Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anemia of chronic inflammation (ACI) is also termed anemia of chronic disease and considered the second most frequent anemia in the world, after iron deficiency anemia (IDA). ACI is also the most common anemia in hospitalized patients, found in conditions associated with an activated immune response, including chronic infections, autoimmune and inflammatory illnesses, and malignancy. The underlying cause of anemia in these diseases is multifactorial, including both direct and indirect inhibition of red blood cell (RBC) production in the bone marrow and mildly decreased RBC life span. The teleological argument for the presence of anemia in these conditions is presumed to be related to iron sequestration, providing an evolutionary advantage in light of the iron dependence of pathogens and rapidly replicating cells. Thus, iron sequestration restricts iron availability and serves to limit the growth of pathogens and malignant cells at the expense of hemoglobin synthesis. Previously considered a diagnosis of exclusion, with treatment mainly focused on the underlying disease, the identification of the peptide hormone hepcidin and its major role in the pathophysiology of ACI have enabled both an enhanced mechanistic understanding and development of novel therapeutics for ACI.
In addition to anemia associated with chronic infections, autoimmune and inflammatory illnesses and cancer, anemia in patients with chronic renal disease, congestive heart failure, and solid organ transplant is also categorized as ACI due to an inflammatory component in these conditions. In contrast, an inflammatory component in idiopathic anemia of the elderly, which often presents with typical features of ACI, remains presumptive. For the purposes of this chapter, we offer a perspective on ACI in its broadest definition. Despite this, the specific details may not apply identically to all diseases on this list (e.g., in chronic renal insufficiency, anemia is in large part driven by inadequate erythropoietin [Epo] or “Epo resistance,” although therapeutic benefit in anemia management may be gained by suppressing hepcidin).
Anemia of chronic inflammation (ACI) remains a diagnosis of exclusion and is characterized as a mild, normocytic normochromic hypoproliferative anemia with hemoglobin ranging from 8 to 10 g/dL ( Fig. 38.1 ). ACI may present, however, with hemoglobin <8 g/dL and sometimes dramatic microcytosis in up to one-third of patients. Typically, elevated inflammatory markers (e.g., C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], and interleukin 6 [IL-6]) and decreased circulating serum iron concentration and transferrin saturation despite ample iron stores (e.g., serum ferritin >100 ng/mL) help to diagnose ACI. ACI may be difficult to differentiate from iron deficiency anemia (IDA). IDA may also coexist with ACI as chronic inflammation, infection, and malignancy may coexist with nutritional deficiency, blood loss, hemolysis, renal failure, and primary bone marrow disorders. IDA in the setting of ACI is typically a consequence of blood loss, either gastrointestinal or genitourinary. For example, chronic inflammatory bowel diseases are frequently associated with both chronic gastrointestinal bleeding secondary to the underlying disease and its treatment (with nonsteroidal anti-inflammatory drugs [NSAIDs] and glucocorticoids). In such cases, localizing and halting the source of bleeding are both required in addition to potential iron replacement. In autoimmune gastritis and celiac disease, iron deficiency may also result from decreased iron absorption. Precise characterization of the diathesis underlying the anemia is thus essential to ascertaining whether administration of iron is likely to improve or resolve the anemia. Furthermore, the underlying pathophysiology of anemia can assist with the decision-making regarding whether enteral or only parenteral iron administration is expected to work.
The traditional gold standard approach to identify iron deficiency anemia (IDA) in anemia of chronic inflammation (ACI) is Prussian-blue staining for iron within macrophages on a bone marrow biopsy specimen. However, this approach has been challenged in light of its invasiveness and high inter-observer variability. In a study of mostly older patients with idiopathic moderate anemia (i.e., hemoglobin 10 g/dL), a bone marrow aspirate with biopsy added little to physical examination and serology. Furthermore, iron-loaded macrophages in bone marrow biopsy specimens correlate poorly with the availability of this iron for erythropoiesis in high hepcidin states, as in ACI. Bone marrow examination may, however, be necessary in some situations to rule out other potential causes of anemia, including malignancy (e.g., myelodysplastic syndrome). Serum ferritin functions as an acute phase reactant. Hence, it alone is insufficiently specific to identify IDA in ACI, unless it is very low (i.e., serum ferritin <10 ng/mL) ( Fig. 38.1 ). Serum ferritin is in equilibrium with cellular ferritin. Thus, serum ferritin increases when iron is sequestered in the liver and splenic macrophages where cellular ferritin expression is increased, as in ACI. Despite this, some degree of elevated ferritin concentration does not exclude a possible response to parenteral iron therapy.
