine production is critical to the intraoperative management of the liver transplant patient. Volume overload, if allowed to occur, results in congestion and eventual failure of the new liver graft. Good urine output enables proper management of volume changes associated with the correction of coagulopathy. Satisfactory renal function assists in maintaining electrolyte and acid-base homeostasis. Acute elevations in serum potassium levels may occur from the rapid transfusion of units of old red blood cells and from the reperfusion of the new liver graft. The role of monitors of cardiac output and volume state can be essential for optimizing renal perfusion and preventing congestion of the new liver graft. The TEE can provide good information on the cardiac chamber sizes and supplement information provided by the pulmonary artery catheter.
Patients with liver cirrhosis frequently have renal dysfunction because of prerenal azotemia or intrinsic renal disease. Prerenal azotemia may be the result of chronic hypovolemia caused by long-term diuretic use and may be treated by volume expansion. Liver disease is also associated with increased renin-angiotensin activity and increased antidiuretic hormone activity, leading to sodium and water retention that is often accompanied by a decreased effective plasma volume. Water retention is greater than sodium retention, resulting in hyponatremia. Secondary aldosteronism also occurs, making hypokalemia and total body potassium depletion common. Serum creatinine level is a significant component of the MELD score, which determines the position of the recipient on the waiting list for an organ; therefore many recipients will have impaired renal function. Those patients with reversible disease such as HRS should recover renal function after transplantation, but those patients who have severe renal damage may require a renal transplant at the same time as the liver transplant. Acute renal dysfunction occurs in up to 78% of liver transplant recipients intraoperatively. Perioperative acute kidney injury may be the result of prerenal, intrinsic renal, postrenal, and exogenous factors ( Table 46-4 and Fig. 46-5 ).
| Prerenal | Absolute or relative renal hypoperfusion; hepatorenal syndrome |
| Intrinsic renal | Glomerular, tubular, vascular, or interstitial damage: ischemia, hypoxia, ischemia/reperfusion injury, inflammation, and hypotension |
| Postrenal | Abdominal compartment syndrome, obstruction of ureters or bladder |
| Exogenous | Drug induced: calcineurin inhibitors |
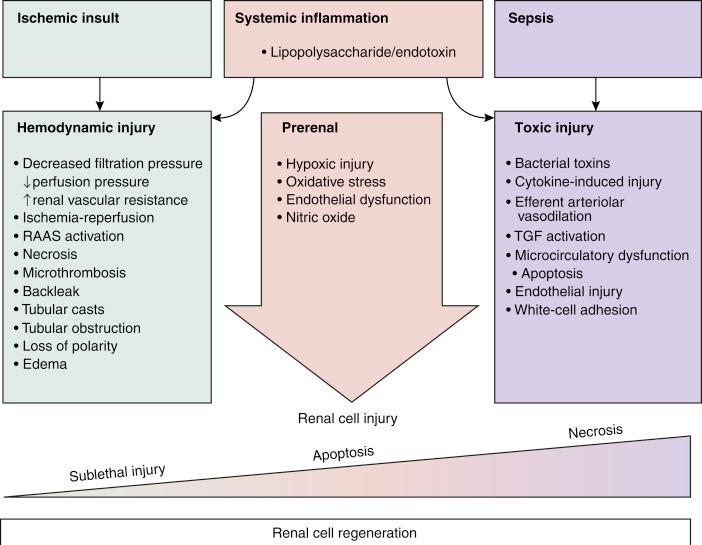
Renal dysfunction may also present as HRS, in which there is no parenchymal damage, but there is profound hypoperfusion of the kidney caused by vasoconstriction in the setting of systemic and splanchnic arterial vasodilatation in patients with advanced cirrhosis. HRS diagnosis is based on the absence of primary renal disease, proteinuria, hypovolemia, and renal hypoperfusion. It is characterized by normal urinary sediment, low urinary sodium level (<10 mEq/L), azotemia, and oliguria. It is important that hypovolemia be ruled out ( Table 46-5 ).
| 1 | Cirrhosis with ascites |
| 2 | Serum creatinine >1.5 mg/dL |
| 3 | No improvement in serum creatinine after 2 days of volume expansion with albumin |
| 4 | Absence of shock |
| 5 | No recent treatment with nephrotoxic drugs |
| 6 | Absence of parenchymal kidney disease as indicated by proteinuria >500 mg/day |
HRS is reversible with liver transplantation but may progress to acute tubular necrosis that is not reversible and could be treated by a combined liver-kidney transplant. Increased sympathetic tone in the renal vasculature plays an important role in the development of this syndrome. Adequate volume replacement is essential in these patients.