While some investigators argue that a ferritin level >50 ng/mL excludes any component of iron deficiency even in inflammatory states, a meta-analysis of iron studies in clinical reports from 1842 subjects with serum ferritin levels >45 ng/mL showed a prevalence of iron deficiency of 6.7% and 3.5% of 1368 subjects with a serum ferritin level >100 ng/mL had iron deficiency. Other researchers have shown that in acute inflammation, even serum ferritin levels >3500 ng/mL can coexist with absent iron stores in bone marrow aspirates.
ACI is independently associated with poor outcomes and a decrease in quality-of-life measures. Standard approaches, such as parenteral iron and erythropoiesis-stimulating agents (ESAs), are not consistently effective. Thus, despite its frequency and significant impact both on morbidity and mortality and new insight into the role of hepcidin, the pathophysiology of ACI remains incompletely understood and currently available therapies are not effectively optimized. The goals of this chapter are to (1) describe the clinical presentation and diagnosis of ACI, (2) elucidate our current understanding of inflammation and iron metabolism and their mechanistic relationship with erythropoiesis, and (3) discuss both the current standard and future potential therapeutic modalities.
Additional parameters have been proposed to differentiate ACI from IDA and provide evidence of concurrent IDA and ACI. Those include soluble transferrin receptor 1 (sTfR1) concentration and sTfR1/log ferritin ( Fig. 38.1 ); these markers have been proposed as estimates of iron-deficient erythropoiesis. For example, circulating sTfR1 concentration is proportional to the cellular expression of the membrane-associated transferrin receptor 1 (TfR1), increases with increased cellular iron requirements and cellular proliferation and is unaffected by inflammation. While a low ratio sTfR1/log ferritin (<1) suggests ACI, sTfR1/log ferritin greater than 2 is indicative of concurrent IDA and ACI. Despite this potential, sTfR1 assays have not been standardized or widely adopted in light of the lack of convincing data of their utility.
A CLIA-approved and CAP-accredited hepcidin assay is available, and a potential standardized international hepcidin reference range is being developed. An algorithm that incorporates hepcidin levels is under development to increase specificity for IDA in ACD. To date, studies reveal that relatively high hepcidin has been associated with lower mean corpuscular hemoglobin in anemic patients and impaired response to ESAs in an animal model of ACI, providing some degree of promise for its utility. Finally, hemoglobin concentration in reticulocytes (CHr), distinct from indices of mature RBCs, can reflect the recent status of iron stores in normal or Epo-induced erythropoiesis. sTfR1-, CHr-, and hepcidin-based algorithms for iron-replete and iron-deficient ACI are being developed. A combination of these will likely be useful in diagnosing and differentiating ACI from ACI with concurrent IDA. For now, none of these additional parameters are fully characterized or yet widely incorporated into clinical practice, and the diagnosis of ACI relies on more standard measures (see Fig. 38.1 ).
ACI may co-occur with many different underlying diseases, including diseases not conventionally considered “inflammatory,” such as coronary artery disease, congestive heart failure, obesity, renal failure, and diabetes; patients with these illnesses often exhibit cytokine abnormalities more typically associated with inflammatory conditions (e.g., elevated interleukin 6 [IL-6] and C-reactive protein [CRP] in kidney disease or CRP and fibrinogen in myocardial infarction). For example, 52% of hospitalized patients with unexplained anemia meet laboratory criteria for ACI, and of the 184 patients admitted to intensive care units, most patients with anemia are found to have laboratory findings consistent with ACI (while 13% also had nutritional causes of anemia [iron, folate, or vitamin B12 deficiency]). Patients older than age 60 years have a 24% 3-year incidence of ACI. The epidemiological studies of ACI are complicated by the heterogeneity of underlying diseases. ACI is also extremely common in cancer; 39% of cancer patients are found to be anemic. Chemotherapy with or without radiation therapy increases the incidence of anemia to 63% and 42%, respectively. Anemia is 56% prevalent in patients positive for human immunodeficiency virus (HIV), directly proportional to the burden of symptoms, and mild anemia is 33% to 60% prevalent in patients with rheumatoid arthritis (RA). Although the clinical setting in which anemia is found helps with the diagnosis of ACI, an underlying inflammatory process could not be identified in 30% of cases.
The pathophysiology of ACI is multifactorial, resulting from the effects of inflammatory cytokines, particularly interleukin-1 (IL-1), IL-6, IL-10, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IFN-α, and IFN-β, all or some of which are increased in most inflammatory processes, i.e., infections, autoimmune disorders, and malignancies. Cytokine levels frequently correlate inversely with hemoglobin, potentially implicating them in the etiology of ACI. Serum TNF-α levels correlate with both disease activity and the degree of anemia in RA. Transgenic mice with endogenously elevated IFN-γ exhibit preferential differentiation of non-erythroid lineage hematopoietic precursors and diminished erythroid burst-forming units (BFU-E). Furthermore, multiple clinical trials using cytokine antagonists in inflammatory diseases demonstrate effective reversal of anemia. For example, in patients with Crohn disease, anti-TNF-α therapy improved both disease activity and anemia. Anti-TNF-α therapy in vitro increased the growth of peripheral blood-derived erythroid progenitors from patients with active disease. Multiple lines of evidence suggest that elevated inflammatory cytokines lead to (1) decreased RBC life span, (2) increased iron sequestration and resultant decreases in iron availability for erythropoiesis and hemoglobin synthesis, (3) direct and indirect inhibition of erythroid progenitor differentiation, and (4) inadequate Epo response to anemia ( Fig. 38.2 ).
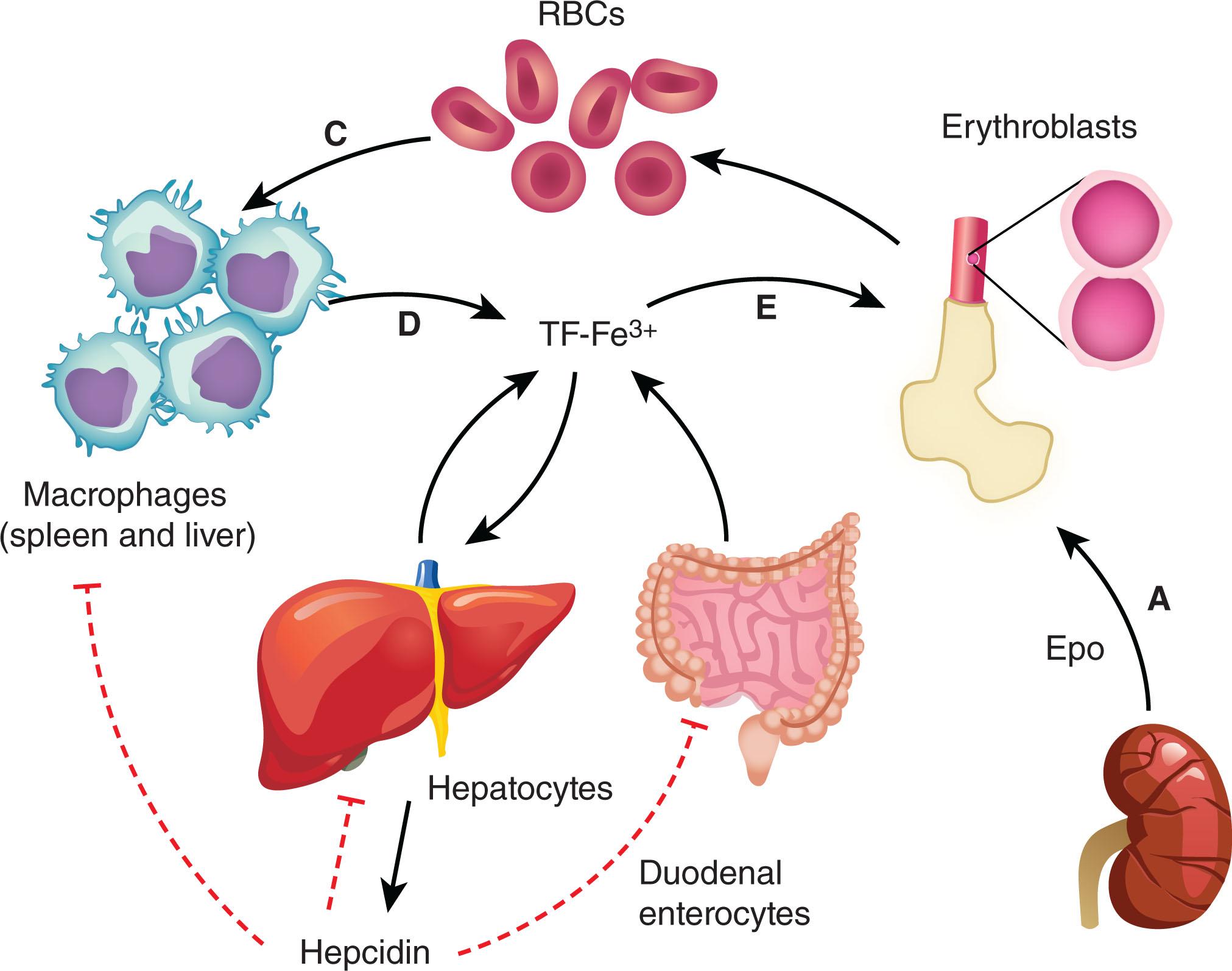
The underlying mechanism for the reduction in RBC life span in ACI is not fully elucidated, but studies support the absence of an intrinsic RBC defect. TNF-α, IFN-γ, and IL-1 specifically enhance erythrophagocytosis by splenic macrophages, leading to a shortened RBC life span (see Fig. 38.2 ). RBCs from ACI patients have a normal life span when infused into normal subjects, suggesting an abnormality extrinsic to the RBC itself. For example, mice with elevated IFN-γ levels (either as a transgene or by exogenous administration) exhibit reduced RBC survival because of RBC removal by cytokine-stimulated macrophages. In addition, chronic sub-lethal doses of TNF-α in rats results in a 25% decline in total RBC mass and decrease in RBC survival. Furthermore, alterations in RBC rheology (deformability and aggregation) are seen in intensive care unit patients, especially those with sepsis. In vivo and in vitro experiments suggest that fever itself can induce rheologic changes in RBCs, leading to increased destruction and up to a 15% decline in RBC mass. Similarly, increased cytokines, for example IL-1, in RA patients are associated with enhanced RBC-phagocytic ability of macrophages.