Endogenous production of creatinine is reduced with end-stage liver disease, and therefore serum creatinine level may not reflect the severity of the renal dysfunction. The patient’s prerenal status must be closely assessed, because reduced plasma volume may exist from chronic use of diuretics. Aggressive rehydration may be necessary to provide and maintain an effective intravascular volume and prevent renal hypoperfusion. Perioperative oliguria should be treated initially as prerenal in origin and a fluid challenge administered. Using an albumin volume expansion and a renal dose infusion of dopamine, together with a mannitol infusion, may optimize urine output in the patient with HRS. A dopamine infusion, however, is not protective of the kidney in this clinical situation. The role of an infusion of fenoldopam, a dopamine-1 receptor agonist, shows great promise not only in renal protection but also by improving mesenteric perfusion and new liver graft vascular perfusion. A completely different approach that is also being examined is the role of splanchnic vasoconstriction in improving HRS. Vasopressin analogues terlipressin and ornipressin are undergoing clinical trial.
If the patient is unresponsive to therapy, early institution of intraoperative continuous venovenous hemodialysis should be considered. It can easily be accomplished in the operating room and greatly facilitates fluid and electrolyte management. If it is not available, all units of banked blood should be washed before infusion to reduce the potassium load. The cell-saver blood salvage system can be used to do this directly in the operating room. The cell-saver system can also be used to “hemodialyze” the patient by drawing off blood through a large-bore central catheter, washing it in the cell saver, and retransfusing it back to the patient. In this manner, volume and potassium can be removed, and the patient undergoes hemoconcentration.
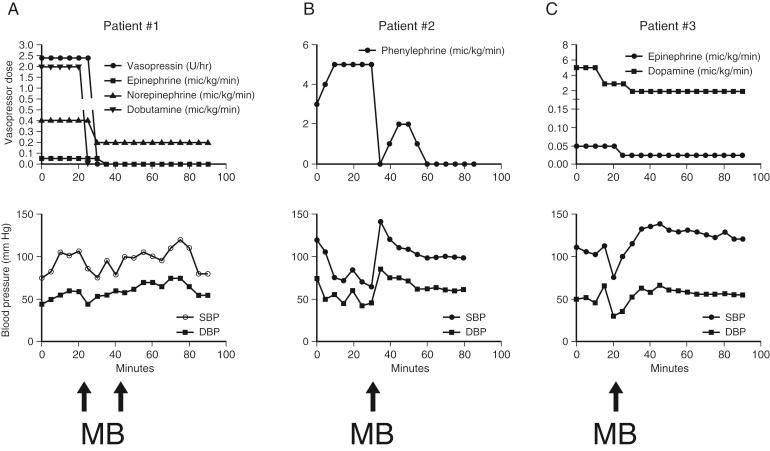
Indications for instituting continuous venovenous hemodialysis during liver transplantation include anuria or severe oliguria, hyperkalemia, hyponatremia, severe metabolic acidosis, volume overload with poor response to diuresis, and abdominal compartment syndrome. Acute kidney injury during liver transplantation contributes significantly to the incidence of postoperative renal failure. Even mild kidney injury, defined as a rise in serum creatinine level above 0.5 mg/dL, is associated with reduced patient and graft survival. Therefore measures to maintain renal perfusion and protect the kidney during liver transplantation are essential. Should venovenous bypass be used intraoperatively to decompress the venous system and maintain cardiac output during the anhepatic phase? The published data do not support using or not using bypass. Maintaining renal perfusion is essential and may be very challenging when faced with massive blood loss and the reperfusion syndrome. Vasopressors that have shown some improvement in renal perfusion and blood pressure during liver transplantation include norepinephrine, vasopressin, and methylene blue ( Fig. 46-6 ). Methylene blue may cause severe serotonin toxicity if administered to patients receiving serotonin reuptake inhibitors.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here