Inflammatory cytokines also directly suppress erythroid precursor proliferation and differentiation (Epo responsiveness is discussed in subsequent sections). As a consequence, the bone marrow’s ability to compensate for enhanced erythrophagocytosis is hampered. Studies support a role for TNF-α, IL-1, IFN-γ, and perhaps IL-6 in this direct inhibition.
First, IFN-γ modulates gene expression involved in the in vitro proliferation of hematopoietic stem cells, thus affecting self-renewal. Second, the addition of IFN-γ to in vitro cultured erythroid progenitors increases the expression of the transcription factor PU.1. Since PU.1 directly interacts with the major erythroid transcription factor GATA-1, it is postulated that IFN-γ treatment decreases proliferation and differentiation of erythroid progenitors by a PU.1 dependent mechanism. Third, serum TNF-α levels inversely correlate with hemoglobin levels and BFU-E number in RA patients. Fourth, treatment with anti-TNF improves proliferation and decreases apoptosis of erythroid progenitor cells. The actual effect of TNF-α on erythropoiesis is likely indirect, mediated by the local release of other cytokines including IFN from accessory cells, since the inhibitory effect of TNF-α on CFU-E was completely abrogated by neutralizing antibodies against IFN-β but not by antibodies to IFN-γ or IL-1. Similarly, the effects of IL-1 also appear to be indirect since the growth of purified CFU-E is inhibited by IL-1 only in the presence of adherent T lymphocytes. This inhibition can be reversed by antibodies to IFN-γ, suggesting that IL-1 leads to lymphocyte secretion of IFN. Lastly, more recent data suggest that IL-6 may have a suppressive effect directly on erythroid precursors.
In in vitro erythroid colony formation assays derived from bone marrows of patients with ACI have shed some light specifically on extrinsic effects on erythroblast suppression. The removal of bone marrow–adherent cells (mostly macrophages and monocytes) leads to increased erythroid colony formation. This effect is lost after co-culture with ACI-adherent cells but unaffected by co-culture with control bone marrow–derived adherent cells. Patients with RA have decreased BFU-E that are inversely proportional to circulating TNF levels. In parallel, culture with serum from patients with RA and anemia inhibits BFU-E proliferation, while serum from non-anemic RA patients does not. While these are not definitive studies and do not specifically delineate the factors involved, they point to an extrinsic effect on erythropoiesis.
Together, these data provide evidence of the complex crosstalk between inflammation and erythropoiesis with significant work yet to be done to enhance clarity and provide a more comprehensive understanding of the underlying molecular mechanisms beyond the mostly correlative evidence thus far.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here